Abstract
Cathepsin D is a lysosomal hydrolase involved in intra- and extracellular proteolysis. This enzyme is aberrantly produced and processed in malignancy, and most notably is over-secreted into the tumor cell microenvironment. This hyper-secretion may lead to excessive degradation of the extracellular matrix, and contribute to tumor progression and metastases. These phenomena have been established in vitro, and there is evidence that Cathepsin D is similarly dysregulated in human breast cancer patients. Because breast cancer lacks an effective screening or surveillance biomarker, here we address the hypothesis that serum Cathepsin D activity may be useful to assess the presence or progression of breast cancer in females. While representative histologic sections from various disease-specific cohorts confirm previous findings that increased Cathepsin D production and secretion correlate with tumor progression, we report no difference in serum Cathepsin D activity between patients who are disease-free, patients with pre-invasive or limited invasive disease, and patients with metastatic disease. Furthermore, in patients with known metastatic disease, there were no clinical variables associated with significantly different serum Cathepsin D activity. however, the immunohistochemical localization of Cathepsin D expression in histopathologic sections from breast cancer patients correlates with disease progression. Based on the serum results, and in contradistinction to Cathepsin D localization in breast cancer tissues, our findings support using Cathepsin D as a reliable histopathology biomarker for disease progression, but not for serum screening.
Keywords: cathepsin D, breast cancer, biomarker, serum, metastases
Introduction
Breast cancer is diagnosed and surveyed by standard imaging modalities such as ultrasound and mammography. Despite the more frequent use of increasingly sensitive imaging techniques (e.g., MRI), diagnosis and subsequent monitoring of breast cancers rely on a mass of tumor cells that can be felt or seen (usually a mass of >1 billion cells1). Breast cancer can be a terminal diagnosis due to the fact that, despite best attempts, early detection has failed and a diagnosis is made at a late stage. As such, there is intense interest in identifying serum (or plasma) biomarkers that may delineate the presence, absence or extent of disease when tumors cannot be palpated or visualized. These biomarkers could be potentially useful for prognosticating (helping to determine outcome), but they are conceivably valuable in surveillance (assessing recurrent disease). This notion is being addressed by numerous proteomic researchers, and though mounting evidence suggests that a variety of serum/plasma biomarkers may be clinically useful in breast cancer,2,3 there are currently no serum/plasma biomarker assays available for systematic clinical use to monitor breast cancer progression.
Cathepsin D (CD) is a well-characterized lysosomal hydrolase, serving critical functions in intra-cellular protein degradation. CD is synthesized as a pro-form (Pro-CD), undergoes several post-translational modifications and ultimately is converted to mature active enzyme in the lysosomes.4,5 Under normal conditions, less than 10% of CD is secreted as Pro-CD into the extra-cellular milieu and is also detected in the serum. In mammary malignancies, however, CD is aberrantly over-produced and hyper-secreted by both malignant and extra-tumoral cells such as macrophages and fibroblasts.6-9 Intracellular CD in breast cancer cells has been shown to have 8–16 times more activity than normal mammary cells.10 In addition, the increased pro-CD affects tumor growth (mitogenic effect), increases angiogenesis and tumor metastases.11-14 Furthermore, secreted pro-CD can be activated by interstitial proteases or a low pH (a hallmark of the tumor microenvironment),15,16 and plays a significant role in matrix degradation and activation of other extracellular proteases.17,18
Histological examination of breast cancer tissue reveals that enhanced CD staining is associated with poor prognosis and survival,19,20 and intracellular CD levels relate directly to metastatic potential.21 In addition, there is compelling evidence on the association of dysregulated CD and clinical outcome, emphasizing its importance and diagnostic (as well as prognostic) promise. The presence of CD in the serum (plasma) has prompted its evaluation as predictive of the presence or progression of breast cancer; however, the accumulated data are often conflicting and inconclusive.22-24 Serum CD activity, though, is a relatively unexplored biologic marker that may hold great promise in the early detection or surveillance of breast cancer. Serum CD activity is elevated in uveal melanoma, colorectal,25,26 bladder27 and lung cancers when compared to their respective controls.28,29 A recent study has addressed CD activity in serum samples of breast cancer patients before and after surgery. These cohorts, stratified by extent of primary tumor and nodal disease, showed that those with larger tumors and more nodal metastases had significantly higher serum CD levels before surgery and this elevated activity returned to baseline six mo after definitive treatment.30
To further examine the utility of serum CD activity in the early diagnosis or surveillance of breast cancer, we have set out to: (1) assess serum CD activity in control and study female patients; (2) determine if any particular cohort exhibits higher CD activity than control patients; (3) identify if any patient and disease-specific variables may be associated with elevated CD activity; and (4) demonstrate, through representative patient-matched histopathology staining, that tissue CD reliably reflects disease status. Ultimately, the goal of this work is to expand on the existing data regarding the utility of serum CD activity and provide a foundation for a standardized CD activity assay to be used for systematic clinical use.
Results
Patient and tumor specific data are displayed in Table 1. There were no significant differences in patient age among study cohorts. Individual tumor characteristics (e.g., T/N/M status, grade, tumor marker positivity, etc.) are indicated in the appropriate columns.
Table 1.
Patient and tumor characteristics; other than age, values represent n of each sample
| Clinical variable | Control | Local disease | Metastatic disease | |
|---|---|---|---|---|
| Age (median, SIQR) | 43, 4 | 55.5, 7 | 49, 8 | |
| Tumor (T/N/M) | ||||
| T1 | - | 10 | 6 | |
| T2 | - | 10 | 10 | |
| T3 | - | 1 | 2 | |
| T4 | - | 0 | 1 | |
| N0 | - | 12 | 2 | |
| N1 | - | 5 | 8 | |
| N2 | - | 4 | 6 | |
| M0 | - | 35 | - | |
| M1 | - | - | 29 | |
| Grade | ||||
| 1 | - | 2 | 3 | |
| 2 | - | 8 | 10 | |
| 3 | - | 19 | 9 | |
| ER status | ||||
| + | - | 14 | 23 | |
| - | - | 14 | 3 | |
| PR status | ||||
| + | - | 13 | 14 | |
| - | - | 14 | 11 | |
| p53 status | ||||
| + | - | 4 | 5 | |
| - | - | 1 | 13 | |
| Her-2-neu status | ||||
| + | - | 5 | 11 | |
| - | - | 17 | 15 | |
| Axillary node status | ||||
| No. + nodes (median, SIQR) | - | 0, 0.5 | 3, 4 | |
| Total no. nodes (median, SIQR) | - | 4, 5.5 | 15, 4.5 | |
| Site of metastasis | ||||
| Bone | - | - | 12 | |
| Brain | - | - | 5 | |
| Lung | - | - | 9 | |
| Liver | - | - | 8 | |
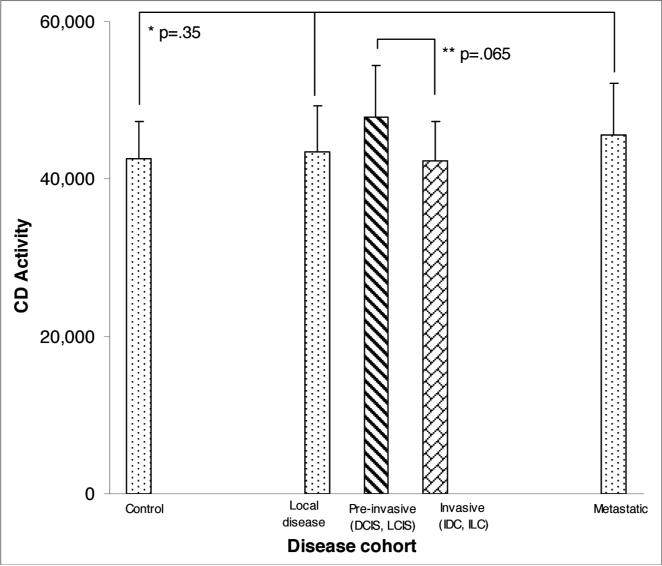
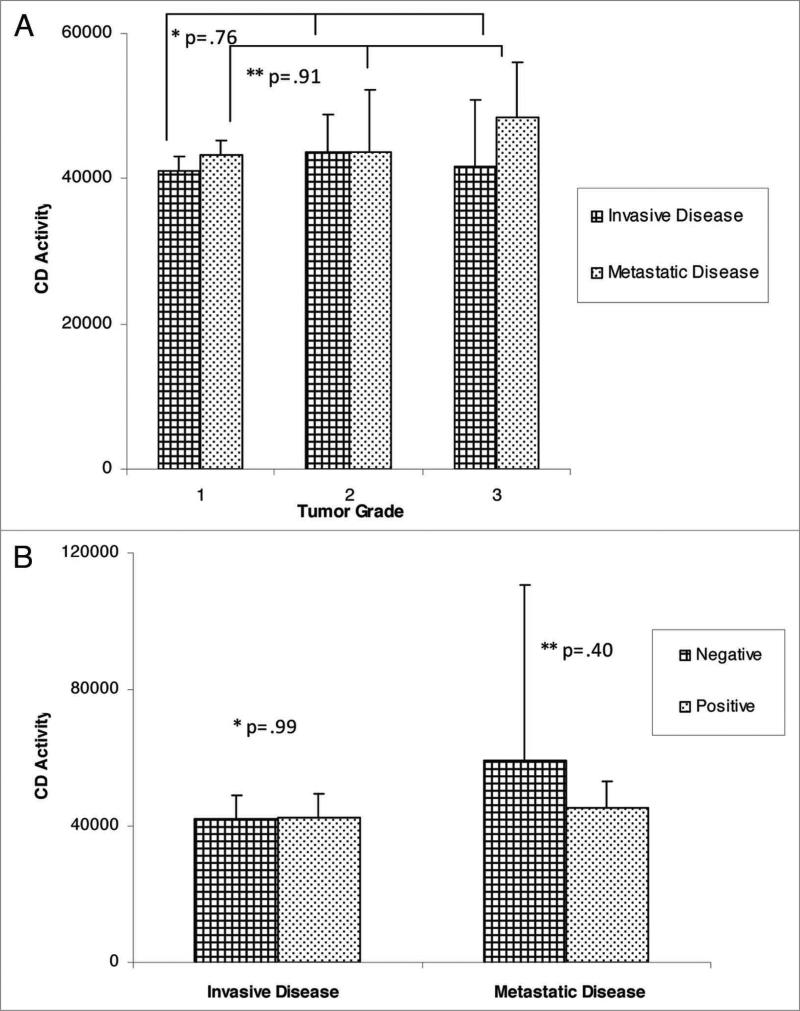
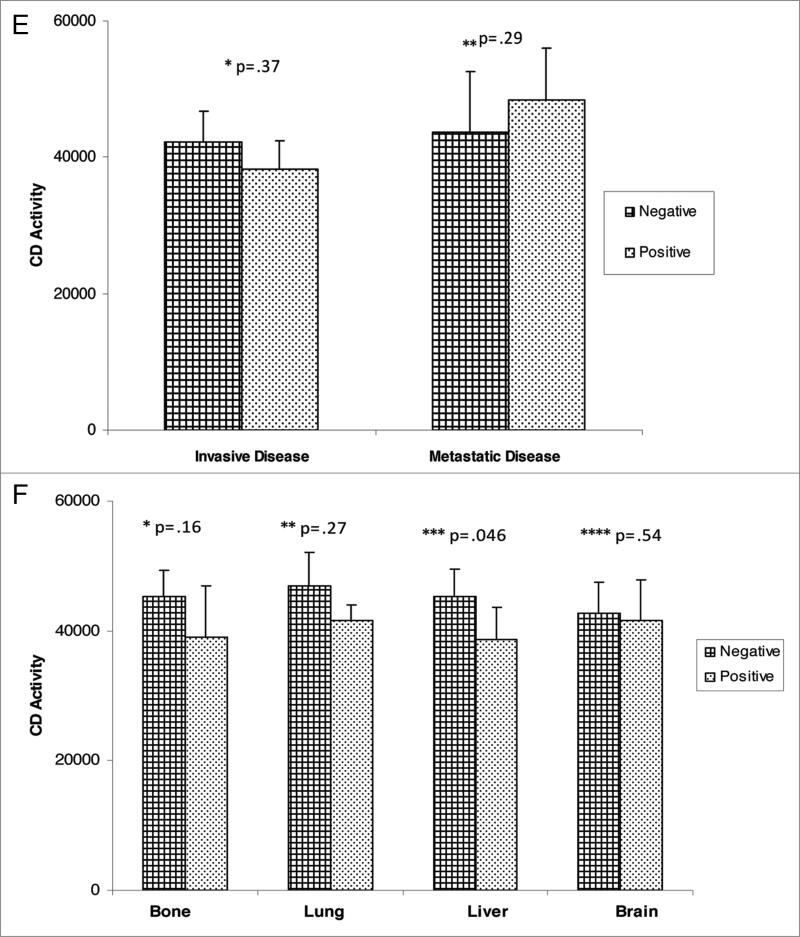
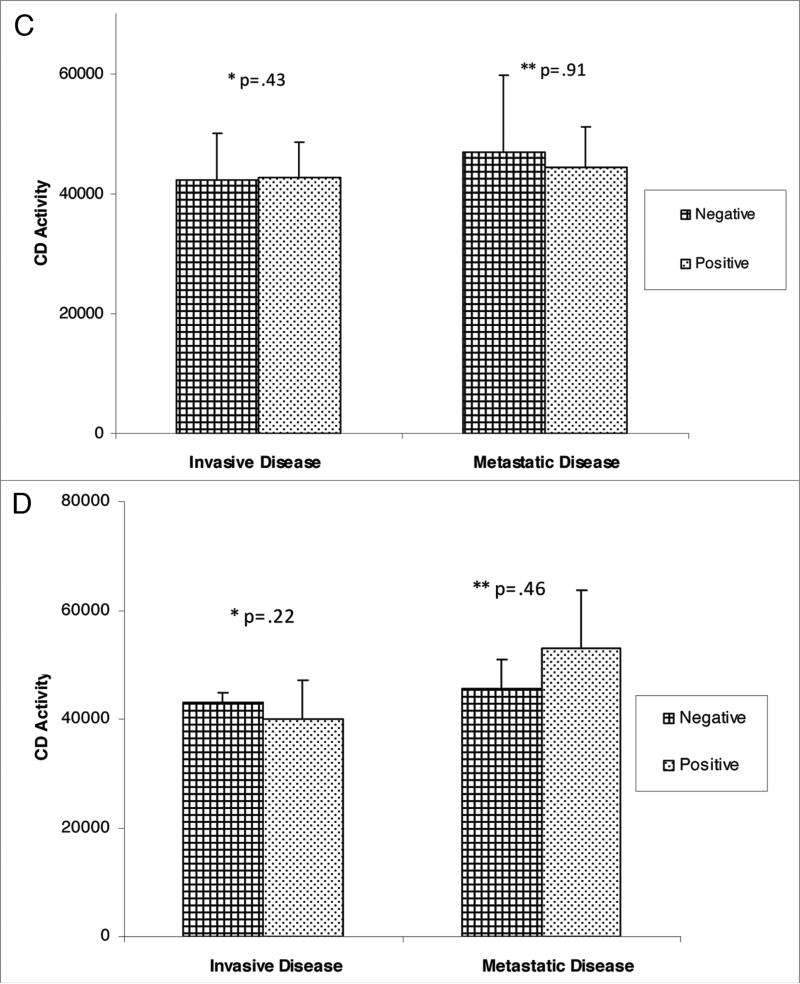
For comparison of extent of disease and CD activity, the median, semi-interquartile range (SIQR) and associated p-values are noted in Figure 1. There was no significant difference in CD activity between disease free control patients with local disease or patients with metastatic disease. Further subset analysis of patients with non-invasive disease (ductal carcinoma in situ, lobular carcinoma in situ, atypical ductal hyperplasia, atypical lobular hyperplasia) versus patients with invasive disease (invasive ductal carcinoma, invasive lobular carcinoma) revealed no statistical difference in CD activity. In addition, CD activity based upon tumor characteristics did not reveal any significant differences (Fig. 2A–F). Variables including ER, PR, p53 and Her-2 status, as well as tumor grade, did not demonstrate elevated CD activity. Similarly, the site of metastasis did not correlate with significant elevation in CD activity.
Figure 1.
CD activity in controls compared to disease cohorts. p-values for control versus local disease and metastatic cohorts is indicated (*), while **denotes comparison between pre-invasive and invasive cohorts.
Figure 2A and B.
CD activity against tumor variables; (a) tumor grade; *: comparison of grades with invasive disease cohort, **comparison of grades in metastatic cohort; (B) ER status.
Figure 2E and F.
CD activity against tumor variables; (e) her-2-neu status; B through e--* and **denote p values in comparing negative and positive variable and (F) site of metastasis (* through ****denotes p value at site of metastasis, whether negative or positive).
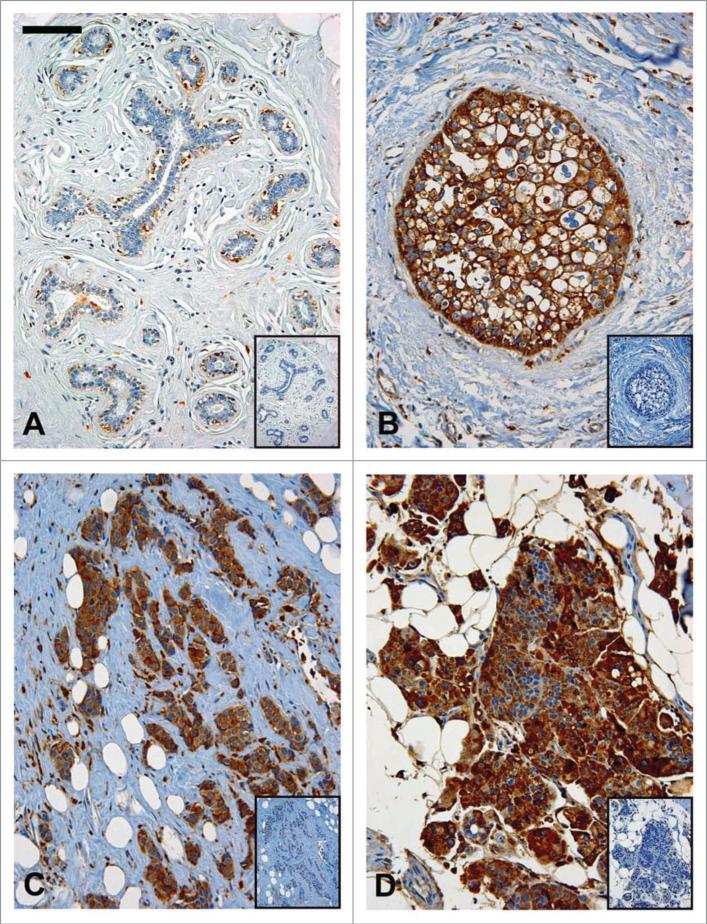
Representative patient-matched histopathology sections immunostained for CD are shown in Figure 3. Patient tissue from: (A) control, (B) DCIS, (C) IDC and (D) a metastasis demonstrate relatively little CD staining in mammary epithelial cells from a control patient while CD staining from patient tumors, of all stages of progression, is prominent. The robust staining pattern of CD in the glandular epithelium seen in this in vivo illustration of CD over- production and hyper-secretion corroborates data mentioned previously that clearly correlate histo-logic progression of breast cancer with aberrant CD regulation.
Figure 3.
Representative patient matched histologic sections, immunostained for Cathepsin D expression; (a) Control mammary tissue demonstrating little CD staining by ductal epithelial cells (negative control inserted); (B) Ductal carcinoma-in-situ, (C) Invasive ductal carcinoma, and (D) primary lesion from patient with metastatic disease all demonstrating marked CD staining in ductal epithelial cells; all images obtained with ×10 magnification; bar = 100 μm.
Discussion
In the past decade investigators have attempted to utilize serum CD levels as a biomarker for early detection, progression and surveillance of breast cancer. The results of these reports are equivocal; there is no clear indication that quantifying CD in the blood discriminates between patients with or without disease. The lack of clarity from these data has spurred efforts to address serum CD activity, rather than protein levels alone. The most compelling data to date have been published by Wozniak and colleagues.30 These authors have utilized a heme-based CD activity assay to demonstrate elevated CD activity in breast cancer patients before surgery, and returning to baseline 6 mo post-operatively.30
Based on these observations, we sought to demonstrate that serum from patients with invasive disease possessed accentuated serum CD activity. Furthermore, we hypothesized that patients with metastatic disease have serum CD activity that exceeds those of patients with limited, local disease. Our efforts to correlate CD activity (not levels) with disease status revealed no statistically significant differences in serum CD activity between control, local disease or metastatic disease cohorts. Additionally, there were no significant differences in serum CD activity between patients with pre-invasive lesions compared to those with primary (not metastatic) lesions.
The discrepancy between our findings and that of Wozniak and coworkers might reflect different patient cohorts and experimental approaches utilized in our respective studies. While their study revealed that patients with tumors 2.5–10 cm or greater than three positive axillary nodes possessed a higher serum CD activity than did patients with tumors smaller than 2.5 cm and 0–3 positive axillary nodes (which in turn demonstrated higher serum CD activity than controls), our study did not show any difference in serum CD activity across any cohort. Notably, our study subjects demonstrated a much broader index of tumor burden than previous studies, and yet no differences, either between or within cohorts, were revealed. In addition, further examination of specific clinical and tumor dependent variables within a cohort of metastatic patients indicated no association between biologic features of the aggressive tumors with elevated CD activity.
The sample size of each individual cohort in our study was adequate, and in fact larger than many other published studies detailing CD activity. While further statistical analysis within patient cohorts decreased our n and made each interquartile value slightly larger, the median values did not change significantly. As depicted in Figure 2, this similarity in serum CD activity was true regardless of the individual clinical variable(s) analyzed in the metastatic cohort.
The noted discrepancy might also be due to two distinct approaches employed for determining CD activity. As indicated, we have used a fluorometric assay which is based upon cleavage of a Cathepsin D-preferred substrate with a sequence RGFFP, conjugated with an AFC tag. When cleaved, this substrate yields the AFC molecule which is quantified with a fluorometer. Comparatively, Wozniak and colleagues utilized the traditional heme-based assay, which relies upon hemoglobin hydrolysis and subsequent measurement of released tyrosine.30
The sensitivity of the assay used in this study is in the nano-molar range, and while this sensitivity does not represent a dramatic improvement over previous activity assays, the methodologic improvement of this assay lies in its specificity. The previous heme-based, tyrosine-releasing assay has been utilized for decades (dating back to the 1950s) and there is no standardized, reliable determination of its specificity in the literature. The assay utilized in our study, however, relies on a very specific peptide bond that is broken (with subsequent release of the AFC tag) by aspartyl proteases.
This work highlights the challenges of studying and applying serum biomarkers to a specific malignancy. The proteomics field has invested countless resources in expanding blood-based screening and surveillance tools, yet few positive findings have been applied to clinical practice. The vast complexity of blood and blood-based assays may account for these difficulties, due to the inherent proteolytic enzymes present, the buffering capacity which may interfere with pH-dependent activation or optimal functioning, or other yet unforeseen biological obstacles in applying current detection methods. Based on our data, we suggest that serum CD activity is not currently a reliable screening or surveillance method for detecting breast cancer presence or progression, and that further positive proof must be made available before relying on CD activity as a reliable serum biomarker. However, we have also demonstrated (and confirmed previous findings) that CD expression in histopathologic sections from breast cancer patients correlates with disease progression, and continued efforts to validate CD expression and/or activity as reliable biomarkers may ultimately be affirmed.
Materials and Methods
Subjects
Approval to enroll patients and conduct our analyses was granted by the Northwestern University Institutional Review Board. Study criteria included women greater than 18 y of age and the willingness to submit a blood sample. Exclusion criteria included male gender and preexisting malignancies other than that of breast origin. The four cohorts studied included: (1) control subjects (n = 27); (2) patients with pre-invasive lesions without evidence of invasion (DCIS, LCIS; n = 11); (3) patients with invasive disease (IDC, ILC; n = 24); and (4) patients with known, documented metastatic lesions (to various sites, n = 29). Patient specific variables analyzed included age, histopathology (where applicable), Tumor-Node-Metastasis (T/N/M) status (where applicable), number of nodes positive, total number of nodes, grade of tumor, ER/PR/p53/Her-2-neu status, and site of metastases (bone, brain, liver, lung or ‘other’) if present. Where patient data were incomplete, the sum of each subset within each cohort may not equal the total cohort size.
Cathepsin D activity
CD activity was assayed using human serum (or plasma) and a CD activity kit (BioVision, Mountain View, CA). Briefly, 50 μL of human serum (or plasma) was added to each well of a 96-well plate. A master mix comprised of a 1:25 dilution of CD substrate:reaction buffer was made (not antibody based), and 52 μL of this mix was added to each assay well. The plate was incubated for one h at 37°C and read with a fluorometer (FLUOstar OPTIMA) using excitation and emission filters of 320 and 460 nm, respectively. Values were properly gain adjusted, and reported as relative fluorescence units (RFU). Non-parametric rank based statistical analyses were used to yield medians and semi-interquartile ranges (SIQR); p values were calculated using a Wilcoxon rank sum test.
Immunohistochemistry
Slides from formalin fixed, paraffin embedded patient tissues were obtained from the Department of Pathology, Northwestern University Feinberg School of Medicine. Briefly, 4 μm thick tumor sections were deparaffinized with xylene, hydrated with graded alcohol, washed and subjected to antigen retrieval with citrate buffer (pH 6.0). The immunostaining was performed on the Microm HMS 710i Autostainer (Thermo Fisher Scientific, Waltham, MA). Slides were incubated with the primary goat anti-human CD antibody (1:80, R&D Systems, AF1014) or with goat IgG as a negative control for 60 min followed by incubation with biotinylated anti-goat secondary antibody (Vector Laboratories, Burlingame, CA), and with streptavidin horseradish peroxidase (Lab Vision, Thermo Fisher Scientific) for 20 min each. The antibody-antigen complex was visualized by using 3,3'-diaminobenzidine (DAB) (Lab Vision, Thermo Scientific Fisher). Slides were counterstained with Mayer's hematoxylin (Dako) and mounted. Images were captured using a Leica DM 4000 B microscope equipped with a Leica DFC 480 5.0 mega pixel CCD camera.
Statistical analysis
Statistics are summarized as medians and semi-interquartile range, half the distance between the 25th and 75th data percentile. Medians are compared across groups using the Kruskal-Wallis test for three groups or the Wilcoxon rank sum test for two groups. Other descriptive statistics are presented as frequencies.
Figure 2C and D.
CD activity against tumor variables; (C) pR status, (D) p53 status.
Acknowledgements
The authors would like to thank Dr. Luigi Strizzi for useful scientific discussions. This work was made possible by NIH/CA grant number 75681.
Abbreviations
- DCIS
ductal carcinoma in situ
- LCIS
lobular carcinoma in situ
- IDC
infiltrating ductal carcinoma
- ILC
infiltrating lobular carcinoma
- CD
cathepsin D
References
- 1.Kumar V, Fausto N, Abbas A. Robbins & Cotran Pathologic Basis of Disease. 7th ed. Saunders; Philadelphia: 2004. [Google Scholar]
- 2.Pitteri SJ, Faca VM, Kelly-Spratt KS, Kasarda AE, Wang H, Zhang Q, et al. Plasma proteome profiling of a mouse model of breast cancer identifies a set of upregulated proteins in common with human breast cancer cells. J Proteome Res. 2008;7:1481–9. doi: 10.1021/pr7007994. [DOI] [PubMed] [Google Scholar]
- 3.Orazine CI, Hincapie M, Hancock WS, Hattersley M, Hanke JH. A proteomic analysis of the plasma glyco-proteins of a mcf-7 mouse xenograft: a model system for the detection of tumor markers. J Proteome Res. 2008;7:1542–54. doi: 10.1021/pr7008516. [DOI] [PubMed] [Google Scholar]
- 4.von Figura K, Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–93. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]
- 5.Kornfeld S. Lysosomal enzyme targeting. Biochem Soc Trans. 1990;18:367–74. doi: 10.1042/bst0180367. [DOI] [PubMed] [Google Scholar]
- 6.Liaudet-Coopman E, Beaujoin M, Derocq D, Garcia M, Glondu-Lassis M, Laurent-Matha V, et al. Cathepsin D: newly discovered functions of a long-standing aspartic protease in cancer and apoptosis. Cancer Lett. 2006;237:167–79. doi: 10.1016/j.canlet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Roger P, Montcourrier P, Maudelonde T, Brouillet JP, Pages A, Laffargue F, et al. Cathepsin D immunostaining in paraffin-embedded breast cancer cells and macrophages: correlation with cytosolic assay. Hum Pathol. 1994;25:863–71. doi: 10.1016/0046-8177(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 8.O'Donoghue AE, Poller DN, Bell JA, Galea MH, Elston CW, Blamey RW, et al. Cathepsin D in primary breast carcinoma: adverse prognosis is associated with expression of cathepsin D in stromal cells. Breast Cancer Res Treat. 1995;33:137–45. doi: 10.1007/BF00682721. [DOI] [PubMed] [Google Scholar]
- 9.Tetu B, Brisson J, Lapointe H, Wang CS, Bernard P, Blanchette C. Cathepsin D expression by cancer and stromal cells in breast cancer: an immunohistochemical study of 1,348 cases. Breast Cancer Res Treat. 1999;55:137–47. doi: 10.1023/a:1006140213493. [DOI] [PubMed] [Google Scholar]
- 10.Capony F, Rougeot C, Montcourrier P, Cavailles V, Salazar G, Rochefort H. Increased secretion, altered processing and glycosylation of pro-cathepsin D in human mammary cancer cells. Cancer Res. 1989;49:3904–9. [PubMed] [Google Scholar]
- 11.Berchem G, Glondu M, Gleizes M, Brouillet JP, Vignon F, Garcia M, et al. Cathepsin-D affects multiple tumor progression steps in vivo: proliferation, angiogenesis and apoptosis. Oncogene. 2002;21:5951–5. doi: 10.1038/sj.onc.1205745. [DOI] [PubMed] [Google Scholar]
- 12.Garcia M, Derocq D, Pujol P, Rochefort H. Overexpression of transfected cathepsin D in transformed cells increases their malignant phenotype and metastatic potency. Oncogene. 1990;5:1809–14. [PubMed] [Google Scholar]
- 13.Liaudet E, Garcia M, Rochefort H. Cathepsin D maturation and its stimulatory effect on metastasis are prevented by addition of KDEL retention signal. Oncogene. 1994;9:1145–54. [PubMed] [Google Scholar]
- 14.Vetvicka V, Vektvickova J, Fusek M. Effect of human procathepsin D on proliferation of human cell lines. Cancer Lett. 1994;79:131–5. doi: 10.1016/0304-3835(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths JR. Are cancer cells acidic? Br J Cancer. 1991;64:425–7. doi: 10.1038/bjc.1991.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wittlin S, Rosel J, Hofmann F, Stover DR. Mechanisms and kinetics of procathepsin D activation. Eur J Biochem. 1999;265:384–93. doi: 10.1046/j.1432-1327.1999.00747.x. [DOI] [PubMed] [Google Scholar]
- 17.Benaud C, Dickson RB, Thompson EW. Roles of the matrix metalloproteinases in mammary gland development and cancer. Breast Cancer Res Treat. 1998;50:97–116. doi: 10.1023/a:1006061115909. [DOI] [PubMed] [Google Scholar]
- 18.Leto G, Gebbia N, Rausa L, Tumminello FM. Cathepsin D in the malignant progression of neoplastic diseases (review). Anticancer Res. 1992;12:235–40. [PubMed] [Google Scholar]
- 19.Esteva FJ, Hortobagyi GN. Prognostic molecular markers in early breast cancer. Breast Cancer Res. 2004;6:109–18. doi: 10.1186/bcr777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isola J, Weitz S, Visakorpi T, Holli K, Shea R, Khabbaz N, et al. Cathepsin D expression detected by immunohistochemistry has independent prognostic value in axillary node-negative breast cancer. J Clin Oncol. 1993;11:36–43. doi: 10.1200/JCO.1993.11.1.36. [DOI] [PubMed] [Google Scholar]
- 21.Pujol P, Maudelonde T, Daures JP, Rouanet P, Brouillet JP, Pujol H, et al. A prospective study of the prognostic value of cathepsin D levels in breast cancer cytosol. Cancer. 1993;71:2006–12. doi: 10.1002/1097-0142(19930315)71:6<2006::aid-cncr2820710614>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 22.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007. 25:5287–312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 23.Brouillet JP, Dufour F, Lemamy G, Garcia M, Schlup N, Grenier J, et al. Increased cathepsin D level in the serum of patients with metastatic breast carcinoma detected with a specific pro-cathepsin D immunoassay. Cancer. 1997;79:2132–6. [PubMed] [Google Scholar]
- 24.Schultz DC, Bazel S, Wright LM, Tucker S, Lange MK, Tachovsky T, et al. Western blotting and enzymatic activity analysis of cathepsin D in breast tissue and sera of patients with breast cancer and benign breast disease and of normal controls. Cancer Res. 1994;54:48–54. [PubMed] [Google Scholar]
- 25.Skrzydlewska E, Sulkowska M, Wincewicz A, Koda M, Sulkowski S. Evaluation of serum cathepsin B and D in relation to clinicopathological staging of colorectal cancer. World J Gastroenterol. 2005;11:4225–9. doi: 10.3748/wjg.v11.i27.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiluk M, Skrzydlewski Z, Kiluk A, Woroniecki A, Rucinska M, Kozlowski L. Activity of cathepsin D in some neoplasms of the gastrointestinal tract. Pol Merkur Lekarski. 1997;2:307–8. [PubMed] [Google Scholar]
- 27.Szajda SD, Darewicz B, Kudelski J, Chlabicz M, Domel T, Chabielska E, et al. Cancer procoagulant and cathepsin D activity in blood serum in patients with bladder cancer. Pol Merkur Lekarski. 2005;18:651–3. [PubMed] [Google Scholar]
- 28.Wozniak A, Drewa T, Rozwodowska M, Drewa G, Lambrecht W, Wisniewska I. Activity of some lysosomal enzymes in serum and in tumors of patients with squamous cell lung carcinoma. Neoplasma. 2002;49:10–5. [PubMed] [Google Scholar]
- 29.Lou X, Xiao T, Zhao K, Wang H, Zheng H, Lin D, et al. Cathepsin D is secreted from M-BE cells: its potential role as a biomarker of lung cancer. J Proteome Res. 2007;6:1083–92. doi: 10.1021/pr060422t. [DOI] [PubMed] [Google Scholar]
- 30.Wozniak A, Mila-Kierzenkowska C, Schachtschabel DO, Wozniak B, Rozwodowska M, Drewa T, et al. Activity of cathepsin D and alpha(1)-antitrypsin in the blood serum of patients with mammary carcinoma. Exp Oncol. 2005;27:233–7. [PubMed] [Google Scholar]
- 31.Vetvicka V, Vetvickova J, Benes P. Role of enzymatically inactive procathepsin D in lung cancer. Anticancer Res. 2004;24:2739–43. [PubMed] [Google Scholar]
- 32.Vashishta A, Ohri SS, Proctor M, Fusek M, Vetvicka V. Role of activation peptide of procathepsin D in proliferation and invasion of lung cancer cells. Anticancer Res. 2006;26:4163–70. [PubMed] [Google Scholar]
- 33.Shen J, Person MD, Zhu J, Abbruzzese HL, Li D. Protein expression profiles in pancreatic adenocarcinoma compared with normal pancreatic tissue and tissue affected by pancreatitis as detected by two-dimensional gel electrophoresis and mass spectrometry. Cancer Res. 2004;64:9018–26. doi: 10.1158/0008-5472.CAN-04-3262. [DOI] [PubMed] [Google Scholar]