Abstract
Rationale
Early-onset drug taking is associated with increased likelihood of addiction, but it is unclear whether early onset is causal in development of addiction. Many other factors are associated with increased risk of addiction and also promote early intake. Here, a rodent model is used to explore the causality of early onset in development of self-administration and addiction-like behavior and to examine factors that promote self-administration.
Methods
We used cocaine self-administration to examine drug taking and addiction-like behavior in adolescent and adult rats a priori characterized for their locomotor responses to novelty and cocaine and behavior in the light–dark task.
Results
Adolescent animals initially sought more cocaine than adults. However, as the adolescents matured, their intake fell and they did not differ from adults in terms of unreinforced lever-pressing, extinction or reinstatement behavior. For both age groups, self-administration was positively correlated with the locomotor response to novelty, the locomotor response to cocaine, and with time in light in the light–dark task. The rats that were insensitive to cocaine's locomotor effects and that spent the least time in light in the light–dark task sought the least cocaine, appearing to be “protected” from the reinforcing effects of cocaine. There was no difference between the two age groups in appearance of this “protected” phenotype.
Conclusions
These results suggest that early onset of drug taking may promote increased use, but does not promote progression to addiction-like behavior. Furthermore, protective factors, such as innate anxiety and insensitivity to cocaine's pharmacological effects, function across developmental stages.
Keywords: Cocaine, Self-administration, Adolescent, Rat, Anxiety, Locomotion, Novelty, Regression
Introduction
Early adolescent onset of drug taking is a risk factor for the development of substance use disorders (SUDs). However, many factors such as comorbid psychopathology and genetics increase the risk of early onset and also increase the risk of addiction (Franken and Hendriks 2000; McGue et al. 2001a; McGue et al. 2001b; Tarter et al. 1999). It is currently unclear whether age-of-onset or other characteristics predominate in predicting risk for addiction. Experimental animal models are valuable in determining the role of early adolescent drug experience on subsequent liability to drug addiction. Previous reports from animal models show that the effects of early drug exposure depend on the behavioral model examined and the drug. Adolescent rodents tend to be more sensitive to conditioned rewarding effects of drugs and are less sensitive to aversive effects (reviewed in (Schramm-Sapyta et al. 2009)). Studies of voluntary consumption reveal drug-specific and schedule-specific effects (see, for example, (Levin et al. 2007; Levin et al. 2003; Shram et al. 2007) for nicotine; (Bell et al. 2006; Brunell and Spear 2005; Doremus et al. 2005; Fullgrabe et al. 2007; Siegmund et al. 2005; Vetter et al. 2007) for ethanol; and (Belluzzi et al. 2005; Frantz et al. 2007; Kantak et al. 2007; Kerstetter and Kantak 2007; Leslie et al. 2004; Perry et al. 2007) for cocaine).
Many other factors that predict drug intake and addiction-like behavior have been examined in adult rodents. Initial self-administration of amphetamine (Piazza et al. 1989) and cocaine (Piazza et al. 2000) is predicted by the locomotor response to novelty. Rats categorized as high responders to a novel environment (HR animals) are more likely than low-responding (LR) animals to acquire self-administration of psychostimulants and also exhibit greater locomotor sensitization (Hooks et al. 1991; Jodogne et al. 1994). However, the locomotor response to novelty does not predict conditioned place preference (Erb and Parker 1994; Gong et al. 1996) or compulsive drug taking (Belin et al. 2008). Another factor which predicts drug intake in adult rats is the locomotor response to experimenter-administered cocaine. Rats with a low locomotor response to a single dose of cocaine (LCRs) take more cocaine on a progressive ratio schedule of reinforcement than high cocaine responders (HCRs) (Mandt et al. 2008). LCRs are also more likely to exhibit locomotor sensitization and conditioned place preference upon repeated exposure (Allen et al. 2007; Sabeti et al. 2003) and are more sensitive to discriminative stimulus effects of cocaine (Klein and Gulley 2009). However, these predictors may change developmentally. Adolescent HCRs to a single exposure are more likely than adolescent LCRs to sensitize upon repeated exposure (Caster et al. 2007).
Many factors predicting drug intake have been identified in adult animals, and adolescence has a well-established role in initiation of drug intake, but the interaction of individual differences and developmental stage has not been fully explored. It is possible that differing factors promote drug intake at differing developmental stages. Few previous studies in rodent models have systematically explored the intersection between individual differences and developmental stage (see (Perry et al. 2006)). One reason that so few studies have examined this intersection is that standard statistical approaches are not powerful enough to detect the predicted effects unless a prohibitively large number of subjects are employed. Most such studies categorize animals as high or low responders (to novelty, cocaine, etc.) and then examine group differences in measures of drug seeking. This approach reduces statistical power because it eliminates most of the variation between subjects, and variation is essential for detecting relationships among measurements.
In the current study, we have employed multiple statistical approaches that allow us to examine the interaction between age group and other predictors while using a manageable sample size. We asked three key questions. First, do cocaine self-administration and addiction-like behaviors differ between adolescents and adults? To address this question, we compared behavior in cocaine self-administration, extinction, and reinstatement between rats that initiated self-administration as adolescents vs. adults. Second, do established predictors of self-administration differentially predict drug seeking in adolescents vs. adults? To address this question, we used multiple regression to assess whether measures related to novelty seeking, cocaine sensitivity, and anxiety are predictive of subsequent self-administration behavior, and whether age group moderated the effects of these factors on drug intake. Third, can we identify populations of rats which are either prone to or protected from high levels of drug intake, and if so, does age group affect the likelihood of being so categorized? To address this question, we used multiple regression to model drug seeking, and examined whether the inclusion of age-group as a main or interaction effect added predictive power to the model.
We observed that adolescents initially self-administered more cocaine, but intake fell as they matured, and they did not exhibit different extinction or reinstatement behavior compared to animals that initiated as adults. Adolescents and adults exhibited similar “compulsive” lever pressing during cocaine non-available periods. We also observed that locomotor response to novelty, locomotor response to cocaine, and time in light in the light–dark task were all positively correlated with drug seeking in self-administration, and that age did not moderate these relationships. Finally, we observed that animals that were highly anxious and insensitive to cocaine's locomotor effects sought the least cocaine, appearing “protected” from cocaine's reinforcing effects. There was no effect of age group on likelihood of being in this “protected” group.
Materials and methods
Rats and housing conditions
Male CD rats were purchased from Charles River Laboratories (Raleigh, NC, USA) at 21 days of age (adolescents) or 58 days of age (adults). They were housed in a temperature- and humidity-controlled vivarium on a reversed 12–12 light–dark cycle (lights off at 7 A.M.). Food and water were initially available ad libitum, and then the rats were food-deprived as indicated below. Rats were pair-housed until implantation of the catheter, after which they were singly housed to avoid chewing on the catheter ports.
General experimental timeline
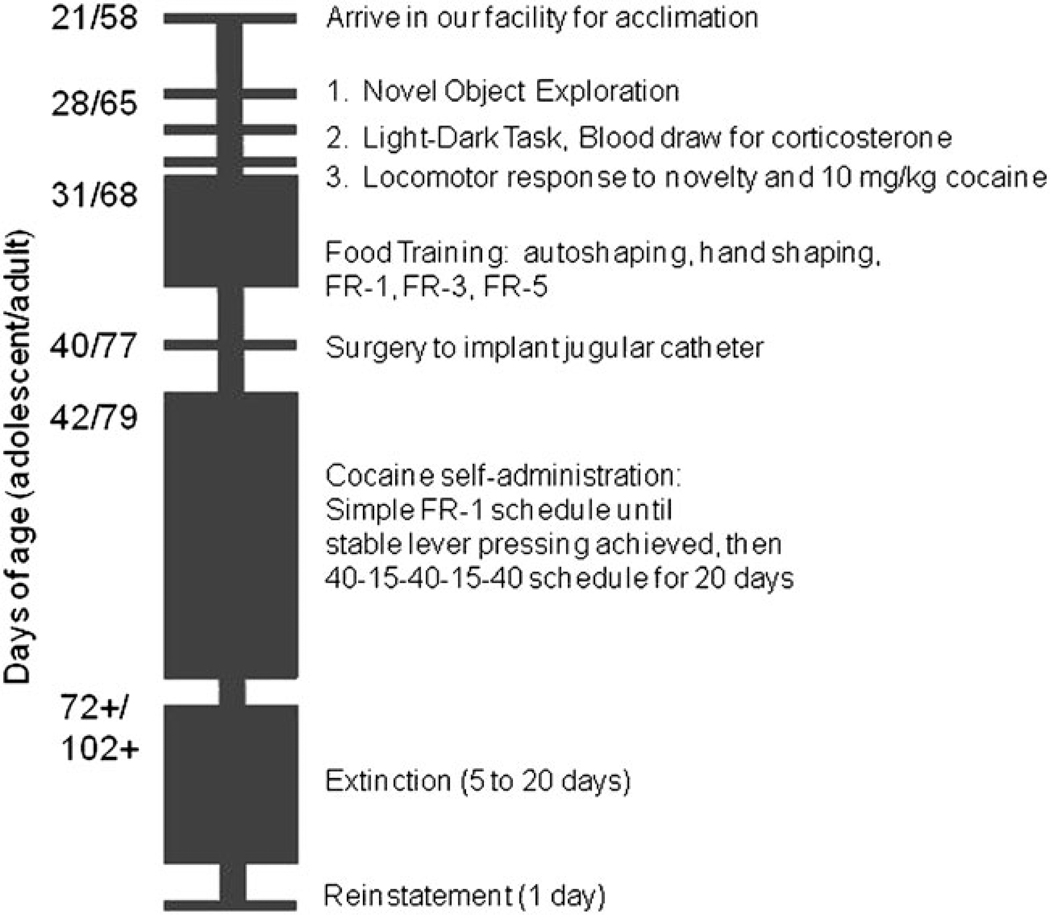
Rats were received from the vendor 1 week before the start of experiments. Beginning at 28 and 65 days of age, they were tested in a prescreen test battery, which included the novel object exploration task (day 1), light–dark task and blood collection for corticosterone analysis (day 2), and novelty- and cocaine-induced locomotion (day 3). Rats were then trained to lever press for food, surgically implanted with a jugular catheter and trained to self-administer cocaine (see timeline, Fig. 1).
Fig. 1.
Experimental time line. See Methods section for details
Prescreen: test battery administered before self-administration training
Novel object exploration
Rats were videotaped interacting with a novel object in their home cage. A full description of this task is available in the Electronic Supplemental Materials. The measure of interest in this task was time (seconds) spent interacting with the novel object out of 5 min.
Light–dark task
Rats were tested in Hamilton–Kinder locomotor boxes fitted with dark inserts, as described (Schramm-Sapyta et al. 2007). They were initially placed in the light half of the chamber and were monitored for 15 min. The measure of interest was time spent in the light half of the chamber, referred to as “time in light.” When necessary, a median split of these data was used to categorize rats for high or low anxiety.
Blood collection from saphenous vein
Blood was collected from the saphenous (leg) vein for analysis of corticosterone levels (Hem et al. 1998). A full description of these methods is available in Electronic Supplemental Materials.
Assessment of locomotor response to novelty
Locomotor response to novelty was assessed using Hamilton–Kinder open-field activity monitors (40×40×40 cm) with corn cob bedding on the floor. Rats were placed in the center of the chamber and activity was recorded for 1 h. Total distance traveled (centimeter) is reported, and is referred to as “novelty locomotion.”
Assessment of locomotor response to cocaine
Immediately after the assessment of novelty locomotion, the rats were injected with 10 mg/kg cocaine, i.p. in 1 ml/kg saline and returned to the Hamilton–Kinder activity monitors. Locomotor activity was recorded for 1 h post-injection. The total distance traveled during this hour (centimeter) is reported and referred to as “cocaine locomotion.”
None of the prescreen measures varied significantly by age group, see Table 1.
Table 1.
Summary of prescreen test battery measures
| Prescreen measurement | Adolescents mean±SEM | Adults mean±SEM | Significant age effect? |
|---|---|---|---|
| Novel object time (s) | 57.3±8.3 | 58.7±7.6 | NS |
| Time in light (s) | 322.6±25.3 | 319.4±22.7 | NS |
| Corticosterone (ng/mL) | 308.2±17.3 | 333.6±15.6 | NS |
| Novelty locomotion (cm) | 25,357±2,337 | 23,785±2,101 | NS |
| Cocaine locomotion (cm) | 36,510±4,465 | 29,012±4,013 | NS |
Self-administration procedure
Food training prior to cocaine self-administration
After the prescreen test battery described above, rats were trained in Med Associates operant conditioning chambers (32×24×20 cm) to receive food reinforcement. Rats were food-deprived from this point until the end of the experiment, receiving approximately 15–20 g of food per day. Rats were trained in a combination of autoshaping, hand shaping, and overnight training as necessary. They were then trained on FR-1, FR-3, and FR-5 schedules of reinforcement in the self-administration harnesses with a requirement of 100 pellets to move to each successive FR schedule. Rats that did not acquire food-seeking behavior or that did not receive at least 100 pellets at each FR stage were excluded from further testing.
Surgery to implant jugular catheter
Rats were anesthetized with either a ketamine/domitor cocktail (0.60 mg/kg ketamine and 0.15 mg/kg domitor, administered i.p.) or the inhalant isoflurane (2–2.5% with 1.25 L oxygen). A heparin and antimicrobial-infused catheter (Instech Solomon, 3Fr.) was inserted in the right jugular vein by standard procedures (see Electronic Supplemental Materials for a full description). The catheter exited dorsally and was attached to a port (Instech, Plastic SoloPort, 16 mm), which was implanted subcutaneously and sutured to the trapezius muscle. Bupivacaine was used prophylactically for pain management, and rats were allowed 2–4 days to recover with ad libitum food and water. Catheters were flushed daily with 0.3-mL sterile lock solution containing 25 IU/mL heparinized saline and 0.4 mg/mL gentamicin. Catheter patency was tested with methohexital (0.3 mL, 5 mg/mL) at the end of the self-administration period or when dramatic changes in lever-pressing behavior were observed. Rats that did not respond immediately to methohexital were excluded from further study.
Self-administration of cocaine
After recovery from surgery, food restriction was reinstated and rats were trained to self-administer cocaine in the same operant conditioning chambers used for food training. In an FR-1 schedule, presses on the active lever resulted in the blinking cue light and infusion of 0.8 mg/kg of cocaine over approximately 6 s, in a volume of approximately 100 µL. There was a 40-s timeout after each infusion during which lever presses were recorded but had no programmed consequences. Acquisition sessions lasted up to 3 h or until the rat received 37 infusions. Sessions were repeated daily until each animal met the following acquisition criteria: a minimum of two infusions per day, with less than 20% variation across two consecutive days and a minimum ratio of 2:1 active:inactive lever presses.
Upon achieving acquisition criteria, rats were switched to a schedule in which cocaine-available periods (40 min) were alternated with cocaine non-available (CNA) periods (15 min) in a 40–15–40–15–40 pattern. Cocaine-available periods began with the illumination of the cue light above the active lever. During the CNA periods, the house light was turned on and the cue light turned off. During CNA, presses on both levers were recorded but had no programmed consequences. Measures of interest during the self-administration sessions were number of infusions obtained, active lever presses during cocaine-available periods, and active lever presses during CNA periods. Inactive lever pressing was examined in each animal to ensure that the 2:1 selectivity for the active lever was maintained. (This self-administration schedule is based on that used by (Deroche-Gamonet et al. 2004)).
Extinction and stress-induced reinstatement
After 20 daily sessions of the 40–15 protocol with cocaine, the drug was replaced with saline for extinction testing. Extinction continued until infusion number fell to less than 20% of the average at the end of the cocaine sessions, or a maximum of 20 extinction sessions. Rats were then left in their home cages for 5 days without exposure to the chambers. On the fifth day, they were deprived of food. On the sixth day, they were tested for reinstatement of lever pressing in the 40–15 protocol with saline. The reinstatement of lever pressing after this schedule of abstinence and food deprivation may reflect either stress-induced reinstatement or reinstatement due to the novelty of returning to the chamber after a 5-day absence. Measures of interest were days to achieve extinction criteria and active lever pressing during reinstatement.
Analysis of brain cocaine levels
A separate group of rats received surgery to implant jugular catheters and ports for analysis of brain and plasma cocaine levels. The surgeries were timed so that recovery occurred at the same ages at which the self-administration sessions began in the previous group (approximately 42 days for adolescents, 79 days for adults). After confirmation of catheter patency with methohexital, rats were injected i.v. with 0.8 mg/kg cocaine in a volume of 100 µL, which was followed with 300-µL saline to ensure entry into the blood stream. This injection procedure required approximately 6 s, comparable to the infusions used in the self-administration chambers. Rats were decapitated under isoflurane anesthesia at 1- or 10-min post-infusion for collection of trunk blood and brain tissue. One milliliter of trunk blood was mixed with 10 µL of saturated NaF and 100 µL of heparin and centrifuged for collection of plasma. Brains were frozen immediately on dry ice in aluminum foil. Plasma and brain tissue samples were frozen at −80°C until they were sent to the Center for Human Toxicology at the University of Utah for analysis of cocaine and metabolite content. Plasma and brain homogenates were analyzed by liquid chromatography-tandem mass spectrometry essentially as described (Lin et al. 2001) with conditions for including the metabolites ecgonine methyl ester and norcocaine (Lin et al. 2003).
Drug treatments
Cocaine was dissolved in 0.9% sterile saline, in a 10 mg/mL solution for i.p. injections, and a concentration determined by the animal's weight for i.v. infusions.
Statistical analysis
The effect of age-of-onset (adolescent vs. adult groups) was examined using ANOVA at the p<0.05 level of significance using Statview statistical software to analyze the raw (untransformed) data. Descriptive statistics are presented as mean±SEM. Linear regression and correlation were performed using JMP statistical software. Normality of distributions was assessed using the Shapiro–Wilk W test for normality. Correlations involving non-normal distributions were assessed in two ways: (1) using Spearman's Rho, a non-parametric test which uses ranks; and (2) by using simple regression on log-transformed data.
Multivariate analysis
Three of the five prescreen test battery measures were normally distributed: the time in light in the light–dark task, circulating corticosterone, and novelty locomotion. The two other measures, novel object time and cocaine locomotion, were right-skewed but normally distributed upon logarithmic transformation.
Of the outcome measures, infusion number was normally distributed, but active lever pressing during food training, cocaine self-administration, and the CNA periods were right-skewed. These latter variables were normally distributed upon logarithmic transformation.
Model building
To build the model predicting self-administration, variables were added one at a time, selected by their ability to significantly improve prediction beyond variables already in the model. All independent variable main effects, as well as interactions with age group, were considered.
Analysis of “protected” vs. “at risk” groups
Rats were categorized as high vs. low locomotor responders to novelty, high vs. low locomotor responders to cocaine, and anxious vs. non-anxious based on median splits of each of these measures. Fisher's exact test was then used to examine whether adolescents vs. adults were more likely to fall into the high vs. low groups. Rats that were included in both the “anxious” group and the low cocaine locomotion group were labeled “protected,” and all others were labeled “at risk.” Fisher's exact test was then used to determine whether adolescents vs. adults were more likely to be categorized as “protected.”
Results
Effect of age-of-onset on self-administration
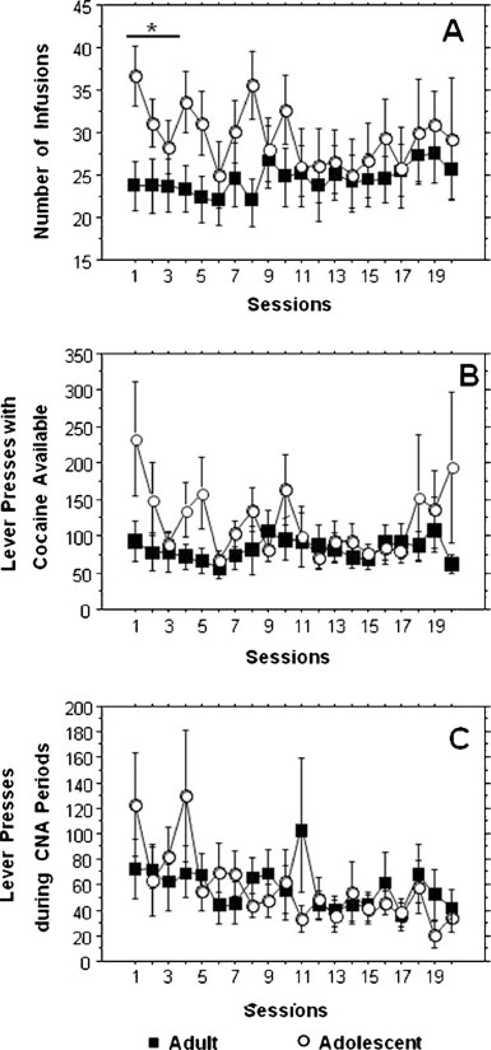
Figure 2 and Table 2 present age-group averages of key variables. There was no effect of age group on days to acquire self-administration behavior. After acquisition, adolescents took more infusions than adults (p=0.04) and tended to perform more active lever presses than adults (p=0.09, NS). Adolescents did not perform more lever presses than adults during the CNA periods. In the late phase of self-administration, as the adolescents matured into adulthood, the intake of the adolescent-onset animals fell to adult levels, and there was no effect of age-of-onset on infusions earned or active lever pressing during cocaine-available or CNA periods. There was no effect of age-of-onset on days to extinction or on active lever pressing during stress-induced reinstatement.
Fig. 2.
Time courses of a infusions earned, b active lever presses, and c lever pressing during CNA periods during the 20 sessions of the 40–15 self-administration protocol. Asterisk, significant age effect
Table 2.
Summary of self-administration outcome measures
| Adolescents mean±SEM | Adults mean±SEM | Significant age effect? | |
|---|---|---|---|
| Early-phase measures: | N=21 | N=26 | |
| Infusions, days 1–3 | 32±3 | 24±3 | p=0.04 |
| Active lever presses, days 1–3 | 157±32 | 84±29 | NS, p=0.09 |
| CNA lever presses, days 1–3 | 87±20 | 76±18 | NS |
| Late-phase measures: | N=11 | N=16 | |
| Infusions, days 18–20 | 30±5 | 27±4 | NS |
| Active lever presses, days 18–20 | 148±49 | 101±41 | NS |
| CNA lever presses days 18–20 | 29±18 | 62±15 | NS |
| Days to extinguish self-administration | 10±2 | 9±2 | NS |
| Active lever presses when extinction criteria were met | 16±9 | 21±8 | NS |
| Active lever pressing during reinstatement test | 28±12 | 34±10 | NS |
Potential confounds
Several confounding factors were considered and eliminated as possible explanations for the age difference in infusion number that we observed.
Differential food deprivation
The difference in infusion number is not attributable to differential food deprivation of the two age groups. As shown in Table 3, adults were generally more food-deprived than adolescents (as a percentage of published standard weights). Greater food deprivation would have led to greater cocaine seeking (Carroll et al. 1979; Carroll et al. 1981; de la Garza et al. 1981; De Vry et al. 1989; Glick et al. 1987; Papasava and Singer 1985), which is the opposite of what we observed.
Table 3.
Weights of rats at each stage of experiment
| Adolescent | Adult | |||||
|---|---|---|---|---|---|---|
| Age (weeks) | Weight (grams) mean±SEM |
% of published standards for this strain |
Age (weeks) | Weight (grams) mean±SEM |
% of published standards for this strain |
|
| Food training | Approximately 5 | 153±4 | 122 | Approximately 10 | 329±4 | 94 |
| Acquisition of cocaine self-administration | 6 | 186±4 | 106 | 11 | 331±5 | 86 |
| Early self-administration | 7 | 202±4 | 91 | 12 | 320±4 | 78 |
| Late self-administration | 10 | 251±6 | 72 | 15 | 315±4 | 68 |
| Extinction and reinstatement | 12 | 262±7 | 63 | 17 | 314±6 | Standard n/a |
Published standards obtained from Charles River Laboratories, http://www.criver.com/SiteCollectionDocuments/rm_rm_c_CD_IGS_rat_weight_chart.pdf
Pharmacokinetics
The difference in cocaine intake between adolescents and adults is also not attributable to a difference in brain uptake or clearance of cocaine. As shown in Fig. 3, there was no effect of age on levels of cocaine in the brain at 1 min or 10 min after i.v. cocaine infusion. In both ages, brain levels were maximal at 1-min post-infusion and fell to approximately one half of that level at 10-min post-infusion. Similar results were obtained for cocaine levels in plasma (data not shown). The metabolite benzoylecgonine appeared in the plasma beginning at 1 min, and reached higher levels at 10 min (data not shown). There was no effect of age group on its appearance.
Fig. 3.
Cocaine concentration in brain homogenate (ng/g) in adolescent and adult rats at 1 and 10-min post-i.v. infusion. Asterisk, significantly different from 1-min group
Contamination from food-seeking behavior
The increased cocaine self-administration in adolescents is also not attributable to generally increased lever-pressing behavior. At the end of food training, the adult animals performed more lever presses than the adolescents (adults, 1,806±184 (mean±SEM); adolescents, 940±125; t test, p=0.0002). We cannot rule out the possibility that the increased infusions earned by adolescents resulted from an “extinction burst” of lever pressing for food, i.e., that the adolescents were not seeking cocaine but failing to extinguish food seeking. This seems unlikely, however, based on the fact that there was a significant age by food-seeking interaction in predicting active lever pressing during initial cocaine self-administration (R2=0.24, p=0.008). In adults, food seeking and cocaine seeking were positively correlated, whereas the two measures were unrelated in adolescents. If failure to extinguish food seeking was motivating the adolescents to perform higher lever presses during the early cocaine sessions, then we would have predicted a positive correlation in the adolescent group.
Ability of prescreen measures to predict drug-seeking behavior
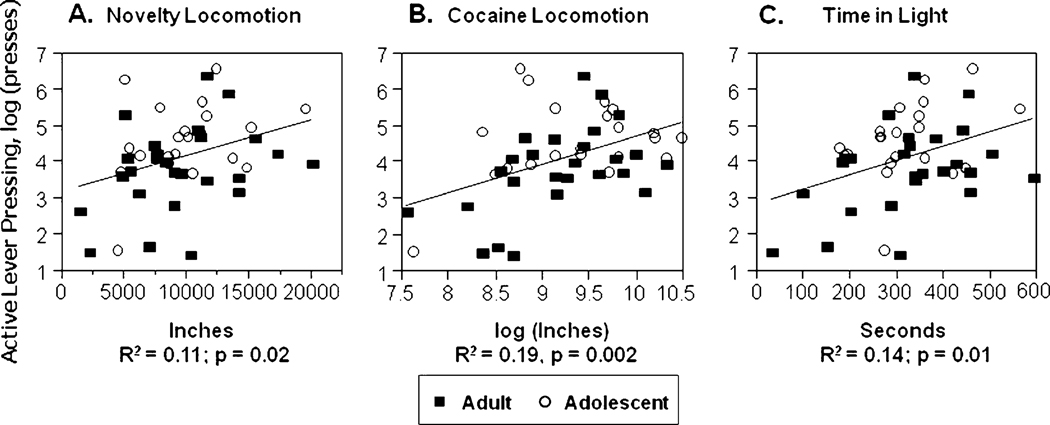
We then examined the ability of our prescreen measures to predict self-administration outcome measures by using multiple regression. With both ages combined, active lever pressing was significantly correlated with novelty locomotion, cocaine locomotion, and time in light in the light–dark task. Infusion number was significantly correlated with novelty locomotion and cocaine locomotion. Lever pressing during the CNA periods was significantly correlated with cocaine locomotion and time in light. See Table 4 and Fig. 4.
Table 4.
Correlations between prescreen and initial self-administration measures
| Prescreen: outcome: | Novel object time | Time in light | Corticosterone (ng/mL) |
Novelty locomotion | Cocaine locomotion (non-normal) |
|---|---|---|---|---|---|
| Infusions | NS | NS | NS | R=0.31* | R=0.43** |
| Active lever pressing | NS | R=0.37** ρ=0.23,NS | NS | R=0.35* ρ=0.329* | R=0.43** ρ=0.39** |
| CNA active Pressing | NS | R=0.24* ρ=0.20,NS | NS | NS | R=0.33* ρ=0.27,NS |
R represents the correlation obtained using log-transformed values, ρ represents Spearman's Rho, obtained from the non-parametric correlation test.
p<0.05;
p<0.01
Fig. 4.
Correlations between active lever pressing and a novelty locomotion; b cocaine locomotion; and c time in light in light dark task. Open circles, adolescent animals, closed squares, adult animals
The overlap in these relationships is not surprising, since there is significant overlap among some of the measures. Among the prescreen measures, novelty locomotion was significantly correlated with time in light in the light–dark task (R2=0.51, p<0.0001), and with cocaine locomotion (R2=0.21, p=0.0013; Spearman's Rho=0.3763, p=0.0091). No other pairs of measures were significantly correlated. Among the self-administration outcome measures, there was also significant covariation. Infusion number was correlated with active lever pressing (R2=0.74, p<0.0001; Spearman's Rho=0.8548, p<0.0001) and also with both active and inactive lever pressing during the CNA periods (R2=0.46, p<0.0001; Spearman's Rho=0.6021, p<0.0001 for active lever pressing; R2=0.18, p=0.0028 for inactive lever pressing). Active lever pressing was also significantly correlated with CNA active lever pressing (R2=0.52, p<0.0001; Spearman's Rho=0.6307, p<0.0001).
Effects of age on relationships between prescreen and self-administration measures
Next, we tested for interactions to determine whether the effect of prescreen measures on self-administration outcomes differed across the two age groups. We focused this analysis on active lever pressing, because it demonstrated the most consistent effects in the simple correlation analyses including both ages. Using multiple regression, no model predicting active lever pressing was significantly improved by the inclusion of age as a main effect or interaction term.
As another statistical approach to test whether age impacted vulnerability to high drug taking, we used Fisher's exact test to determine whether adolescents or adults were more likely to be included in the high cocaine locomotion group, the high novelty locomotion group, or the non-anxious group (the three categories that predicted high active lever pressing). HCRs included 11 adult rats and 12 adolescent rats, whereas LCRs included 15 adults and 9 adolescents (NS by Fisher's exact test, p=0.24). High novelty responders included 12 adults and 11 adolescents, low novelty responders included 14 adults and 10 adolescents (NS by Fisher's exact test, p=0.45). The non-anxious group included 14 adults and 9 adolescents, whereas the anxious group included 12 adults and 12 adolescents (NS by Fisher's exact test, p=0.85). Thus, there is no difference in the likelihood of falling into the high cocaine locomotion, high novelty locomotion, or non-anxious category for adolescents vs. adults.
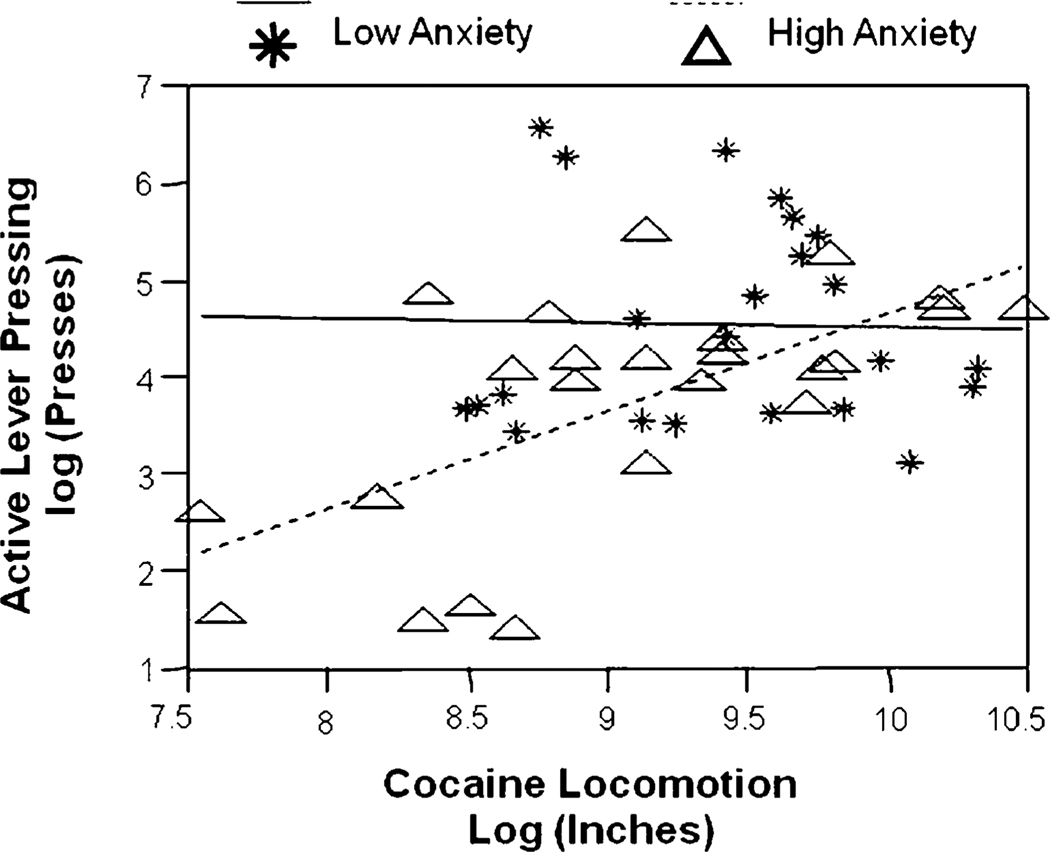
We next used multiple regression to determine what combination of the prescreen measures best predicted active lever pressing. Based on the pairwise correlations described above, cocaine locomotion was the best single predictor, generating the highest R2 value. We then tested whether addition of other measures significantly improved prediction of active lever pressing in that model. We found that the interaction of anxiety level with cocaine locomotion most strongly predicted active lever pressing. As shown in Fig. 5, in anxious rats, sensitivity to cocaine locomotion was an important determinant of active lever pressing: anxious rats pressed the lever more if they were more sensitive to cocaine's locomotor effects. However, non-anxious animals pressed the lever at high rates regardless of their sensitivity to cocaine locomotion. It appeared that highly anxious, cocaine-locomotor-insensitive rats were protected from the reinforcing effects of cocaine because they performed fewer active lever presses than all other groups. We then tested whether each animal's likelihood of falling into this “protected” category was influenced by age. By Fisher's exact test, adolescents and adults are equally likely to be included in this category. (10 out of 26 adults met these criteria, 5 out of 21 adolescents met the criteria, NS, p=0.23.)
Fig. 5.
Interaction between behavior in the light–dark task and locomotor response to cocaine predicts active lever pressing. Asterisks and solid line, low-anxiety animals; triangles and dashed line, high-anxiety animals, based on median split of time in light in light–dark task. (Full model R2=0.37; p=0.0002.)
Discussion
This study combines an examination of the role of age-of-onset with individual characteristics to predict vulnerability to drug taking in rats. We observed, first, that adolescent onset of drug taking in rats led to initially higher drug intake, but did not affect later drug taking or addiction-like behavior. Second, common predictors of drug intake (novelty seeing, cocaine sensitivity, anxiety) predicted drug taking comparably in adolescents and adults. Third, we identified a “protected” phenotype consisting of high anxiety and low cocaine sensitivity, which predicted low cocaine seeking in both ages.
Our finding of initial differences in cocaine intake between adolescents and adults is slightly at odds with previous papers (Belluzzi et al. 2005; Frantz et al. 2007; Kantak et al. 2007; Kerstetter and Kantak 2007; Leslie et al. 2004) that have demonstrated no difference in cocaine self-administration between adolescent and adult rats. All of these reports describe methods involving less food training (prior to self-administration) than the current study, and we attribute, at least partly, the difference to that factor. The differences we observed were not attributable to pharmacokinetics, differential food deprivation, or general increased lever-pressing behavior.
The multivariate analysis of individual characteristics must be described as exploratory to reflect the fact that many measures were taken from each animal, thereby increasing the risk of false-positive results. Despite that caveat, we found several interesting relationships. First, we, like others, observed that locomotion in an inescapable novel environment predicts early-phase self-administration of psychostimulants (Klebaur et al. 2001; Piazza et al. 1989; Piazza et al. 2000). This likely reflects the relationship between locomotion and ability to learn an operant task (Mitchell et al. 2005), and does not generalize to other forms of novelty exploration (Klebaur et al. 2001). Similarly, we observed that exploration of a novel open field, but not of a novel object in the home cage was related to cocaine self-administration. The locomotor response to novelty has been reported to not predict “addiction-like” behaviors (Belin et al. 2008), and may be related to general activity and ability to learn an operant response (Mitchell et al. 2005) but see (Marinelli 2005).
Second, we observed that a high locomotor response to experimenter-administered cocaine predicted high cocaine seeking in subsequent self-administration. Other researchers have reported that cocaine-induced locomotion predicts locomotor sensitization (Sabeti et al. 2003), CPP (Allen et al. 2007), progressive ratio drug seeking (Mandt et al. 2008), and discriminative stimulus effects (Klein and Gulley 2009). In all of these reports, low initial response to cocaine predicted greater subsequent cocaine-related behavior. The current studies obtained the opposite result: rats with a high locomotor response to cocaine obtained more infusions during the 40–15 schedule of reinforcement than those with a low locomotor response to cocaine. There are several experimental differences that may account for this discrepancy, particularly differential food training, differing unit doses of cocaine, and the use of the 40–15 schedule for drug delivery. These discrepancies suggest that there are many complex relationships among cocaine-related behaviors and that dosage and prior experience likely contribute to observed relationships.
Third, low anxiety predicted higher drug seeking. This is consistent with one previous report (Bush and Vaccarino 2007). In light of many reports demonstrating that cocaine is anxiogenic (Ettenberg and Geist 1993; Geist and Ettenberg 1990; Panlilio et al. 2007), we hypothesize that cocaine may become more aversive in animals with high baseline anxiety, which may limit its reinforcing effects. It is well-established that treatment with anxiolytic drugs increases cocaine-seeking behavior (Ettenberg and Geist 1991; Maier et al. 2008). However, one published report suggests the opposite relationship: that greater baseline anxiety increases the rewarding effects of cocaine (Pelloux et al. 2009). Clearly, the relationship between anxiety and cocaine is a complicated one.
The major finding of this paper is that anxiety and cocaine sensitivity affect cocaine self-administration similarly in adolescent and adult rats. This was demonstrated by lack of an age×prescreen interaction and lack of improvement of the statistical model predicting active lever pressing by inclusion of age. This result suggests that age of onset is not causal in development of drug-seeking behavior in rodents, but rather that other factors are more determinative of these behaviors.
Finally, we identified a “protected” phenotype before initiation of self-administration training. Rats that are highly anxious and not sensitive to cocaine's locomotor effects subsequently performed the fewest active lever presses in self-administration. Age did not moderate the likelihood of being in this “protected” category.
The covariation we observed among the behaviors observed sheds light on the underlying constructs that these tasks measure. For example, the correlation between infusion number and both active and inactive lever pressing during the cocaine-non-available periods suggests that CNA lever pressing may not be a measure of compulsive drug seeking, as previously suggested (Deroche-Gamonet et al. 2004), but may instead reflect locomotor effects of the drug received in the prior cocaine-available block. Future experiments should incorporate a CNA block before the first cocaine-available block. Second, the lack of correlation between novelty locomotion and novel object exploration in the home cage suggests that the two forms of novelty-seeking are not measuring the same underlying construct. Finally, the lack of correlation between behavior in the light–dark task and corticosterone level suggests that corticosterone may not be uniquely indicative of fear, but may represent some more global effect, such as arousal.
Humans who initiate drug use at an early age are more likely to develop SUDs (Brown et al. 2004; DeWit et al. 2000; Lewinsohn et al. 1999; Lynskey et al. 2003; Meyer and Neale 1992; Patton et al. 2004; Prescott and Kendler 1999; Robins and Przybeck 1985). However, there are several genetic and environmental risk factors that also hasten the onset of drug taking. Thus, it is unclear from the epidemiologic literature whether early onset per se is sufficient to increase risk of addiction. The findings described here indicate that individual factors are more of a driver of drug intake than developmental stage, at least for cocaine. Taken together, these results indicate that drug abuse prevention efforts should be targeted to individuals who are most at risk, as early as possible, rather than to all members of a certain age cohort.
Supplementary Material
Acknowledgments
The authors wish to thank Reynold Francis for assistance with corticosterone assays, and Ann Petro, Susan Slade, and Cori Wells for assistance with surgeries. This work was funded by NIDA, DA020729 to NLSS and NIDA 019114 to CMK.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-011-2216-5) contains supplementary material, which is available to authorized users.
Contributor Information
Nicole L. Schramm-Sapyta, Email: Nicole.schrammsapyta@duke.edu, Department of Psychiatry, Duke University Medical Center, Durham, NC 27710, USA.
Marty C. Cauley, Department of Psychiatry, Duke University Medical Center, Durham, NC 27710, USA
Dalene K. Stangl, Statistical Science, Duke University Medical Center, Durham, NC 27710, USA
Susan Glowacz, Department of Psychiatry, Duke University Medical Center, Durham, NC 27710, USA.
K. Amy Stepp, Department of Psychiatry, Duke University Medical Center, Durham, NC 27710, USA.
Edward D. Levin, Department of Psychiatry, Duke University Medical Center, Durham, NC 27710, USA
Cynthia M. Kuhn, Pharmacology and Cancer Biology, Duke University Medical Center, Durham, NC 27710, USA
References
- Allen RM, Everett CV, Nelson AM, Gulley JM, Zahniser NR. Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague–Dawley rats. Pharmacol Biochem Behav. 2007;86:37–44. doi: 10.1016/j.pbb.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Brown TL, Flory K, Lynam DR, Leukefeld C, Clayton RR. Comparing the developmental trajectories of marijuana use of African American and Caucasian adolescents: patterns, antecedents, and consequences. Exp Clin Psychopharmacol. 2004;12:47–56. doi: 10.1037/1064-1297.12.1.47. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Bush DE, Vaccarino FJ. Individual differences in elevated plus-maze exploration predicted progressive-ratio cocaine self-administration break points in Wistar rats. Psychopharmacology. 2007;194:211–219. doi: 10.1007/s00213-007-0835-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205:319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. J Pharmacol Exp Ther. 1981;217:241–247. [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. A single high dose of cocaine induces differential sensitization to specific behaviors across adolescence. Psychopharmacology. 2007;193:247–260. doi: 10.1007/s00213-007-0764-5. [DOI] [PubMed] [Google Scholar]
- de la Garza R, Bergman J, Hartel CR. Food deprivation and cocaine self-administration. Pharmacol Biochem Behav. 1981;15:141–144. doi: 10.1016/0091-3057(81)90353-1. [DOI] [PubMed] [Google Scholar]
- De Vry J, Donselaar I, Van Ree JM. Food deprivation and acquisition of intravenous cocaine self-administration in rats: effect of naltrexone and haloperidol. J Pharmacol Exp Ther. 1989;251:735–740. [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Hance J, Offord DR, Ogborne A. The influence of early and frequent use of marijuana on the risk of desistance and of progression to marijuana-related harm. Prev Med. 2000;31:455–464. doi: 10.1006/pmed.2000.0738. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Erb SM, Parker LA. Individual differences in novelty-induced activity do not predict strength of amphetamine-induced place conditioning. Pharmacol Biochem Behav. 1994;48:581–586. doi: 10.1016/0091-3057(94)90317-4. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of self- administered cocaine. Psychopharmacology. 1991;103:455–461. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacol Biochem Behav. 1993;44:191–198. doi: 10.1016/0091-3057(93)90298-8. [DOI] [PubMed] [Google Scholar]
- Franken IH, Hendriks VM. Early-onset of illicit substance use is associated with greater axis-II comorbidity, not with axis-I comorbidity. Drug Alcohol Depend. 2000;59:305–308. doi: 10.1016/s0376-8716(99)00132-5. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O'Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Fullgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 2007;86:320–326. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Geist TD, Ettenberg A. A simple method for studying intravenous drug reinforcement in a runaway. Pharmacol Biochem Behav. 1990;36:703–706. doi: 10.1016/0091-3057(90)90278-p. [DOI] [PubMed] [Google Scholar]
- Glick SD, Hinds PA, Carlson JN. Food deprivation and stimulant self-administration in rats: differences between cocaine and d-amphetamine. Psychopharmacology. 1987;91:372–374. doi: 10.1007/BF00518194. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Justice JB., Jr Locomotor response to novelty does not predict cocaine place preference conditioning in rats. Pharmacol Biochem Behav. 1996;53:191–196. [PubMed] [Google Scholar]
- Hem A, Smith AJ, Solberg P. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret and mink. Lab Anim. 1998;32:364–368. doi: 10.1258/002367798780599866. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav. 1991;38:467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Jodogne C, Marinelli M, Le Moal M, Piazza PV. Animals predisposed to develop amphetamine self-administration show higher susceptibility to develop contextual conditioning of both amphetamine-induced hyperlocomotion and sensitization. Brain Res. 1994;657:236–244. doi: 10.1016/0006-8993(94)90973-3. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Goodrich CM, Uribe V. Influence of sex, estrous cycle, and drug-onset age on cocaine self-administration in rats (Rattus norvegicus) Exp Clin Psychopharmacol. 2007;15:37–47. doi: 10.1037/1064-1297.15.1.37. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Kantak KM. Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacology. 2007;194:403–411. doi: 10.1007/s00213-007-0852-6. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol. 2001;12:267–275. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Klein DA, Gulley JM. Reduced sensitivity to the locomotor-stimulant effects of cocaine is associated with increased sensitivity to its discriminative stimulus properties. Behav Pharmacol. 2009;20:67–77. doi: 10.1097/FBP.0b013e3283242fdd. [DOI] [PubMed] [Google Scholar]
- Leslie FM, Loughlin SE, Wang R, Perez L, Lotfipour S, Belluzzia JD. Adolescent development of forebrain stimulant responsiveness: insights fromanimal studies. Ann NYAcad Sci. 2004;1021:148–159. doi: 10.1196/annals.1308.018. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Brown RA. Level of current and past adolescent cigarette smoking as predictors of future substance use disorders in young adulthood. Addiction. 1999;94:913–921. doi: 10.1046/j.1360-0443.1999.94691313.x. [DOI] [PubMed] [Google Scholar]
- Lin SN, Moody DE, Bigelow GE, Foltz RL. A validated liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry method for quantitation of cocaine and benzoylecgonine in human plasma. J Anal Toxicol. 2001;25:497–503. doi: 10.1093/jat/25.7.497. [DOI] [PubMed] [Google Scholar]
- Lin SN, Walsh SL, Moody DE, Foltz RL. Detection and time course of cocaine N-oxide and other cocaine metabolites in human plasma by liquid chromatography/tandem mass spectrometry. Anal Chem. 2003;75:4335–4340. doi: 10.1021/ac030037c. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, Statham DJ, Martin NG. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Maier EY, Ledesma RT, Seiwell AP, Duvauchelle CL. Diazepam alters cocaine self-administration, but not cocaine-stimulated locomotion or nucleus accumbens dopamine. Pharmacol Biochem Behav. 2008;91:202–207. doi: 10.1016/j.pbb.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandt BH, Schenk S, Zahniser NR, Allen RM. Individual differences in cocaine-induced locomotor activity in male Sprague–Dawley rats and their acquisition of and motivation to self-administer cocaine. Psychopharmacology. 2008;201:195–202. doi: 10.1007/s00213-008-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M. The many facets of the locomotor response to a novel environment test: theoretical comment on Mitchell, Cunningham, and Mark (2005) Behav Neurosci. 2005;119:1144–1151. doi: 10.1037/0735-7044.119.4.1144. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res. 2001a;25:1166–1173. [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001b;25:1156–1165. [PubMed] [Google Scholar]
- Meyer JM, Neale MC. The relationship between age at first drug use and teenage drug use liability. Behav Genet. 1992;22:197–213. doi: 10.1007/BF01066999. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Cunningham CL, Mark GP. Locomotor activity predicts acquisition of self-administration behavior but not cocaine intake. Behav Neurosci. 2005;119:464–472. doi: 10.1037/0735-7044.119.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Solinas M, Matthews SA, Goldberg SR. Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacology. 2007;32:646–657. doi: 10.1038/sj.npp.1301109. [DOI] [PubMed] [Google Scholar]
- Papasava M, Singer G. Self-administration of low-dose cocaine by rats at reduced and recovered body weight. Psychopharmacology. 1985;85:419–425. doi: 10.1007/BF00429657. [DOI] [PubMed] [Google Scholar]
- Patton GC, McMorris BJ, Toumbourou JW, Hemphill SA, Donath S, Catalano RF. Puberty and the onset of substance use and abuse. Pediatrics. 2004;114:e300–e306. doi: 10.1542/peds.2003-0626-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Costentin J, Duterte-Boucher D. Anxiety increases the place conditioning induced by cocaine in rats. Behav Brain Res. 2009;197:311–316. doi: 10.1016/j.bbr.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Perry JL, Anderson MM, Nelson SE, Carroll ME. Acquisition of i.v. cocaine self-administration in adolescent and adult male rats selectively bred for high and low saccharin intake. Physiol Behav. 2007;91:126–133. doi: 10.1016/j.physbeh.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacology. 2006;186:235–245. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose–response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: a noncausal association. Alcohol Clin Exp Res. 1999;23:101–107. [PubMed] [Google Scholar]
- Robins LN, Przybeck TR. Age of onset of drug use as a factor in drug and other disorders. NIDA Res Monogr. 1985;56:178–192. [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J Pharmacol Exp Ther. 2003;305:180–190. doi: 10.1124/jpet.102.047258. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology. 2007;191:867–877. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology. 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008;33:739–748. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Tarter R, Vanyukov M, Giancola P, Dawes M, Blackson T, Mezzich A, Clark DB. Etiology of early age onset substance use disorder: a maturational perspective. Dev Psychopathol. 1999;11:657–683. doi: 10.1017/s0954579499002266. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.