Abstract
Intracellular pathogens subvert the host cell cytoskeleton to promote their own survival, replication, and dissemination. Study of these microbes has led to many discoveries about host cell biology, including the identification of cytoskeletal proteins, regulatory pathways, and mechanisms of cytoskeletal function. Actin is a common target of bacterial pathogens, but recent work also highlights the use of microtubules, cytoskeletal motors, intermediate filaments, and septins. The study of pathogen interactions with the cytoskeleton has illuminated key cellular processes such as phagocytosis, macropinocytosis, membrane trafficking, motility, autophagy, and signal transduction.
Introduction
Pathogenic microorganisms offer many advantages for elucidating cytoskeletal function and regulation. They exploit actin, microtubules, septins, and intermediate filaments (IFs) in diverse ways. They provide clear functional read-outs, such as infection efficiency or formation of distinct cytoskeletal structures. Finally, microbes often produce locally focused or exaggerated signals, facilitating the dissection of pathways that might be more diffuse or moderate in the host. Pathogens have helped us assign function to cytoskeletal proteins, discover new regulatory modes, and unravel temporal and mechanistic interplay between factors controlling filament dynamics.
In this review, we discuss four infectious processes that have shed light on the host cytoskeleton. The first is pathogen invasion, which exploits cellular uptake pathways that rely on actin, such as phagocytosis and macropinocytosis. Emerging evidence suggests that microtubules and septins also play roles in distinct entry pathways. The second process is establishment of a replication niche, which subverts cytoskeletal functions that normally operate during membrane trafficking and cellular defense. Third, the actin-based motility (ABM) of pathogens through the cytoplasm mimics vesicle rocketing and has also illuminated host cell migration, whereas pathogen motility on the outer surface of the plasma membrane mimics a receptor tyrosine kinase signaling pathway. Finally, pathogen dissemination is an emerging field for which the endogenous processes are not defined. We draw examples from three decades of cellular microbiology, focusing on recent developments and on cases where pathogens (mostly bacteria) played particularly noteworthy roles in key discoveries.
Bacterial invasion of host cells: Many doors, many keys
Entry pathways converge on actin polymerization.
Diverse bacteria invade nonphagocytic cells by stimulating endogenous uptake processes, such as phagocytosis and macropinocytosis. Actin polymerization is central to both of these processes, driving plasma membrane extensions that engulf external cargo. Invading bacteria use a multitude of signaling molecules upstream of actin polymerization, and thus have contributed broadly to our understanding of actin regulation.
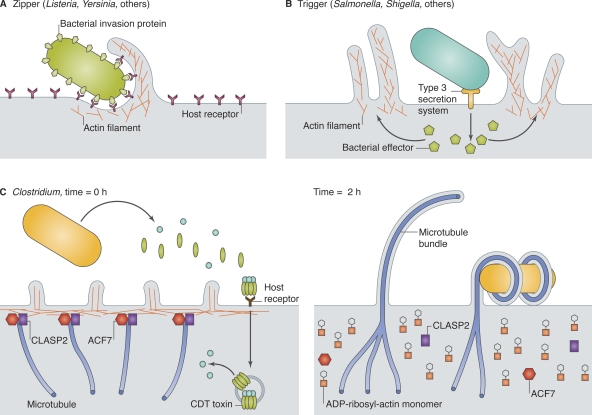
Bacterial invasion pathways have historically been classified into “zipper” and “trigger” categories (Cossart and Sansonetti, 2004). Zipper mechanisms, best studied for Listeria monocytogenes and Yersinia spp., occur when specialized bacterial surface proteins bind host receptors that signal across the membrane to a phagocytic pathway, producing limited membrane rearrangement closely apposed to the entering bacterium (Fig. 1 A). In trigger mechanisms, exemplified by Salmonella enterica serovar Typhimurium (Salmonella) and Shigella flexneri, the pathogen injects effector proteins across the host membrane, often via the syringe-like type 3 secretion system (T3SS), inducing a bloom of actin-rich membrane ruffles that engulf the bacterium and nearby particles (Fig. 1 B). Study of Salmonella invasion established that ruffles directly mediate macropinocytosis, a process in which extracellular cargo is taken up nonselectively, and that ruffles produced by endogenous mechanisms, such as by growth factors, have identical uptake behavior (Francis et al., 1993).
Figure 1.
Bacteria exploit actin and microtubules to promote invasion and adherence. (A) Zippering bacteria express an invasion protein on their surface, which binds to a host receptor and initiates actin-dependent phagocytosis. (B) Triggering bacteria inject protein effector(s) across the host cell membrane, usually via a T3SS, leading to actin-dependent macropinocytosis. (C) Clostridium difficile transferase (CDT), a binary toxin, is endocytosed by intestinal epithelial cells, and the A subunit is released into the cytosol (left). CDT toxin ADP-ribosylates actin, promoting actin filament disassembly, effacement of microvilli, and release of cortical proteins that normally capture and stabilize microtubules. Unrestrained microtubule growth produces cellular extensions that wrap around external bacteria.
Another lesson learned from bacterial entry is the central importance of the Arp2/3 complex in plasma membrane remodeling. The Arp2/3 complex, when activated by nucleation-promoting factors (NPFs), nucleates branched actin networks. NPFs can be recruited to the plasma membrane and activated by Rho-family GTPases, leading to the formation of structures such as ruffles and phagocytic cups. Most invasive pathogens signal to the Arp2/3 complex during entry, although some (notably Salmonella) also exploit Arp2/3-independent actin assembly pathways (Hayward and Koronakis, 1999; Zhou et al., 1999; Hänisch et al., 2011). Recent work continues to impart information about the pathways that regulate the Arp2/3 complex. For instance, when the contributions of several NPFs to Salmonella invasion were quantified, genetic deletion of N-WASP was found to double expression of the NPF WASH in uninfected cells (Hänisch et al., 2010). This suggests that WASH can partially compensate for the loss of N-WASP and is one of many examples of cytoskeletal plasticity.
A general lesson about actin polymerization is that it requires vigilant modulation to maintain cell function and viability. Salmonella illustrates this point, as it injects at least six effectors that control actin rearrangements during entry (Haraga et al., 2008; McGhie et al., 2009). Two effectors (SopE and SopE2) activate Rho GTPases by mimicking host guanine nucleotide exchange factors (GEFs; Hardt et al., 1998; Stender et al., 2000), while another (SopB/SigD) activates GTPases indirectly, via inositol phosphatase activity (Zhou et al., 2001; Terebiznik et al., 2002). Two effectors (SipA and SipC) directly interact with actin, nucleating and stabilizing filaments near the entry site (Hayward and Koronakis, 1999; Zhou et al., 1999; Lilic et al., 2003; McGhie et al., 2004). After a burst of polymerization, a sixth effector (SptP) down-regulates Rho-family proteins by promoting their GTPase activity, restoring normal actin structure at the cell cortex (Fu and Galán, 1999). The balance between Rho activation and inhibition is regulated both spatially (Patel et al., 2009) and temporally (Kubori and Galán, 2003). Thus, triggered entry is not a sudden event like the firing of a gun, but an elaborate, choreographed process.
Is actin the only gatekeeper?
Although actin polymerization is critical for uptake processes, roles for other filament networks are being uncovered. Microtubule-dependent bacterial invasion has been reported for Campylobacter jejuni and Citrobacter freundii (Oelschlaeger et al., 1993), two pathogenic strains of Escherichia coli (Donnenberg et al., 1990; Dhakal and Mulvey, 2009), and even the well-studied Listeria (Guzman et al., 1995; Kuhn, 1998) and Salmonella (Aiastui et al., 2010). Whether actin is also required in some of these cases is unsettled. A precise role for microtubules during entry has been elusive, in part because the pathway(s) appear to be cell-type and strain specific.
In the past few years, progress has been made toward defining a mechanistic basis for microtubule dependence during bacterial entry. Several toxins made by Clostridium spp. were found to induce the temporary formation of long, microtubule-filled projections that entwine bacteria and promote adherence (Fig. 1 C; Schwan et al., 2009). Although Clostridium do not invade cells, adherence is a prerequisite of invasion, so microtubule-based projections could promote entry by other pathogens. Interestingly, the toxins affect microtubules indirectly: they ADP-ribosylate actin, leading to actin filament disassembly, followed by release from the cell cortex of proteins that normally capture and stabilize microtubules (CLASP2 and ACF7). For a few hours after toxin application, microtubule-based projections dominate the cell morphology, followed by cell shrinking and rounding. Actin-depolymerizing drugs also induce microtubule-based projections, albeit at lower levels. The exaggerated effect produced by Clostridium toxins could provide a tool for dissecting the interplay between actin and microtubules at the cell periphery.
Intermediate filaments and septins might also contribute to bacterial entry. The intermediate filament vimentin has been implicated in invasion by Salmonella (Murli et al., 2001) and Escherichia coli K1 (Chi et al., 2010). Moreover, depletion of septin-2 reduced invasion efficiency of Listeria and Shigella, and several septins localized around invading bacteria (Mostowy et al., 2009). For Listeria, the effect of septin depletion was specific to one of two receptor-mediated internalization pathways and varied with cell type, suggesting that septins participate in discrete entry pathways.
Cross talk between actin, microtubules, intermediate filaments, and/or septins occurs in many cellular contexts (Rodriguez et al., 2003; Chang and Goldman, 2004; Li and Gundersen, 2008; Gilden and Krummel, 2010; Spiliotis, 2010), complicating the demarcation of roles for each filament type. Further complications arise from findings that blur distinctions between different entry pathways; for instance, zippering bacteria such as Listeria exploit the clathrin-mediated endocytic machinery, which is classically associated with smaller cargo (Veiga and Cossart, 2005; Veiga et al., 2007). A systems biology approach, as proposed for viral entry (Damm and Pelkmans, 2006), could help untangle the pathways, and might clarify the variability across cell types. In this approach, RNAi knockdown of numerous host genes is combined with infection by a panel of pathogens to define “functional modules,” or host genes that function together during invasion. A broad panel of bacteria could reveal the number of separable entry pathways available in the host and define which cytoskeletal factors participate in each.
Barring the door: Extracellular pathogens disrupt actin to prevent phagocytosis.
In contrast to Salmonella, which dampens its effects on the cytoskeleton to maintain host cell viability, extracellular bacteria often treat cells more harshly. Many extracellular bacteria deliver toxins into host cells; many of these toxins covalently modify cytoskeletal factors such as actin and Rho-family GTPases, preventing uptake of the pathogen by phagocytic cells. Despite their destructiveness, these toxins’ modes of action pertain to endogenous processes. From them, host signaling molecules and post-translational modifications have been identified. For instance, Rac, a member of the Rho family, was discovered as a substrate of the Clostridium botulinum C3 toxin (Didsbury et al., 1989), and the toxin was instrumental in elucidating the roles of Rac and Rho as signaling hubs, with Rac controlling membrane ruffles (Ridley et al., 1992) and Rho controlling stress fibers and focal adhesions (Ridley and Hall, 1992). Numerous post-translational modifications—including phosphorylation, glucosylation, adenylylation (AMPylation), ADP-ribosylation, proteolysis, and deamidation—can disable or activate Rho proteins. Both microbial and host proteins use these regulatory modes, as discussed in a recent review (Visvikis et al., 2010).
A newly discovered variation on Rho modification is used by Photorhabdus luminescens, which ADP-ribosylates RhoA, preventing GTP hydrolysis and putting RhoA in a constitutively active state (Lang et al., 2010). Previously characterized toxins that ADP-ribosylate Rho proteins, such as C3 toxin, target a different residue and inhibit Rho function. A second Photorhabdus toxin ADP-ribosylates actin, preventing β-thymosin from sequestering actin monomers, thus promoting polymerization (Lang et al., 2010). Again, the toxin targets a different residue and has the opposite effect on actin function compared with previously studied toxins. Together, these two Photorhabdus toxins promote rampant, disruptive actin rearrangements and inhibit phagocytosis. Although the relevance of these forms of regulation to endogenous processes is not clear, it is interesting that phagocytosis can be blocked by both up- and down-regulating actin polymerization. This emphasizes the theme that actin polymerization must be carefully controlled to produce useful results, and excessive assembly can bring the network to a halt.
Constructing a niche
After invasion, many intracellular pathogens remain within the membrane-bound entry compartment, modifying it to suit their needs. This requires subversion of diverse host pathways to acquire resources for growth while manipulating phagosome maturation to prevent destruction within lysosomes. Again Salmonella provides a useful example, as it expresses multiple effectors with overlapping and antagonistic effects on the cytoskeleton (Bakowski et al., 2008; McGhie et al., 2009). We review how study of Salmonella led to the identification of a kinesin-interacting partner and potential new roles for cytoskeletal motors in membrane trafficking. We then describe recent insights into IFs and septins, including possible roles in autophagy regulation, gleaned from the study of Chlamydia trachomatis, Shigella, and others.
Salmonella regulates membrane trafficking via kinesin-1 and SKIP.
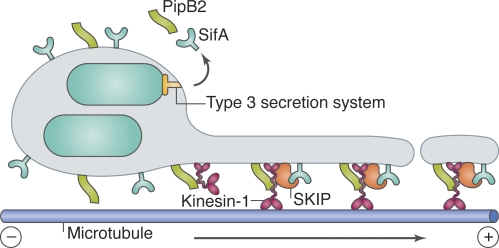
Salmonella replicates in a perinuclear compartment called the Salmonella-containing vacuole (SCV), from which membrane tubules called Sifs (Salmonella-induced filaments) extend. SCV integrity was known to require microtubules, microtubule motors, and bacterial effectors secreted by the T3SS, including SifA. A key interacting partner of SifA is a host protein of previously unknown function called SKIP (SifA and kinesin-interacting protein; Boucrot et al., 2005). As the name suggests, SKIP interacts with kinesin-1 in vitro and is required for SCV integrity. However, the role of kinesin in SCV maintenance was puzzling because its recruitment appeared to be regulated both negatively (by SifA; Boucrot et al., 2005) and positively (by another effector, PipB2; Henry et al., 2006). A clue was provided by the discovery that late after infection some SCVs moved toward the cell periphery before dissemination, in a manner dependent on microtubules, kinesin-1, and PipB2 (Szeto et al., 2009). Moreover, in uninfected cells, SKIP was found to promote anterograde movement of late endosomes/lysosomes along microtubules (Jackson et al., 2008; Dumont et al., 2010), possibly through control of membrane tubulation (Ohlson et al., 2008) or scission (Dumont et al., 2010). At a mechanistic level, SKIP binds the late endosomal GTPase Rab9, and SifA might mimic Rab9 (Jackson et al., 2008). A model for SCV maintenance was proposed in which PipB2 recruits inactive kinesin-1 to SCV membranes, where a complex containing kinesin, SKIP, and SifA forms (Fig. 2). SKIP could then activate kinesin, possibly through its interaction with the kinesin light chain, promoting the release of kinesin-associated vesicles (Dumont et al., 2010). The lack of kinesin on SCVs could thus be explained by its rapid dispersal on SCV-derived vesicles. The precise biochemical mechanism of SKIP and kinesin on SCVs remains to be determined. It will be interesting to test this model and to see if analogous mechanisms operate on endogenous SKIP/Rab9-positive compartments.
Figure 2.
Model for kinesin-1 and SKIP activity on SCV membranes. The T3SS-secreted Salmonella effectors PipB2 and SifA recruit kinesin-1 and SKIP, respectively, to SCV membranes. It has been proposed that SKIP might then activate kinesin-1 by binding to the kinesin light chain, and that SKIP–kinesin-1 complexes promote tubulation and/or scission of SCV-derived membrane compartments, which are transported toward the plus end of microtubules.
Another interesting parallel between SCVs and endogenous compartments involves the actin-based motor myosin II, which was unexpectedly implicated in SCV positioning and integrity (Wasylnka et al., 2008). In uninfected cells, myosin II, together with Rab6, contributes to vesicle fission from the Golgi apparatus (Miserey-Lenkei et al., 2010). By analogy, in Salmonella-infected cells, myosin II might promote the release of SCV-derived vesicles, perhaps in cooperation with SKIP, kinesin-1, and SifA. The involvement of both myosin and kinesin in SCV-derived vesicle formation could point to new mechanisms of cooperation between actin- and microtubule-based motors.
Filament “cages”: Nest or trap?
Actin and IFs also contribute to the establishment of replicative niches. In some cases, IFs, alone or with actin, form stabilizing cages around pathogen-containing vacuoles, apparently protecting them from host recognition. For example, Chlamydia trachomatis induces relocalization of the IFs vimentin, cytokeratin-8, and cytokeratin-18, as well as actin filaments, to the Chlamydia inclusion membrane (Kumar and Valdivia, 2008). IF and actin assembly around the inclusion are interdependent, and disruption of either leads to release of bacteria into the cytoplasm, triggering host defense mechanisms. Chlamydia regulates IF assembly in a novel way: the bacterial protease CPAF locally cleaves IF head domains, reducing their cohesiveness. Although the head domain of vimentin is essential for filament assembly in vitro (Herrmann et al., 1996), the IFs cleaved by CPAF form complexes in vitro, and the head domain remains associated with cages (Kumar and Valdivia, 2008). Thus, proteolysis at an unidentified site in the head domain could represent a way to regulate the elasticity of IF networks, allowing cage expansion while maintaining structural integrity.
Protective IF cages also coalesce around other intracellular bacteria. Salmonella vacuoles recruit the same three IF proteins as Chlamydia (vimentin, cytokeratin-8, and cytokeratin-18), and IF disruption results in SCV dispersal (Guignot and Servin, 2008). Vimentin cages form around Anaplasma phagocytophilum inclusions, and a bacterial effector binds vimentin and indirectly promotes survival (Sukumaran et al., 2011), supporting the hypothesis that caging is a protective, pathogen-controlled “nesting” process.
In contrast, a distinct type of cage acts as a host-mediated trap that promotes destruction of the pathogen by autophagy. A proportion of cytosolic Shigella become wrapped in septin filaments, in a myosin II–dependent manner, concurrently with acquisition of autophagy markers (Mostowy et al., 2010). Although initial cage assembly requires actin polymerization, an inverse correlation exists between the presence of a cage and productive ABM, and also between myosin activity and motility, indicating that a balance of cytoskeletal forces determines the fate of each bacterium. Because septin cages initially require, but then antagonize actin polymerization, the authors looked for septins around other bacteria that undergo ABM. Septin cages were detected around Mycobacterium marinum but not Listeria or Rickettsia conorii, suggesting that caging is influenced by bacterial factors, which could provide routes to understand its regulation. The connection between septin cages and autophagy suggests a new cellular function for septins. Given that cellular aggresomes, which are vimentin cages surrounding protein aggregates (Wileman, 2007), are also linked to autophagy, it is tempting to speculate that certain filament assemblies simultaneously immobilize cytoplasmic contents and mark them for autophagy. If this is true, then pathogens would be expected to evolve countermeasures to modify filament traps and avoid autophagy. For example, if vimentin acts as an autophagy signal, recruitment of additional filaments (such as cytokeratins or actin) might mask this signal. Intracellular pathogens, by serving as both targets and manipulators of autophagy, are invaluable tools for investigating its regulation.
Tiny rocket scientists: Diverse pathogens “discovered” actin-based motility
Some bacterial species escape from the phagocytic vacuole and replicate freely in the host cytoplasm. A subset of these pathogens expresses factors that trigger actin polymerization against their surface, producing mechanical force that propels them through the cell and facilitates spread to neighboring cells. This form of motility evolved independently in Listeria, Shigella, Rickettsia, and Burkholderia, which each use a distinct mechanism driven primarily by a single bacterial protein (Fig. 3 A), demonstrating that, in contrast to vacuole positioning, ABM may be simpler to achieve. Some viruses—vaccinia and other poxviruses (Cudmore et al., 1995; Dodding and Way, 2009), and a baculovirus (Ohkawa et al., 2010)—also use ABM, although poxviruses trigger it from outside the cell. The study of pathogen ABM, particularly of Listeria, revolutionized our understanding of cellular actin-based propulsion, such as vesicle rocketing and lamellipodia-driven cell migration. We review discoveries that established the ABM field, and then highlight recent studies that shed new light on actin dynamics.
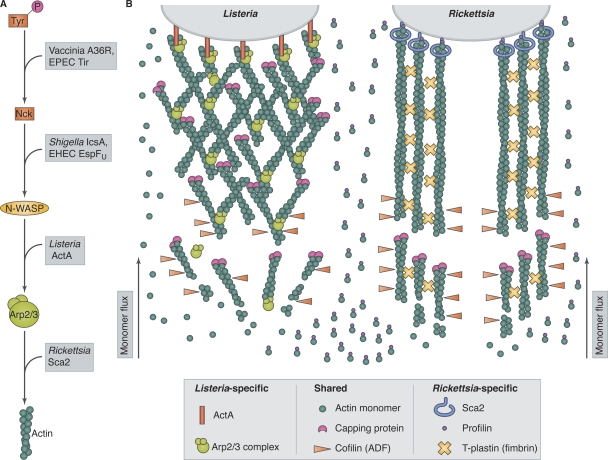
Figure 3.
Pathogens use distinct actin-based motility mechanisms. (A) Pathogens intercept actin assembly pathways at different levels. Vaccinia virus and EPEC express proteins (A36R and Tir) that mimic host phosphotyrosine motifs to recruit the adaptor protein Nck. Shigella and EHEC produce proteins (IcsA and EspFU) that recruit and activate N-WASP. Listeria ActA mimics N-WASP to activate the host Arp2/3 complex. Rickettsia bypasses host nucleators using the formin-like protein Sca2 to interact directly with actin. (B) The actin-based motilities of Listeria and Rickettsia have distinct host protein requirements. Listeria expresses ActA on its surface, which activates the host Arp2/3 complex, producing branched actin filaments. Actin monomers or profilin–actin complexes can polymerize onto filament ends in Listeria tails. Rickettsia expresses the formin-like protein Sca2 on its surface, which nucleates unbranched actin filaments and requires profilin for filament elongation. T-plastin is also important for Rickettsia tail formation. Both systems require capping protein and cofilin (ADF).
Motility through the cytoplasm.
Actin’s importance for eukaryotic cell migration has been established for decades. It was inferred from the study of Listeria actin “comet tails” (Tilney and Portnoy, 1989) that the motive force for lamellipodia protrusion is derived from polymerization itself, rather than myosin activity along a cytoskeletal track (Theriot et al., 1992). However, the mechanisms of actin filament nucleation, organization, and disassembly in lamellipodia were unknown. The comet tails produced by Listeria ActA or Shigella IcsA were highly similar to each other, suggesting that pathways controlling actin tail morphology were controlled by the host (Kocks et al., 1995). It was clear that identifying host binding partners of ActA or IcsA would advance our understanding of cellular actin dynamics.
The first ActA binding partner identified was the Arp2/3 complex, a conserved seven-subunit complex that was sufficient for actin assembly at the Listeria surface (Welch et al., 1997). At the biochemical level, the Arp2/3 complex weakly promoted actin nucleation on its own and was strongly activated by ActA (Welch et al., 1998). It was later determined that Shigella also exploits the Arp2/3 complex, but via a distinct mechanism: Shigella factors bind and activate host N-WASP, which recruits Arp2/3 and actin (Egile et al., 1999; Leung et al., 2008). The importance of Arp2/3 in cellular processes was quickly recognized, and endogenous proteins such as WASP/N-WASP and WAVE were shown to activate Arp2/3 complex in the same manner as ActA (Goley and Welch, 2006). Collectively, these Arp2/3 activators became known as NPFs. Additional NPFs, such as WHAMM, WASH, and JMY, continue to be identified and characterized, uncovering new roles for the Arp2/3 complex in processes such as ER-to-Golgi transport and endosome trafficking (Rottner et al., 2010).
Recapitulating Arp2/3 complex–mediated motility of bacteria from purified components requires additional activities besides nucleation. Sustained movement was achieved using the following components: Arp2/3 complex to nucleate filaments; actin-depolymerizing factor (ADF, also known as cofilin) to accelerate depolymerization and maintain the actin monomer pool; and capping protein to prevent nonproductive growth of filaments away from the bacterial surface (Fig. 3 B; Loisel et al., 1999). Profilin, which binds actin monomers and enhances depolymerization from pointed ends, increased the rate of movement but was not strictly required. This landmark study laid the foundation for elucidating the biochemical and biophysical bases of force production by actin assembly, which directly informs the cellular process of vesicle rocketing (Marchand et al., 1995; Merrifield et al., 1999; Taunton et al., 2000). Moreover, the nucleation machinery and signaling molecules involved in ABM are substantially similar to those driving the protrusion of lamellipodia and pseudopodia, although these structures differ from rocketing particles in size and shape (Borisy and Svitkina, 2000; Pollard and Borisy, 2003; Bugyi et al., 2008). Reconstitution of motility also provided a tractable system to dissect the activities of other proteins that regulate actin dynamics. To list just a few examples, it has been used to demonstrate filament capping by twinfilin (Helfer et al., 2006), severing by villin (Revenu et al., 2007), and enhancement of the N-WASP–Arp2/3 interaction by the adaptor protein Grb2 (Carlier et al., 2000).
There is more to learn from pathogens moving through cytoplasm. 22 years after Tilney’s micrographs of Listeria ABM, the biophysics of its propulsion are still being debated and tested (Mogilner, 2006; Dickinson, 2009). Furthermore, alternative mechanisms of ABM were recently discovered. Rickettsia is the first pathogen found to bypass the Arp2/3 complex for ABM (Serio et al., 2010), instead encoding its own formin-like nucleator (Haglund et al., 2010; Kleba et al., 2010). This type of motility requires a different set of host factors compared with Listeria and Shigella (Fig. 3 B). Specifically, profilin and the actin-bundling protein T-plastin (fimbrin) are critical for Rickettsia ABM (Serio et al., 2010). Rickettsia may subvert an endogenous motility pathway, as host formins mediate the movement of oocyte chromosomes toward the cortex during meiosis I (Li et al., 2008) and might play a role in ER positioning (Chhabra et al., 2009). Whether Rickettsia ABM will prove useful in understanding the transport of cellular cargoes remains to be determined.
Motility across the membrane.
A distinct “surfing” form of ABM is used by extracellular vaccinia virus and pathogenic strains of E. coli, which intercept receptor tyrosine kinase signaling pathways, resulting in the formation of a moving, actin-rich pedestal beneath the pathogen. Each of these microbes encodes a transmembrane protein that becomes phosphorylated by Src- and Abl-family kinases, leading to recruitment of the adaptor proteins Nck1 and Nck2, which activate N-WASP to stimulate Arp2/3-mediated actin polymerization (Frischknecht et al., 1999; Campellone, 2010). Studies of vaccinia and enteropathogenic E. coli (EPEC) demonstrated the physiological relevance of the phosphotyrosine/Nck/N-WASP pathway for signaling to actin assembly. After its elucidation, this pathway was found to be essential in kidney podocytes, signaling through the host receptor nephrin to produce the actin-rich cellular extensions that are critical for kidney filtration function (Jones et al., 2006). Stimulated T cell antigen receptors (TCRs) also induce actin polymerization via Nck, although the pathway uses WASP instead of N-WASP (Barda-Saad et al., 2005). Nck also signals to actin downstream of other receptor tyrosine kinases, including neuronal axon guidance receptors such as Ephrin A4 (Fawcett et al., 2007) and growth factor receptors such as PDGF-R (Rivera et al., 2006; Ruusala et al., 2008), although the downstream pathways and potential roles of Nck are not clearly defined. Nonetheless, pathogen signaling through Nck/N-WASP parallels several endogenous processes.
Study of surfing pathogens has revealed dynamic interplay between Arp2/3, NPFs, and their regulatory partners. Vaccinia virus was used to examine the recruitment and turnover of Nck, N-WASP, WASP-interacting protein (WIP), and Grb2 during motility (Weisswange et al., 2009). Surprisingly, although Nck and WIP are thought to recruit N-WASP, the turnover rate for N-WASP was much slower than for Nck and WIP, implying that other interactions stabilize N-WASP in vaccinia tails. Moreover, N-WASP did not turn over when its ability to stimulate Arp2/3-mediated nucleation was disrupted, suggesting that interaction with the Arp2/3 complex is required to dissociate N-WASP from its binding partners. Presumably, this requirement also applies to N-WASP–mediated nucleation on rocketing vesicles, and possibly to other NPFs, although these hypotheses remain to be tested. These molecular interactions have implications at the level of virus motility. The rate of N-WASP exchange correlated positively with the rate of virus motility, but inversely with the number of tails, illustrating the need to balance speed with stability to achieve productive motility.
Recent work with EPEC has revealed membrane phosphoinositide signals that regulate actin assembly. The EPEC protein that recruits Nck, called Tir, also binds host phosphoinositide 3-kinase (Sason et al., 2009; Selbach et al., 2009) and the inositol-5-phosphatase SHIP2 (Smith et al., 2010). The combined activities of these two enzymes can convert PI(4,5)P2 to PI(3,4)P2, the predominant membrane phosphoinositide in wild-type pedestals. When SHIP2 recruitment is prevented, PI(3,4,5)P3 accumulates instead, and bacteria are associated with multiple, aberrantly long pedestals (Smith et al., 2010), suggesting that PI(3,4)P2 down-regulates signaling after an initial burst of PI(3,4,5)P3-enhanced actin polymerization. The lipid requirements for EPEC pedestal formation might correspond to endogenous processes, particularly to TCR activation (Smith-Garvin et al., 2009) and down-regulation (Smith et al., 2010) and nephrin-mediated signaling (Huber et al., 2003; Zhu et al., 2008). The clear read-out provided by EPEC pedestals could be useful for investigating the mechanisms by which PI(3,4)P2 regulates actin assembly.
Enterohemorrhagic E. coli (EHEC), although closely related to EPEC, uses a distinct mechanism of pedestal formation that has illuminated N-WASP regulation. Instead of Nck, EHEC Tir recruits the bacterial effector EspFU, which contains 2–6 repeated sequences that bind and activate N-WASP. Dissection of EspFU demonstrated the critical role of multivalency in N-WASP activation, as the repeated EspFU peptides activate efficiently only when they can recruit multiple copies of N-WASP (Sallee et al., 2008). Isolated EspFU peptides bind N-WASP but do not promote robust actin polymerization (Campellone et al., 2008; Sallee et al., 2008). The importance of oligomerization as a universal mode of NPF regulation is supported by in vitro work showing that dimerized NPF activation domains have ∼100 times greater affinity for (Padrick et al., 2008) and activity toward the Arp2/3 complex (Higgs and Pollard, 2000; Padrick et al., 2008) compared with monomeric NPFs. Oligomerization could also explain results in uninfected cells in which artificial clustering of WASP or an upstream binding partner at the plasma membrane stimulated actin polymerization (Castellano et al., 1999; Rivera et al., 2004).
As with cytoplasmic ABM, more remains to be learned from surfing pathogens. For instance, even the well-studied molecule EPEC Tir contains peptides whose effects on actin pedestals are not understood (Campellone, 2010). Intriguingly, both EPEC and host nephrin use secondary mechanisms of actin assembly in addition to the primary phosphotyrosine/Nck-dependent pathway, so additional parallels might exist between the two systems. Finally, EHEC EspFU can be used to explore how cells generate plasma membrane protrusions independently of tyrosine kinase signaling.
New directions: Pathogen exit and dissemination
Pathogens can quickly consume a host cell’s resources and must spread to new cells to continue their life cycle. Although host cell lysis can promote spread, it is often advantageous for microorganisms to exit cells in a controlled, nonlytic manner. Less is known about molecular mechanisms of dissemination compared with entry or intracellular motility, but several exit strategies use the actin cytoskeleton (Fig. 4). It is likely that normal host cell processes are exploited during exit, but these processes are poorly defined. This makes pathogen exit an exciting area for future research.
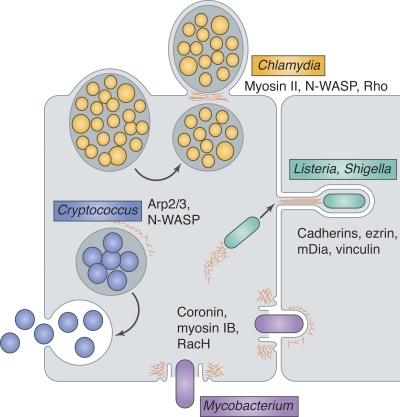
Figure 4.
Exit strategies of diverse intracellular pathogens use actin. Chlamydia (yellow) vacuoles are extruded through a cortical constriction, and the plasma membrane seals around the constriction point in a manner dependent on actin (red), releasing a double-membrane–bound bacterial compartment. The extrusion pathway appears to require myosin II and N-WASP for initiation and Rho for detachment of the extruded vacuole from the host cell. Listeria and Shigella (green), propelled by actin-based motility, enter plasma membrane protrusions and are taken up by neighboring cells. Cadherins, ezrin, mDia, and vinculin have been implicated in protrusion formation. Mycobacterium (purple) exits cells through a plasma membrane break surrounded by a barrel-shaped ejectosome rich in actin, myosin IB, and coronin. In host cells lacking RacH, ejectosomes are not detected and mycobacterial spreading is impaired. Cryptococcus (blue) phagosomes fuse with the plasma membrane, and intermittent actin polymerization around the phagosome, apparently mediated by N-WASP and the Arp2/3 complex, inhibits this fusion.
Bacteria that undergo ABM, such as Listeria and Shigella, subsequently enter long, plasma membrane–bound protrusions that extend from infected cells into adjacent cells (Tilney and Portnoy, 1989; Kadurugamuwa et al., 1991) and escape into the neighboring cell’s cytoplasm (Robbins et al., 1999; Monack and Theriot, 2001). Within protrusions, actin tail filaments become longer and more densely bundled (Sechi et al., 1997; Gouin et al., 1999) and more stable (Robbins et al., 1999). Host adherens junction proteins, such as vinculin (Kadurugamuwa et al., 1991) and cadherins (Sansonetti et al., 1994), have been implicated in protrusion-mediated spread. Because cadherin cytoplasmic domains link to actin filaments via vinculin and other junction components, it was speculated that junction proteins might bind comet tails, altering actin filament organization and promoting the formation and rigidity of protrusions. Analogously, disruption of ezrin, an actin membrane linker that localizes to Listeria protrusions but not cytoplasmic tails, results in short, crumpled protrusions (Pust et al., 2005). In contrast, the junction protein Tuba—which has N-WASP binding, scaffolding, and GEF activity—negatively regulates protrusion formation (Rajabian et al., 2009), apparently by promoting cortical tension in epithelial cell layers (Otani et al., 2006; Rajabian et al., 2009). The Listeria effector InlC disrupts Tuba–N-WASP binding, relaxing cortical tension and promoting protrusions. Thus, protrusion formation appears to require an initial relaxation of cortical rigidity, followed by promotion of rigidity, presumably by a different set of factors, around the bacterial actin tail.
Recently, the formin-family actin nucleators Dia1 and Dia2 were found to support dissemination of Shigella (Heindl et al., 2010). As with ezrin, Dia1 and Dia2 localize to actin in protrusions but not to cytoplasmic tails, and disruption of Dia reduces the frequency and length of protrusions. The involvement of formins, which generate long, unbranched actin filaments, is consistent with the parallel filament bundles found in protrusions. Together, the effects of formins, ezrin, vinculin, and cadherin on protrusions but not cytoplasmic tails suggest that a distinct set of actin regulatory factors interacts with motile bacteria after they contact the plasma membrane. Further study is required to determine which factors help build protrusions and how the transition in actin organization is regulated. Moreover, how these cytoskeletal alterations promote uptake by neighboring cells is unclear. In uninfected cells, uptake of vesicles derived from neighboring cells was observed and was named paracytophagy (Robbins et al., 1999), but paracytophagy’s role in cells and its connection to protrusion-mediated spread have not been described.
Another actin-dependent mode of exit is the extrusion of Chlamydia inclusions into the extracellular space (Hybiske and Stephens, 2007). Compartments containing a few to hundreds of Chlamydia, surrounded by both inclusion and plasma membranes, were observed to balloon out from a constriction point in the host cell, eventually being released into the media. Extrusion requires actin, N-WASP, and myosin II, and the final pinching-off step requires the GTPase Rho. Chlamydia have a second, lytic exit strategy, and usually both mechanisms are used with equal frequency. Curiously, the authors found that jasplakinolide, which blocks actin depolymerization but not polymerization, abrogated the lytic strategy and quickly induced extrusions, even early in infection when Chlamydia do not normally exit. This suggests that robust actin polymerization is sufficient to induce extrusion, which implies that it is a host-driven process. However, the endogenous function of extrusion, if any, remains a mystery.
A third actin-dependent exit strategy was recently described for Mycobacterium marinum and M. tuberculosis (Hagedorn et al., 2009). These bacteria, which spend part of their life cycle free in the host cytoplasm, exited host amoebae through a barrel-shaped structure rich in actin, myosin IB, and coronin, which the authors called an ejectosome. Ejection occurred without long membrane extensions, did not require a comet tail or a recipient cell, and took only a few minutes to complete, distinguishing it from protrusion-mediated spread. Ejection resulted in plasma membrane breakage and exit into either the media or an adjacent cell, which formed a phagocytic cup–like structure around the invading bacterium. In spite of membrane rupture, leakage of cellular contents did not occur, apparently due to a tight septum formed by the actin ring. The authors proposed that ejectosomes might have originated as a plasma membrane resealing mechanism. Actin polymerization and actomyosin contraction occur around plasma membrane wounds (Mandato and Bement, 2001), but this process uses myosin II (Mandato and Bement, 2001; Togo and Steinhardt, 2004), whereas myosin II was not detected at ejectosomes. Thus, the connection between ejectosomes and plasma membrane repair pathways requires further investigation.
A membrane-sealing function might also explain the cycles of transient actin polymerization observed around phagosomes containing the fungal pathogen Cryptococcus neoformans (Johnston and May, 2010) or bacteria such as Listeria (Yam and Theriot, 2004). In the case of Cryptococcus, actin “flashes” follow phagosome permeabilization events, in an Arp2/3 complex– and WASP/N-WASP–dependent manner. Cryptococcus exits cells by phagosomal fusion with the plasma membrane, and fusion is usually preceded by phagosome permeabilization. In contrast to the above strategies, disruption of actin assembly enhances fungal exit, implying that host cells polymerize actin to limit dissemination. Flashes were also observed on phagosomes containing transferrin-coated beads, on mechanically induced plasma membrane wounds, and at membrane invaginations around particles too large to phagocytose (Yam and Theriot, 2004), demonstrating that flashing is an endogenous process, induced by membrane breakage and possibly by the presence of large internalized particles. Although dynamic actin accumulates around plasma membrane wounds, the role of actin polymerization on phagosomes is less clear. Actin might contribute to membrane repair, or could form a barrier to limit mixing of vesicle contents with cytosol while other repair mechanisms occur. In either case, actin polymerization appears to help maintain the integrity of phagosomes.
Collectively, these reports will lead to further discoveries about the roles of actin in ushering pathogens or other particles out of cells, including insights into membrane resealing. As mentioned in the Introduction, the endogenous pathways exploited during pathogen exit are not defined, and it is currently unknown if other cytoskeletal filaments are involved in these processes.
Future perspectives
Given the numerous ways in which pathogens have contributed to our understanding of the cytoskeleton, it is obvious that future study of pathogen–cytoskeleton interactions will uncover important new insights. In particular, pathogens might reveal clues to the role(s) of actin in the nucleus, an area that is just beginning to be explored (Skarp and Vartiainen, 2010). For instance, baculovirus replication requires nuclear translocation and polymerization of actin (Goley et al., 2006). Furthermore, Anaplasma phagocytophilum induces phosphorylation of actin in its host, leading to increased nuclear G-actin and phospho-actin–dependent up-regulation of a host gene required for bacterial survival (Sultana et al., 2010). Pathogens have additional tricks up their sleeves that were not discussed in this review, such as destabilization (Coureuil et al., 2009) or reinforcement (Kim et al., 2009) of intercellular junctions, as well as promotion of host cell motility (Worley et al., 2006), suggesting that insights into these processes will be forthcoming. Advancements will also come from technological improvements, for example in imaging methods, as well as new approaches, such as systems-level analyses and mathematical modeling. In addition to revealing fundamental cellular mechanisms, future studies of the host–pathogen relationship will enhance our understanding of pathogenesis and disease, and may lead to improved diagnostics and treatments for microbial infections.
Acknowledgments
The authors thank K. Campellone and E. Benanti for critical reading of the manuscript. Illustrations provided by Neil Smith: neil@neilsmithillustration.co.uk.
This work was funded by NIH-NIAID grant R01 AI074760.
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- ABM
- actin-based motility
- CDT
- Clostridium difficile transferase
- EPEC
- enteropathogenic Escherichia coli
- EHEC
- enterohemorrhagic Escherichia coli
- GEF
- guanine-nucleotide exchange factor
- IF
- intermediate filament
- NPF
- nucleation-promoting factor
- SCV
- Salmonella-containing vacuole
- T3SS
- type 3 secretion system
- TCR
- T cell antigen receptor
References
- Aiastui A., Pucciarelli M.G., García-del Portillo F. 2010. Salmonella enterica serovar typhimurium invades fibroblasts by multiple routes differing from the entry into epithelial cells. Infect. Immun. 78:2700–2713 10.1128/IAI.01389-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowski M.A., Braun V., Brumell J.H. 2008. Salmonella-containing vacuoles: directing traffic and nesting to grow. Traffic. 9:2022–2031 10.1111/j.1600-0854.2008.00827.x [DOI] [PubMed] [Google Scholar]
- Barda-Saad M., Braiman A., Titerence R., Bunnell S.C., Barr V.A., Samelson L.E. 2005. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat. Immunol. 6:80–89 10.1038/ni1143 [DOI] [PubMed] [Google Scholar]
- Borisy G.G., Svitkina T.M. 2000. Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 12:104–112 10.1016/S0955-0674(99)00063-0 [DOI] [PubMed] [Google Scholar]
- Boucrot E., Henry T., Borg J.P., Gorvel J.P., Méresse S. 2005. The intracellular fate of Salmonella depends on the recruitment of kinesin. Science. 308:1174–1178 10.1126/science.1110225 [DOI] [PubMed] [Google Scholar]
- Bugyi B., Le Clainche C., Romet-Lemonne G., Carlier M.F. 2008. How do in vitro reconstituted actin-based motility assays provide insight into in vivo behavior? FEBS Lett. 582:2086–2092 10.1016/j.febslet.2008.02.065 [DOI] [PubMed] [Google Scholar]
- Campellone K.G. 2010. Cytoskeleton-modulating effectors of enteropathogenic and enterohaemorrhagic Escherichia coli: Tir, EspFU and actin pedestal assembly. FEBS J. 277:2390–2402 10.1111/j.1742-4658.2010.07653.x [DOI] [PubMed] [Google Scholar]
- Campellone K.G., Cheng H.C., Robbins D., Siripala A.D., McGhie E.J., Hayward R.D., Welch M.D., Rosen M.K., Koronakis V., Leong J.M. 2008. Repetitive N-WASP-binding elements of the enterohemorrhagic Escherichia coli effector EspF(U) synergistically activate actin assembly. PLoS Pathog. 4:e1000191 10.1371/journal.ppat.1000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M.F., Nioche P., Broutin-L’Hermite I., Boujemaa R., Le Clainche C., Egile C., Garbay C., Ducruix A., Sansonetti P., Pantaloni D. 2000. GRB2 links signaling to actin assembly by enhancing interaction of neural Wiskott-Aldrich syndrome protein (N-WASp) with actin-related protein (ARP2/3) complex. J. Biol. Chem. 275:21946–21952 10.1074/jbc.M000687200 [DOI] [PubMed] [Google Scholar]
- Castellano F., Montcourrier P., Guillemot J.C., Gouin E., Machesky L., Cossart P., Chavrier P. 1999. Inducible recruitment of Cdc42 or WASP to a cell-surface receptor triggers actin polymerization and filopodium formation. Curr. Biol. 9:351–360 10.1016/S0960-9822(99)80161-4 [DOI] [PubMed] [Google Scholar]
- Chang L., Goldman R.D. 2004. Intermediate filaments mediate cytoskeletal crosstalk. Nat. Rev. Mol. Cell Biol. 5:601–613 10.1038/nrm1438 [DOI] [PubMed] [Google Scholar]
- Chhabra E.S., Ramabhadran V., Gerber S.A., Higgs H.N. 2009. INF2 is an endoplasmic reticulum-associated formin protein. J. Cell Sci. 122:1430–1440 10.1242/jcs.040691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi F., Jong T.D., Wang L., Ouyang Y., Wu C., Li W., Huang S.H. 2010. Vimentin-mediated signalling is required for IbeA+ E. coli K1 invasion of human brain microvascular endothelial cells. Biochem. J. 427:79–90 10.1042/BJ20091097 [DOI] [PubMed] [Google Scholar]
- Cossart P., Sansonetti P.J. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 304:242–248 10.1126/science.1090124 [DOI] [PubMed] [Google Scholar]
- Coureuil M., Mikaty G., Miller F., Lécuyer H., Bernard C., Bourdoulous S., Duménil G., Mège R.M., Weksler B.B., Romero I.A., et al. 2009. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 325:83–87 10.1126/science.1173196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudmore S., Cossart P., Griffiths G., Way M. 1995. Actin-based motility of vaccinia virus. Nature. 378:636–638 10.1038/378636a0 [DOI] [PubMed] [Google Scholar]
- Damm E.M., Pelkmans L. 2006. Systems biology of virus entry in mammalian cells. Cell. Microbiol. 8:1219–1227 10.1111/j.1462-5822.2006.00745.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal B.K., Mulvey M.A. 2009. Uropathogenic Escherichia coli invades host cells via an HDAC6-modulated microtubule-dependent pathway. J. Biol. Chem. 284:446–454 10.1074/jbc.M805010200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson R.B. 2009. Models for actin polymerization motors. J. Math. Biol. 58:81–103 10.1007/s00285-008-0200-4 [DOI] [PubMed] [Google Scholar]
- Didsbury J., Weber R.F., Bokoch G.M., Evans T., Snyderman R. 1989. rac, a novel ras-related family of proteins that are botulinum toxin substrates. J. Biol. Chem. 264:16378–16382 [PubMed] [Google Scholar]
- Dodding M.P., Way M. 2009. Nck- and N-WASP-dependent actin-based motility is conserved in divergent vertebrate poxviruses. Cell Host Microbe. 6:536–550 10.1016/j.chom.2009.10.011 [DOI] [PubMed] [Google Scholar]
- Donnenberg M.S., Donohue-Rolfe A., Keusch G.T. 1990. A comparison of HEp-2 cell invasion by enteropathogenic and enteroinvasive Escherichia coli. FEMS Microbiol. Lett. 57:83–86 10.1111/j.1574-6968.1990.tb04179.x [DOI] [PubMed] [Google Scholar]
- Dumont A., Boucrot E., Drevensek S., Daire V., Gorvel J.P., Poüs C., Holden D.W., Méresse S. 2010. SKIP, the host target of the Salmonella virulence factor SifA, promotes kinesin-1-dependent vacuolar membrane exchanges. Traffic. 11:899–911 10.1111/j.1600-0854.2010.01069.x [DOI] [PubMed] [Google Scholar]
- Egile C., Loisel T.P., Laurent V., Li R., Pantaloni D., Sansonetti P.J., Carlier M.F. 1999. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146:1319–1332 10.1083/jcb.146.6.1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J.P., Georgiou J., Ruston J., Bladt F., Sherman A., Warner N., Saab B.J., Scott R., Roder J.C., Pawson T. 2007. Nck adaptor proteins control the organization of neuronal circuits important for walking. Proc. Natl. Acad. Sci. USA. 104:20973–20978 10.1073/pnas.0710316105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C.L., Ryan T.A., Jones B.D., Smith S.J., Falkow S. 1993. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 364:639–642 10.1038/364639a0 [DOI] [PubMed] [Google Scholar]
- Frischknecht F., Moreau V., Röttger S., Gonfloni S., Reckmann I., Superti-Furga G., Way M. 1999. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 401:926–929 10.1038/44860 [DOI] [PubMed] [Google Scholar]
- Fu Y., Galán J.E. 1999. A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 401:293–297 10.1038/45829 [DOI] [PubMed] [Google Scholar]
- Gilden J., Krummel M.F. 2010. Control of cortical rigidity by the cytoskeleton: emerging roles for septins. Cytoskeleton (Hoboken). 67:477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley E.D., Welch M.D. 2006. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7:713–726 10.1038/nrm2026 [DOI] [PubMed] [Google Scholar]
- Goley E.D., Ohkawa T., Mancuso J., Woodruff J.B., D’Alessio J.A., Cande W.Z., Volkman L.E., Welch M.D. 2006. Dynamic nuclear actin assembly by Arp2/3 complex and a baculovirus WASP-like protein. Science. 314:464–467 10.1126/science.1133348 [DOI] [PubMed] [Google Scholar]
- Gouin E., Gantelet H., Egile C., Lasa I., Ohayon H., Villiers V., Gounon P., Sansonetti P.J., Cossart P. 1999. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J. Cell Sci. 112:1697–1708 [DOI] [PubMed] [Google Scholar]
- Guignot J., Servin A.L. 2008. Maintenance of the Salmonella-containing vacuole in the juxtanuclear area: a role for intermediate filaments. Microb. Pathog. 45:415–422 10.1016/j.micpath.2008.09.007 [DOI] [PubMed] [Google Scholar]
- Guzman C.A., Rohde M., Chakraborty T., Domann E., Hudel M., Wehland J., Timmis K.N. 1995. Interaction of Listeria monocytogenes with mouse dendritic cells. Infect. Immun. 63:3665–3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn M., Rohde K.H., Russell D.G., Soldati T. 2009. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science. 323:1729–1733 10.1126/science.1169381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund C.M., Choe J.E., Skau C.T., Kovar D.R., Welch M.D. 2010. Rickettsia Sca2 is a bacterial formin-like mediator of actin-based motility. Nat. Cell Biol. 12:1057–1063 10.1038/ncb2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänisch J., Ehinger J., Ladwein M., Rohde M., Derivery E., Bosse T., Steffen A., Bumann D., Misselwitz B., Hardt W.D., et al. 2010. Molecular dissection of Salmonella-induced membrane ruffling versus invasion. Cell. Microbiol. 12:84–98 10.1111/j.1462-5822.2009.01380.x [DOI] [PubMed] [Google Scholar]
- Hänisch J., Kölm R., Wozniczka M., Bumann D., Rottner K., Stradal T.E. 2011. Activation of a RhoA/myosin II-dependent but Arp2/3 complex-independent pathway facilitates Salmonella invasion. Cell Host Microbe. 9:273–285 10.1016/j.chom.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Haraga A., Ohlson M.B., Miller S.I. 2008. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 6:53–66 10.1038/nrmicro1788 [DOI] [PubMed] [Google Scholar]
- Hardt W.D., Chen L.M., Schuebel K.E., Bustelo X.R., Galán J.E. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 93:815–826 10.1016/S0092-8674(00)81442-7 [DOI] [PubMed] [Google Scholar]
- Hayward R.D., Koronakis V. 1999. Direct nucleation and bundling of actin by the SipC protein of invasive Salmonella. EMBO J. 18:4926–4934 10.1093/emboj/18.18.4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindl J.E., Saran I., Yi C.R., Lesser C.F., Goldberg M.B. 2010. Requirement for formin-induced actin polymerization during spread of Shigella flexneri. Infect. Immun. 78:193–203 10.1128/IAI.00252-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer E., Nevalainen E.M., Naumanen P., Romero S., Didry D., Pantaloni D., Lappalainen P., Carlier M.F. 2006. Mammalian twinfilin sequesters ADP-G-actin and caps filament barbed ends: implications in motility. EMBO J. 25:1184–1195 10.1038/sj.emboj.7601019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T., Couillault C., Rockenfeller P., Boucrot E., Dumont A., Schroeder N., Hermant A., Knodler L.A., Lecine P., Steele-Mortimer O., et al. 2006. The Salmonella effector protein PipB2 is a linker for kinesin-1. Proc. Natl. Acad. Sci. USA. 103:13497–13502 10.1073/pnas.0605443103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H., Häner M., Brettel M., Müller S.A., Goldie K.N., Fedtke B., Lustig A., Franke W.W., Aebi U. 1996. Structure and assembly properties of the intermediate filament protein vimentin: the role of its head, rod and tail domains. J. Mol. Biol. 264:933–953 10.1006/jmbi.1996.0688 [DOI] [PubMed] [Google Scholar]
- Higgs H.N., Pollard T.D. 2000. Activation by Cdc42 and PIP(2) of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J. Cell Biol. 150:1311–1320 10.1083/jcb.150.6.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber T.B., Hartleben B., Kim J., Schmidts M., Schermer B., Keil A., Egger L., Lecha R.L., Borner C., Pavenstädt H., et al. 2003. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol. Cell. Biol. 23:4917–4928 10.1128/MCB.23.14.4917-4928.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybiske K., Stephens R.S. 2007. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc. Natl. Acad. Sci. USA. 104:11430–11435 10.1073/pnas.0703218104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.K., Nawabi P., Hentea C., Roark E.A., Haldar K. 2008. The Salmonella virulence protein SifA is a G protein antagonist. Proc. Natl. Acad. Sci. USA. 105:14141–14146 10.1073/pnas.0801872105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S.A., May R.C. 2010. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS Pathog. 6:e1001041 10.1371/journal.ppat.1001041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Blasutig I.M., Eremina V., Ruston J.M., Bladt F., Li H., Huang H., Larose L., Li S.S., Takano T., et al. 2006. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 440:818–823 10.1038/nature04662 [DOI] [PubMed] [Google Scholar]
- Kadurugamuwa J.L., Rohde M., Wehland J., Timmis K.N. 1991. Intercellular spread of Shigella flexneri through a monolayer mediated by membranous protrusions and associated with reorganization of the cytoskeletal protein vinculin. Infect. Immun. 59:3463–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Ogawa M., Fujita Y., Yoshikawa Y., Nagai T., Koyama T., Nagai S., Lange A., Fässler R., Sasakawa C. 2009. Bacteria hijack integrin-linked kinase to stabilize focal adhesions and block cell detachment. Nature. 459:578–582 10.1038/nature07952 [DOI] [PubMed] [Google Scholar]
- Kleba B., Clark T.R., Lutter E.I., Ellison D.W., Hackstadt T. 2010. Disruption of the Rickettsia rickettsii Sca2 autotransporter inhibits actin-based motility. Infect. Immun. 78:2240–2247 10.1128/IAI.00100-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C., Marchand J.B., Gouin E., d’Hauteville H., Sansonetti P.J., Carlier M.F., Cossart P. 1995. The unrelated surface proteins ActA of Listeria monocytogenes and IcsA of Shigella flexneri are sufficient to confer actin-based motility on Listeria innocua and Escherichia coli respectively. Mol. Microbiol. 18:413–423 10.1111/j.1365-2958.1995.mmi_18030413.x [DOI] [PubMed] [Google Scholar]
- Kubori T., Galán J.E. 2003. Temporal regulation of Salmonella virulence effector function by proteasome-dependent protein degradation. Cell. 115:333–342 10.1016/S0092-8674(03)00849-3 [DOI] [PubMed] [Google Scholar]
- Kuhn M. 1998. The microtubule depolymerizing drugs nocodazole and colchicine inhibit the uptake of Listeria monocytogenes by P388D1 macrophages. FEMS Microbiol. Lett. 160:87–90 10.1111/j.1574-6968.1998.tb12895.x [DOI] [PubMed] [Google Scholar]
- Kumar Y., Valdivia R.H. 2008. Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds. Cell Host Microbe. 4:159–169 10.1016/j.chom.2008.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang A.E., Schmidt G., Schlosser A., Hey T.D., Larrinua I.M., Sheets J.J., Mannherz H.G., Aktories K. 2010. Photorhabdus luminescens toxins ADP-ribosylate actin and RhoA to force actin clustering. Science. 327:1139–1142 10.1126/science.1184557 [DOI] [PubMed] [Google Scholar]
- Leung Y., Ally S., Goldberg M.B. 2008. Bacterial actin assembly requires toca-1 to relieve N-wasp autoinhibition. Cell Host Microbe. 3:39–47 10.1016/j.chom.2007.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Gundersen G.G. 2008. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat. Rev. Mol. Cell Biol. 9:860–873 10.1038/nrm2522 [DOI] [PubMed] [Google Scholar]
- Li H., Guo F., Rubinstein B., Li R. 2008. Actin-driven chromosomal motility leads to symmetry breaking in mammalian meiotic oocytes. Nat. Cell Biol. 10:1301–1308 10.1038/ncb1788 [DOI] [PubMed] [Google Scholar]
- Lilic M., Galkin V.E., Orlova A., VanLoock M.S., Egelman E.H., Stebbins C.E. 2003. Salmonella SipA polymerizes actin by stapling filaments with nonglobular protein arms. Science. 301:1918–1921 10.1126/science.1088433 [DOI] [PubMed] [Google Scholar]
- Loisel T.P., Boujemaa R., Pantaloni D., Carlier M.F. 1999. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 401:613–616 10.1038/44183 [DOI] [PubMed] [Google Scholar]
- Mandato C.A., Bement W.M. 2001. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J. Cell Biol. 154:785–797 10.1083/jcb.200103105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand J.B., Moreau P., Paoletti A., Cossart P., Carlier M.F., Pantaloni D. 1995. Actin-based movement of Listeria monocytogenes: actin assembly results from the local maintenance of uncapped filament barbed ends at the bacterium surface. J. Cell Biol. 130:331–343 10.1083/jcb.130.2.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhie E.J., Hayward R.D., Koronakis V. 2004. Control of actin turnover by a salmonella invasion protein. Mol. Cell. 13:497–510 10.1016/S1097-2765(04)00053-X [DOI] [PubMed] [Google Scholar]
- McGhie E.J., Brawn L.C., Hume P.J., Humphreys D., Koronakis V. 2009. Salmonella takes control: effector-driven manipulation of the host. Curr. Opin. Microbiol. 12:117–124 10.1016/j.mib.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield C.J., Moss S.E., Ballestrem C., Imhof B.A., Giese G., Wunderlich I., Almers W. 1999. Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nat. Cell Biol. 1:72–74 10.1038/9048 [DOI] [PubMed] [Google Scholar]
- Miserey-Lenkei S., Chalancon G., Bardin S., Formstecher E., Goud B., Echard A. 2010. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat. Cell Biol. 12:645–654 10.1038/ncb2067 [DOI] [PubMed] [Google Scholar]
- Mogilner A. 2006. On the edge: modeling protrusion. Curr. Opin. Cell Biol. 18:32–39 10.1016/j.ceb.2005.11.001 [DOI] [PubMed] [Google Scholar]
- Monack D.M., Theriot J.A. 2001. Actin-based motility is sufficient for bacterial membrane protrusion formation and host cell uptake. Cell. Microbiol. 3:633–647 10.1046/j.1462-5822.2001.00143.x [DOI] [PubMed] [Google Scholar]
- Mostowy S., Nam Tham T., Danckaert A., Guadagnini S., Boisson-Dupuis S., Pizarro-Cerdá J., Cossart P. 2009. Septins regulate bacterial entry into host cells. PLoS ONE. 4:e4196 10.1371/journal.pone.0004196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S., Bonazzi M., Hamon M.A., Tham T.N., Mallet A., Lelek M., Gouin E., Demangel C., Brosch R., Zimmer C., et al. 2010. Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe. 8:433–444 10.1016/j.chom.2010.10.009 [DOI] [PubMed] [Google Scholar]
- Murli S., Watson R.O., Galán J.E. 2001. Role of tyrosine kinases and the tyrosine phosphatase SptP in the interaction of Salmonella with host cells. Cell. Microbiol. 3:795–810 10.1046/j.1462-5822.2001.00158.x [DOI] [PubMed] [Google Scholar]
- Oelschlaeger T.A., Guerry P., Kopecko D.J. 1993. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. USA. 90:6884–6888 10.1073/pnas.90.14.6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa T., Volkman L.E., Welch M.D. 2010. Actin-based motility drives baculovirus transit to the nucleus and cell surface. J. Cell Biol. 190:187–195 10.1083/jcb.201001162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlson M.B., Huang Z., Alto N.M., Blanc M.P., Dixon J.E., Chai J., Miller S.I. 2008. Structure and function of Salmonella SifA indicate that its interactions with SKIP, SseJ, and RhoA family GTPases induce endosomal tubulation. Cell Host Microbe. 4:434–446 10.1016/j.chom.2008.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T., Ichii T., Aono S., Takeichi M. 2006. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J. Cell Biol. 175:135–146 10.1083/jcb.200605012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrick S.B., Cheng H.C., Ismail A.M., Panchal S.C., Doolittle L.K., Kim S., Skehan B.M., Umetani J., Brautigam C.A., Leong J.M., Rosen M.K. 2008. Hierarchical regulation of WASP/WAVE proteins. Mol. Cell. 32:426–438 10.1016/j.molcel.2008.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J.C., Hueffer K., Lam T.T., Galán J.E. 2009. Diversification of a Salmonella virulence protein function by ubiquitin-dependent differential localization. Cell. 137:283–294 10.1016/j.cell.2009.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T.D., Borisy G.G. 2003. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 112:453–465 10.1016/S0092-8674(03)00120-X [DOI] [PubMed] [Google Scholar]
- Pust S., Morrison H., Wehland J., Sechi A.S., Herrlich P. 2005. Listeria monocytogenes exploits ERM protein functions to efficiently spread from cell to cell. EMBO J. 24:1287–1300 10.1038/sj.emboj.7600595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabian T., Gavicherla B., Heisig M., Müller-Altrock S., Goebel W., Gray-Owen S.D., Ireton K. 2009. The bacterial virulence factor InlC perturbs apical cell junctions and promotes cell-to-cell spread of Listeria. Nat. Cell Biol. 11:1212–1218 10.1038/ncb1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenu C., Courtois M., Michelot A., Sykes C., Louvard D., Robine S. 2007. Villin severing activity enhances actin-based motility in vivo. Mol. Biol. Cell. 18:827–838 10.1091/mbc.E06-05-0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A.J., Hall A. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 70:389–399 10.1016/0092-8674(92)90163-7 [DOI] [PubMed] [Google Scholar]
- Ridley A.J., Paterson H.F., Johnston C.L., Diekmann D., Hall A. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 70:401–410 10.1016/0092-8674(92)90164-8 [DOI] [PubMed] [Google Scholar]
- Rivera G.M., Briceño C.A., Takeshima F., Snapper S.B., Mayer B.J. 2004. Inducible clustering of membrane-targeted SH3 domains of the adaptor protein Nck triggers localized actin polymerization. Curr. Biol. 14:11–22 10.1016/j.cub.2003.12.033 [DOI] [PubMed] [Google Scholar]
- Rivera G.M., Antoku S., Gelkop S., Shin N.Y., Hanks S.K., Pawson T., Mayer B.J. 2006. Requirement of Nck adaptors for actin dynamics and cell migration stimulated by platelet-derived growth factor B. Proc. Natl. Acad. Sci. USA. 103:9536–9541 10.1073/pnas.0603786103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J.R., Barth A.I., Marquis H., de Hostos E.L., Nelson W.J., Theriot J.A. 1999. Listeria monocytogenes exploits normal host cell processes to spread from cell to cell. J. Cell Biol. 146:1333–1350 10.1083/jcb.146.6.1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez O.C., Schaefer A.W., Mandato C.A., Forscher P., Bement W.M., Waterman-Storer C.M. 2003. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat. Cell Biol. 5:599–609 10.1038/ncb0703-599 [DOI] [PubMed] [Google Scholar]
- Rottner K., Hänisch J., Campellone K.G. 2010. WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol. 20:650–661 10.1016/j.tcb.2010.08.014 [DOI] [PubMed] [Google Scholar]
- Ruusala A., Pawson T., Heldin C.H., Aspenström P. 2008. Nck adapters are involved in the formation of dorsal ruffles, cell migration, and Rho signaling downstream of the platelet-derived growth factor beta receptor. J. Biol. Chem. 283:30034–30044 10.1074/jbc.M800913200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee N.A., Rivera G.M., Dueber J.E., Vasilescu D., Mullins R.D., Mayer B.J., Lim W.A. 2008. The pathogen protein EspF(U) hijacks actin polymerization using mimicry and multivalency. Nature. 454:1005–1008 10.1038/nature07170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P.J., Mounier J., Prévost M.C., Mège R.M. 1994. Cadherin expression is required for the spread of Shigella flexneri between epithelial cells. Cell. 76:829–839 10.1016/0092-8674(94)90358-1 [DOI] [PubMed] [Google Scholar]
- Sason H., Milgrom M., Weiss A.M., Melamed-Book N., Balla T., Grinstein S., Backert S., Rosenshine I., Aroeti B. 2009. Enteropathogenic Escherichia coli subverts phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate upon epithelial cell infection. Mol. Biol. Cell. 20:544–555 10.1091/mbc.E08-05-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan C., Stecher B., Tzivelekidis T., van Ham M., Rohde M., Hardt W.D., Wehland J., Aktories K. 2009. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 5:e1000626 10.1371/journal.ppat.1000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechi A.S., Wehland J., Small J.V. 1997. The isolated comet tail pseudopodium of Listeria monocytogenes: a tail of two actin filament populations, long and axial and short and random. J. Cell Biol. 137:155–167 10.1083/jcb.137.1.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach M., Paul F.E., Brandt S., Guye P., Daumke O., Backert S., Dehio C., Mann M. 2009. Host cell interactome of tyrosine-phosphorylated bacterial proteins. Cell Host Microbe. 5:397–403 10.1016/j.chom.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Serio A.W., Jeng R.L., Haglund C.M., Reed S.C., Welch M.D. 2010. Defining a core set of actin cytoskeletal proteins critical for actin-based motility of Rickettsia. Cell Host Microbe. 7:388–398 10.1016/j.chom.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarp K.P., Vartiainen M.K. 2010. Actin on DNA-an ancient and dynamic relationship. Cytoskeleton (Hoboken). 67:487–495 [DOI] [PubMed] [Google Scholar]
- Smith K., Humphreys D., Hume P.J., Koronakis V. 2010. Enteropathogenic Escherichia coli recruits the cellular inositol phosphatase SHIP2 to regulate actin-pedestal formation. Cell Host Microbe. 7:13–24 10.1016/j.chom.2009.12.004 [DOI] [PubMed] [Google Scholar]
- Smith-Garvin J.E., Koretzky G.A., Jordan M.S. 2009. T cell activation. Annu. Rev. Immunol. 27:591–619 10.1146/annurev.immunol.021908.132706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis E.T. 2010. Regulation of microtubule organization and functions by septin GTPases. Cytoskeleton (Hoboken). 67:339–345 [DOI] [PubMed] [Google Scholar]
- Stender S., Friebel A., Linder S., Rohde M., Mirold S., Hardt W.D. 2000. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol. Microbiol. 36:1206–1221 10.1046/j.1365-2958.2000.01933.x [DOI] [PubMed] [Google Scholar]
- Sukumaran B., Mastronunzio J.E., Narasimhan S., Fankhauser S., Uchil P.D., Levy R., Graham M., Colpitts T.M., Lesser C.F., Fikrig E. 2011. Anaplasma phagocytophilum AptA modulates Erk1/2 signalling. Cell. Microbiol. 13:47–61 10.1111/j.1462-5822.2010.01516.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana H., Neelakanta G., Kantor F.S., Malawista S.E., Fish D., Montgomery R.R., Fikrig E. 2010. Anaplasma phagocytophilum induces actin phosphorylation to selectively regulate gene transcription in Ixodes scapularis ticks. J. Exp. Med. 207:1727–1743 10.1084/jem.20100276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto J., Namolovan A., Osborne S.E., Coombes B.K., Brumell J.H. 2009. Salmonella-containing vacuoles display centrifugal movement associated with cell-to-cell transfer in epithelial cells. Infect. Immun. 77:996–1007 10.1128/IAI.01275-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taunton J., Rowning B.A., Coughlin M.L., Wu M., Moon R.T., Mitchison T.J., Larabell C.A. 2000. Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J. Cell Biol. 148:519–530 10.1083/jcb.148.3.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terebiznik M.R., Vieira O.V., Marcus S.L., Slade A., Yip C.M., Trimble W.S., Meyer T., Finlay B.B., Grinstein S. 2002. Elimination of host cell PtdIns(4,5)P(2) by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat. Cell Biol. 4:766–773 10.1038/ncb854 [DOI] [PubMed] [Google Scholar]
- Theriot J.A., Mitchison T.J., Tilney L.G., Portnoy D.A. 1992. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 357:257–260 10.1038/357257a0 [DOI] [PubMed] [Google Scholar]
- Tilney L.G., Portnoy D.A. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109:1597–1608 10.1083/jcb.109.4.1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo T., Steinhardt R.A. 2004. Nonmuscle myosin IIA and IIB have distinct functions in the exocytosis-dependent process of cell membrane repair. Mol. Biol. Cell. 15:688–695 10.1091/mbc.E03-06-0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga E., Cossart P. 2005. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat. Cell Biol. 7:894–900 10.1038/ncb1292 [DOI] [PubMed] [Google Scholar]
- Veiga E., Guttman J.A., Bonazzi M., Boucrot E., Toledo-Arana A., Lin A.E., Enninga J., Pizarro-Cerdá J., Finlay B.B., Kirchhausen T., Cossart P. 2007. Invasive and adherent bacterial pathogens co-Opt host clathrin for infection. Cell Host Microbe. 2:340–351 10.1016/j.chom.2007.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvikis O., Maddugoda M.P., Lemichez E. 2010. Direct modifications of Rho proteins: deconstructing GTPase regulation. Biol. Cell. 102:377–389 10.1042/BC20090151 [DOI] [PubMed] [Google Scholar]
- Wasylnka J.A., Bakowski M.A., Szeto J., Ohlson M.B., Trimble W.S., Miller S.I., Brumell J.H. 2008. Role for myosin II in regulating positioning of Salmonella-containing vacuoles and intracellular replication. Infect. Immun. 76:2722–2735 10.1128/IAI.00152-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisswange I., Newsome T.P., Schleich S., Way M. 2009. The rate of N-WASP exchange limits the extent of ARP2/3-complex-dependent actin-based motility. Nature. 458:87–91 10.1038/nature07773 [DOI] [PubMed] [Google Scholar]
- Welch M.D., Iwamatsu A., Mitchison T.J. 1997. Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature. 385:265–269 10.1038/385265a0 [DOI] [PubMed] [Google Scholar]
- Welch M.D., Rosenblatt J., Skoble J., Portnoy D.A., Mitchison T.J. 1998. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science. 281:105–108 10.1126/science.281.5373.105 [DOI] [PubMed] [Google Scholar]
- Wileman T. 2007. Aggresomes and pericentriolar sites of virus assembly: cellular defense or viral design? Annu. Rev. Microbiol. 61:149–167 10.1146/annurev.micro.57.030502.090836 [DOI] [PubMed] [Google Scholar]
- Worley M.J., Nieman G.S., Geddes K., Heffron F. 2006. Salmonella typhimurium disseminates within its host by manipulating the motility of infected cells. Proc. Natl. Acad. Sci. USA. 103:17915–17920 10.1073/pnas.0604054103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam P.T., Theriot J.A. 2004. Repeated cycles of rapid actin assembly and disassembly on epithelial cell phagosomes. Mol. Biol. Cell. 15:5647–5658 10.1091/mbc.E04-06-0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Mooseker M.S., Galán J.E. 1999. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science. 283:2092–2095 10.1126/science.283.5410.2092 [DOI] [PubMed] [Google Scholar]
- Zhou D., Chen L.M., Hernandez L., Shears S.B., Galán J.E. 2001. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol. Microbiol. 39:248–259 10.1046/j.1365-2958.2001.02230.x [DOI] [PubMed] [Google Scholar]
- Zhu J., Sun N., Aoudjit L., Li H., Kawachi H., Lemay S., Takano T. 2008. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 73:556–566 10.1038/sj.ki.5002691 [DOI] [PubMed] [Google Scholar]