Abstract
Bacterial flagellins are potent inducers of innate immune responses in the mouse lung because they bind to TLR5 expressed on the apical surfaces of airway epithelial cells. TLR engagement leads to the initiation of a signaling cascade that results in a pro-inflammatory response with subsequent up-regulation of several cytokines and leads to adaptive immune responses. We examined the ability of two soluble flagellins, a monomeric flagellin expressed in E. coli and a highly purified polymeric flagellin directly isolated from Salmonella, to enhance the efficacy of influenza vaccines in mice. Here we demonstrate that both flagellins co-administered intranasally with inactivated A/PR/8/34 (PR8) virus induced robust increases of systemic influenza-specific IgA and IgG titers and resulted in a more comprehensive humoral response as indicated by the increase of IgG2a and IgG2b subclass responses. Groups immunized with the adjuvanted vaccines were fully protected against high dose lethal challenge by homologous virus whereas inactivated PR8 alone conferred only partial protection. Finally we show that shortly after immunization the adjuvanted vaccines induced a dramatic increase in pro-inflammatory cytokines in the lung, resulting in extensive lung infiltration by granulocytes and monocytes/macrophages. Our results reveal a promising perspective for the use of both soluble monomeric and polymeric flagellin as mucosal vaccine adjuvants to improve protection against influenza epidemics as well as a range of other infectious diseases.
1. Introduction
Influenza virus is a common pathogen of the respiratory system which infects millions of people worldwide [1–3] and causes thousands of hospitalizations and deaths every year. Because of the limited duration of vaccine-induced immunity and the ability of the virus to undergo antigenic changes (antigenic drift) in its surface proteins hemagglutinin (HA) and neuraminidase (NA), annual vaccination is recommended with special emphasis on children, the elderly as well as immunocompromised individuals and patients with chronic underlying diseases.
The limited mucosal immunity induced by the current inactivated split vaccine which is used in humans via the systemic route and the age-restricted use of the intranasally administered live attenuated vaccine against seasonal influenza infections pose limitations in the current vaccine effectiveness. Ideally, the initial host immune responses should be localized in the mucosa to block the virus early during infection before it spreads to other contacts [4, 5]. Therefore there is a need for an appropriate adjuvant to enhance the local and systemic immune responses induced by the inactivated influenza vaccine as well as to induce long-lived and memory responses. In recent years, the study of the mechanisms which regulate the innate immune system has revealed a new group of receptors which are found on the surfaces of antigen presenting cells (APC) [6] and belong to the Toll-like receptor family (TLR) [4, 5]. These molecules recognize pathogen-associated molecular patterns (PAMPs) such as lipoteichoic acids and lipid peptides, double stranded RNA, lipopolysaccharides, flagellin and unmethylated CpG. The binding of these ligands to their cognate TLR initiates a signaling cascade that results in the activation and maturation of antigen-presenting cells (APCs) which turn on the adaptive immune responses [6–9].
Flagellin is a highly conserved bacterial protein that elicits TLR5-dependent inflammatory responses in both animals and plants [10, 11] and plays an important role in bacterial pathogenicity by facilitating motility and adhesion to host mucosal tissues [12–15]. Recent studies have shown that mucosal administration of flagellin is associated with strong adaptive immune responses, mainly Th2-type, with the production of mucosal [16–19] and systemic IgA. The mucosal adjuvant properties correlate with the capacity of flagellin to activate TLR5-positive DCs, neutrophils or epithelial cells [16, 17, 20, 21]. Intranasal administration of flagellin stimulates the signaling of TLR5 in lung epithelial cells and pneumonocytes [22]. TLR5 mediates the production of inflammatory mediators that affect neutrophil recruitment [18], playing a critical role in the clearance of pulmonary infection by flagellated bacteria [23].
The development of an adjuvanted influenza vaccine using a TLR ligand such as flagellin to induce robust and broad immune responses is a very attractive idea, particularly for vaccines administered intranasally because of the presence of TLR5 in the mucosal surfaces of the respiratory compartment. Recombinant flagellin in a soluble or fused form has been reported to play an important role as adjuvant for novel vaccines against epitope-based influenza vaccines [24], Yersinia pestis [15, 25], Plasmodium falciparum [26], Tetanus toxoid [17] and West Nile virus (WNV) [27].
It was previously reported that many flagellated bacteria readily release flagellin monomers into their milieu [28, 29]. A conserved short stretch of 13 amino acid residues buried in the flagellar filament serves as the TLR5 recognition site suggesting that monomeric flagellin, but not the filamentous molecule, stimulates TLR5 [30].
Here we compared the adjuvant properties and potency of recombinant monomeric flagellin derived from S. enterica serovar typhimurium and purified polymeric flagellin isolated from S. enterica serovar enteritidis for an intranasally administered inactivated influenza virus vaccine (A/PR/8/34, HIN1 subtype).
2. Materials and methods
2.1. Construction of recombinant soluble flagellin plasmid
To construct a soluble flagellin-expressing plasmid, the full-length flagellin gene from S. typhimurium (a gift from Dr. Alan Aderem) was cloned into the expression vector pET-22b (Novagen, EMD Biosciences, Madison, WI) under the T7 promoter and lac operator with NdeI/Xho1 in frame with histidine tag. The primers used for the PCR were: forward, 5′-TCGTCATATGGCACAAGTCATTAA-3′,5′-GCCACTCGAGACGCAGTAAAGAGAG -3′ (Nde I and Xho1 sites are underlined). The sequence of the resulting construct was confirmed by DNA sequencing.
2.2. Purification of soluble flagellin
E. coli strain BL was transformed with the flagellin expressing plasmid by 1 mM IPTG induction (21). Five hours post-induction, cells were harvested and used for the preparation of flagellin with a Nickel bead column (Qiagen, Valencia, CA). Affinity purification of His-tagged flagellin was performed according to the manufacturer’s instructions.
2.3. Preparation of polymeric flagellin from S. enteritidis
Flagella filament protein (FliC) from S. enteritidis wild type was prepared as reported [31, 32]. Briefly S. enteritidis strain was grown aerobically on 0.5% semi-solid swarm agar for several passages and further inoculated in a stationary phase of 10 ml Luria broth (LB) tubes at 37°C for 17 h. The inoculum was transferred to 500 ml of LB broth and incubated for 16 h at 200 rpm. The bacterial cells were harvested and resuspended in phosphate buffered saline (PBS). The pH of the suspension was adjusted to 2.0 with 1M HCl and continuously stirred at RT for 30 min to separate the flagella from the bacteria. The bacteria were further pelleted and the supernatant was centrifuged at 100,000 g for 1 h at 4°C to sediment any insoluble material. The soluble monomeric flagellin in the supernatant was collected and the pH was adjusted back to 7.2, followed by precipitation with 65% saturation of ammonium sulfate (Sigma, St. Louis, MO) at 4°C overnight. The precipitate was then centrifuged at 15,000 g for 15 min at 4°C, dialyzed against PBS. It was stored at −80°C until further use.
2.4. Flagellin quality control
All samples were tested for purity by SDS PAGE gel electrophoresis followed by Coomassie and silver staining to detect any LPS in the preparation. Our preparations were tested for endotoxin levels with the chromogenic limulus amedocyte lysate kit (LAL, quantitative assay) (Charles River Labs, Wilmington, MA) according to the manufacturer’s instructions (lowest sensitivity levels 0.125 EU/ml). 1 μg/ml of purified filamentous flagellin preparation was applied to a formvar carbon coated grid for 1 min, and immediately stained with 1% uranyl acetate for 30 seconds. The samples were examined by transmission electron microscopy.
2.5. Cell and virus stocks
Madin-Darby canine kidney (MDCK) cells (ATCC CCL 34, American Type Culture Collection) were maintained in Dulbecco’s Modified Eagle’ Medium (DMEM) (Mediatech Inc., Herndon, VA) containing 10% calf serum. Influenza virus stocks (A/PR/8/34) were prepared as described [33]. The purity of the virus was determined by SDS PAGE gel electrophoresis followed by Coomassie blue staining and electron microscopy. The hemagglutination (HA) activity was determined using 0.5% w/v chicken RBC in phosphate buffered saline (PBS) pH 7.2 as previously described [34]. The purified virus was inactivated with 0.1% (vol/vol) final concentration of formalin, incubated for 72 hrs at 4°C, and then dialyzed against PBS buffer. Inactivation of virus was confirmed by plaque assay in MDCK cells [35].
The mouse adapted A/PR/8/34 strain was passaged 8 times in Balb/c mice and the LD50 was determined in the same mouse strain. The viral titer was determined by plaque assay in MDCK cells.
2.7. TLR5-specific bioactivity assay
A RAW264.7 cell-based assay (ATCC, Manassas, VA) [27] with modifications was used to determine the bioactivity of monomeric (mFliC) and polymeric flagellin (pFlag) as described [36]. The RAW264.7 cell line is a mouse macrophage cell line which expresses TLR2 and 4, but not TLR5 [5]. In brief, 80%-confluent RAW264.7 (TLR5-negative) cells in a 75-cm2 T flask were transfected with 10 μg of plasmid pUNO-hTLR5 expressing the human TLR5 (InvivoGen, San Diego, CA) by use of the transfection reagent Lipofectamine (Invitrogen) by following the manufacturer’s instructions. Six hours post-transfection, cells were removed from the T flask with a cell scraper and seeded into 96-well plates by using 5 ×104 cells/well in 100 μl of fresh medium. Non transfected RAW264.7 cells (TLR5 negative) were also seeded into 96-well plates for comparison. The TLR5-positive and negative cells were incubated with 100 μl of serially diluted purified recombinant monomeric flagellin (mFliC) or flagellar filament protein (pFlag), and supernatants were collected after 24 h. Levels of tumor necrosis factor alpha (TNF-α) production stimulated by the flagellins in both TLR5 positive and TLR5 negative cell cultures was determined by enzyme-linked immunosorbent assay (ELISA). TLR5 bioactivity was expressed as the level of TNF-α production of TLR5-positive cells from which was compared to that of TLR5-negative cells stimulated by recombinant monomeric flagellin (mFliC) or the flagellar filament protein (pFlag).
2.8. Immunizations
Female Balb/c mice 6–8 weeks of age (24 per group) (Charles River Laboratory, Wilmington, MA) were anesthetized with isoflurane and then intranasally immunized with purified inactivated influenza virus (PR8) alone or in the presence of monomeric (10 μg) or polymeric flagellin (1 μg) isolated from S. enteritidis to assess immune responses. Two independent sets of experiments were conducted. Eighteen (18) animals received a priming immunization (i.n.) of virus with or without monomeric or polymeric flagellin followed by one boost at week 4. Six animals were immunized as above and sacrificed 16 hours later for the detection of early immune responses. The control groups were immunized similarly with 10 μg monomeric or 1 μg polymeric flagellin. Experiments were conducted in accordance with the Emory University Institutional Animal Care and Use Committee guidelines.
2.9. Challenge of mice with influenza virus
To determine post-challenge survival rates and recall immune responses, 12 mice per group were challenged 8 weeks after the last immunization by intranasal instillation of 25 μl of virus with 10×LD50 (6 mice per group) or 40×LD50 (6 mice per group) of live mouse adapted PR8 virus and monitored for 14 days. The challenged mice were monitored for signs of morbidity (body weight changes, fever and hunched posture) and mortality. Mice were weighed immediately before and daily after challenge. A weight loss of over 25% in all infected mice was used as the experimental end-point, and mice were euthanized according to IACUC guidelines.
2.10. Sample collection
The animals were bled at 2 weeks after prime. Blood was collected from the retro-orbital plexus with non-heparinized microcapillaries (Fisher Scientific, Pittsburgh, PA) after systemic anesthesia with ketamine (Hospira Inc., Lake Forest, IL) and xylaxine (Lloyd Laboratories, Shenandoah, IA) cocktail. Bronchoalveolar lavage fluid (BALF) was collected by flushing the bronchial tree through the trachea with 0.5 ml DMEM. Lung suspensions were prepared by homogenization and removal of the cell debris with cell strainer (BD Falcon, Chelmsford, MA) and collecting the cells suspended in 1 ml DMEM. The spleens were processed into single cell suspensions and suspended in complete RPMI 1640 [37]. All samples were treated with red blood cell lysis buffer (Sigma, St. Louis, MO). Saliva was collected after intraperitoneal injection of 2 μg of carbamoylcholine chloride to stimulate secretion. Samples were mixed with the protease inhibitor phenylmethylsulfonyl fluoride (1 mM) (Sigma, St. Louis, MO) and stored at −20°C until assayed.
2.11. Evaluation of humoral immune responses
Sera, saliva, BALF were assayed for anti-PR8 IgG, IgG1 and IgG2a, IgG2b, IgG3, IgA and IgM antibody levels by enzyme-linked immunosorbent assay (ELISA) as described [33]. Purified mouse isotype antibodies and goat anti-mouse-HRP for ELISA were purchased from Southern Biotechnology Associates (Birmingham, AL). Optical density was read at 450 nm. We determined the hemagglutination inhibition (HAI) and neutralizing antibody titers, which are both used as indicators of protective immune responses to influenza virus, as described previously [33]. The HAI titer was read as the reciprocal of the highest dilution of serum that conferred inhibition of hemagglutination. For the neutralization assay dilutions of sera were incubated with approximately 100 plaque forming unit of PR8 virus for 1 hr at room temperature. The mixtures were then applied to monolayers of confluent MDCK cells, and a standard plaque reduction assay was performed. Neutralizing antibody titers were calculated as the highest dilution that resulted in a 50% reduction of the number of plaques formed, as compared to controls.
2.12. Evaluation of mucosal and systemic cellular immune responses
2.12.1. ELISPOT assays
Freshly isolated splenocytes (0.5–1.0×106/200 μl complete RPMI) were prepared from immunized mice which were sacrificed 4 days post-boost. They were cultured for 36 hours in the presence of inactivated influenza virus at a final concentration of 1 μg/ml in complete RPMI medium [38]. The number of IFN-γ and IL-4 secreting cells was counted in an ELISPOT reader (Cellular Technology).
2.12.2. Cytokine ELISA
Lungs and BALF were collected at 16 h post-vaccination of mice with influenza virus with or without the flagellins to measure cytokine production. The lungs were individually processed and lung suspensions were prepared in DMEM medium. A panel of cytokines (TNF-α, IFN-γ, IL-12p40/IL-23, IL-6, MIP-2 and IL-10) was selected to study inflammation. TNF-α, IFN-γ, IL-12p40 and IL-6 were purchased from eBioscience, (San Diego, CA), MIP-2 from R&D Systems (Minneapolis, MN) and IL-10 from BD Bioscience (San Jose, CA). Cytokine determination was performed according to the manufacturer’s instructions.
2.13. Wright-Giemsa stain of lung cells
Bronchoalveolar lavage fluid was centrifuged and the cell pellet was smeared on glass-frosted slides, air dried and stained with modified Wright-Giemsa for light microscopy. Triplicate samples were prepared from each immunized group. The cell types were enumerated and calculated from 500 total nucleated cells.
2.14. Statistics
The statistical significance between groups was calculated by two-tailed unpaired Students t tests and p≤0.05 was considered significant. Unless otherwise stated, data were pooled from at least two independent experiments.
3. Results
The immunogenicity of the flagellar proteins isolated from several motile bacteria has been extensively studied although the mechanisms of the intracellular signaling and subsequent host cell immune responses have not been yet fully elucidated. The adjuvant properties of flagellin have also been shown in few vaccines namely epitope-based influenza vaccines, Yersinia pestis, malaria and West Nile virus. Inactivated influenza virus vaccine has been routinely used in humans via the intramuscular or the subcutaneous route. Soluble adjuvants are important tools as vaccine enhancers for non-replicating antigens and our aim was to explore the ability of soluble flagellins to enhance the vaccine potency though the intranasal route.
3.1. Preparation and characterization of monomeric and polymeric flagellins
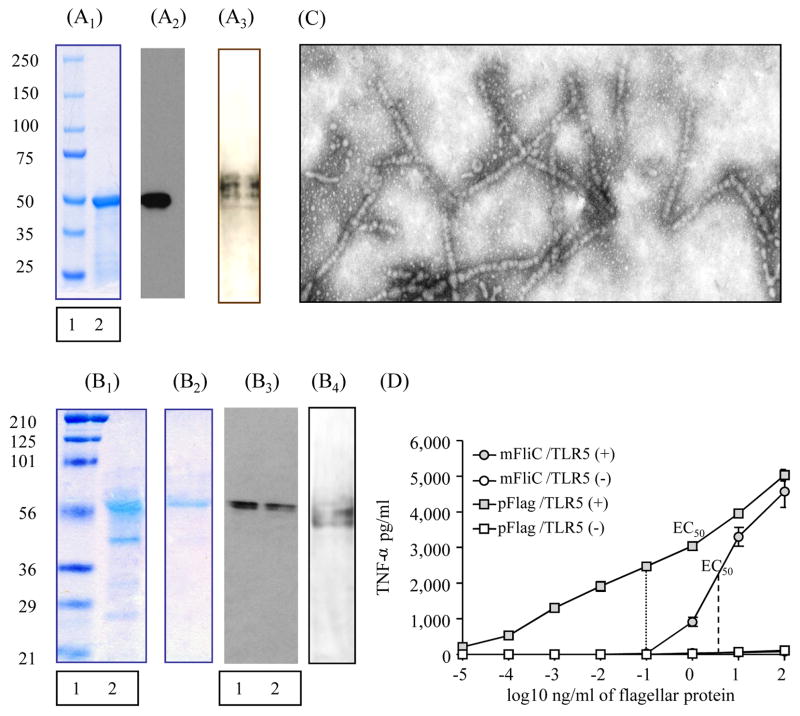
We characterized the expression and the purity of flagellin preparations with Western blot, Coomassie stain, Silver stain and where necessary with electron microscopy. The levels of expression as well as the purity of mFliC, as confirmed by Coomassie stain (Fig. 1A1) and Western blot (Fig. 1A2), showed a single band at the size of approximately 50 kDa. Coomassie stain of the soluble polymeric flagellin (pFlag) upon denaturing SDS gel electrophoresis showed a doublet band at the size of approximately 50–55 kDa (Fig. 1B1) whereas the monomeric form produced by heating of the polymer (Fig. 1B2) demonstrated a single band. The SDS gel showed the expected flagellin doublet (Fig. 1B3, lanes 1,2). The silver stain of both preparations did not show any contamination by LPS (Fig. 1A3 and 1B4). The efficiency of polymerization and purification of flagellin was verified by electron microscopy. Figure 1C depicts the characteristic formation of the flagellar filament protein. The preparation of flagellin after ammonium sulfate precipitation showed the characteristic long filamentous forms.
Fig. 1. Characterization of soluble monomeric and polymeric flagellin.
(A1) Coomassie Blue staining of recombinant monomeric flagellin purified with Nickel affinity column. Lane 1: pre-stained SDS broad range protein marker, lane 2: band of monomeric flagellin, 5 μg (mFliC). (A2) SDS-poyacrylamide gel electrophoresis followed by Western blot of purified mFliC, 1 μg protein: flagellin band is probed with rabbit anti-flagellin antibody and goat anti-mouse IgG HRP. (A3)Silver stain of monomeric flagellin. (B1) Coomassie Blue staining of purified polymeric flagellin (pFlag). Lane 1: pre-stained SDS broad range protein marker (Precision Plus, Bio-Rad, Richmond, CA), lane 2: band of pFlag after ammonium sulfate precipitation, 5 μg. (B2) Band of pFlag, monomerized after heating at 65°C, 2 μg of protein. (B3) Western blot of purified pFlag: flagellin band is probed with mouse anti-flagellin polyclonal antibody and goat anti-mouse IgG HRP. Lane 1: 2 μg protein, lane 2: 1 μg protein loaded (B4) Silver stain of polymeric flagellin. (C) Electron microscopy of purified pFlag. (D) A RAW264.7 cell-based assay was used to determine the bioactivity of mFliC and pFlag. TLR5 bioactivity was expressed as shown by TNF-α production by TLR5 positive cells, subtracting that of TLR5 negative cells stimulated by the flagellins. The experiment was performed in triplicate samples in serial 10-fold dilutions per sample. EC50 (concentration which produces 50% of maximal activity). To confirm that the adjuvanticity of our preparations was attributed to the activation of TLR5 receptor by flagellin and not to residual endotoxin lipopolysaccharide (LPS), we tested for LPS levels and found that they were negligible (≤0.125 EU/ml).
3.2. TLR5 activation by monomeric and polymeric flagellin
To evaluate the flagellar filament protein (pFlag) from S. enteritidis wild type and the recombinant monomeric flagellin (mFliC) expressed in E. coli cells as valid TLR5 ligands we confirmed their ability to activate the TLR5 receptors using a mouse macrophage cell line RAW264.7-based assay [36]. The EC50 (concentration which produces 50% of maximal bioactivity) of mFliC was 5–6 ng/ml, whereas the EC50 of pFlag was approximately 0.1 ng/ml (Fig. 1D).
3.3. Co-administration of flagellin with inactivated influenza virus induces enhanced humoral immune responses
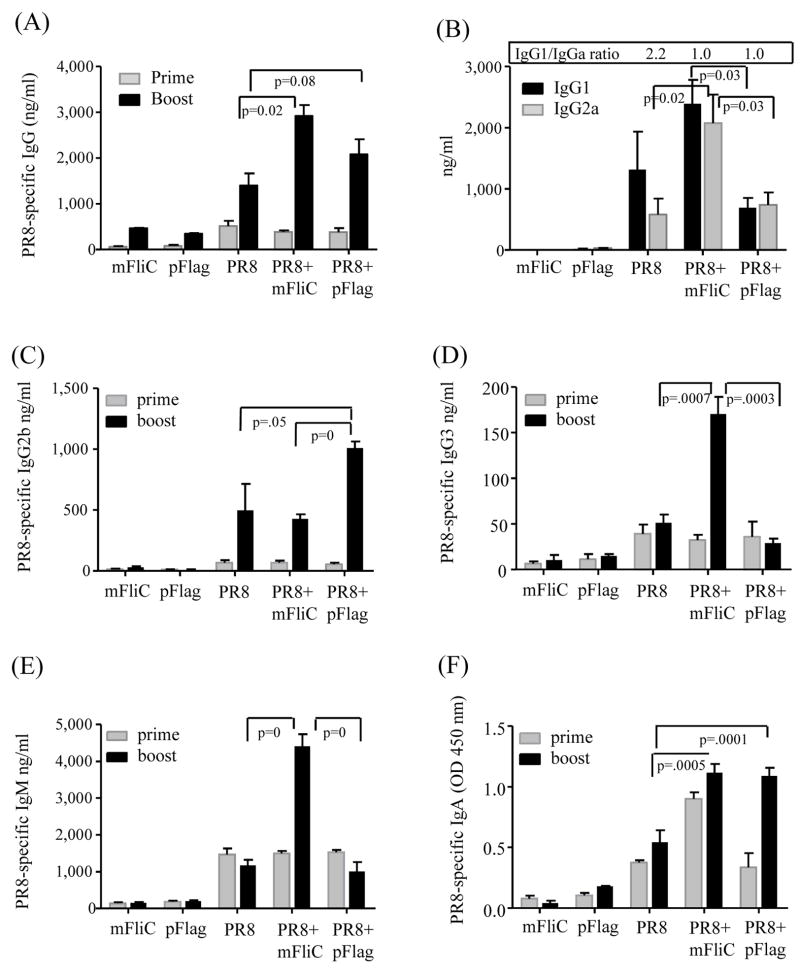
To study the possible adjuvant effects of flagellins, we immunized Balb/c mice with inactivated influenza virus alone or in the presence of either mFliC or pFlag. As control groups, we used mice immunized with mFliC or pFlag alone. Since the bioactivity of pFlag was at least 1 log higher than that of mFliC we adjusted the dose of mFiC to 10 μg and of pFlag to 1 μg. The serum anti-influenza titers were similar among the vaccinated groups after priming (Fig. 2). Two weeks after boost, the co-administration of pFlag or mFliC induced approximately a 2-fold increase in the PR8-specific titers as compared to the PR8 group alone (Fig. 2A). The IgG1/IgG2a ratio in the group of mice vaccinated with PR8 alone was 2.2 whereas with addition of mFliC or pFlag this ratio was 1.0 (Fig. 2B). The profile of the isotype class switching after boost demonstrated that mFliC enhanced both the Th1 and Th2 responses as shown by the increases of IgG1 and IgG2a titers compared to the PR8+pFlag group (p=0.03 for both isotypes) or PR8 group (p=0.02 for IgG2a). Since both flagellins enhanced the anti-PR8 specific titers, but the sum of IgG1 and IgG2a was much lower in the presence of pFlag, we examined other Ig isotypes elicited by this group. We found that the PR8+pFlag group had twice the IgG2b concentration in the serum as compared to animals vaccinated with PR8 (p=0.05) or PR8+mFliC (p=0) (Fig. 2C). The PR8+mFliC group showed very pronounced IgG3 and IgM titers (p<0.0005) compared to the other groups (Fig. 2D, 2E). Finally the IgA levels induced by both adjuvanted vaccines were similar and significantly higher than the levels observed in the PR8 group (p≤0.0005) (Fig. 2F).
Fig. 2.
Total anti-influenza serum IgG and isotype profiles after intranasal prime and boost. (A) Serum IgG titers. (B) Serum IgG1 and IgG2a isotypes. (C) Serum IgG2b titers; (D) serum IgG3 titers; (E) Serum IgM titers; (F) Serum IgA titers; Sera were collected at 2 weeks after each immunization; IgG, IgG isotypes and IgM were determined with quantitative ELISA and expressed as ng/ml. IgA titers were recorded as O.D. values at 450 nm. PR8, inactivated influenza virus alone; PR8+mFliC, inactivated virus plus 10 μg monomeric recombinant flagellin (mFliC); PR8+pFlag, inactivated virus plus 1 μg polymeric flagellin (pFlag); mFliC, 10 μg monomeric recombinant flagellin; pFlag, 1 μg polymeric flagellin. The results are the average of two independent experiments. Bars represent averages and standard errors from six mice per group.
3.4. Flagellins demonstrate potent adjuvant activity boosting the protective influenza-specific antibody levels
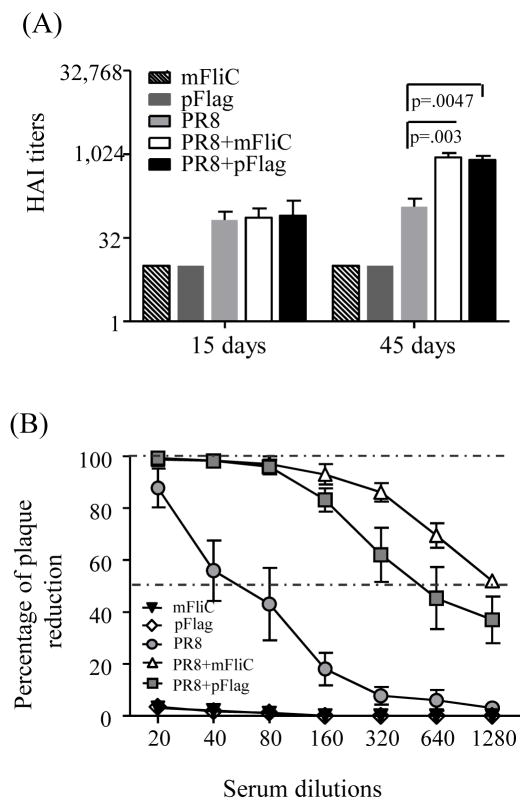
The hemagglutination inhibition titers (HAI) detected in the sera of mice 2 weeks after the 1st immunization dose were well above 40, without any notable differences among the groups that received inactivated virus alone or in combination with flagellin. The 2nd immunization induced robust responses in all groups; in particular the groups that received either adjuvanted vaccine had 8 to 9-fold higher HAI titers than the group immunized with PR8 alone (Fig. 3A).
Fig. 3.
Influenza-specific hemagglutination inhibition titers (HAI) and virus neutralizing activity. (A) HAI titers of sera from immunized mice. Mouse sera collected at 2 weeks after each immunization were used to determine HAI titers as described in Methods. Data represented here are serum HAI titers from individual mice from two independent experiments. Bars represent the arithmetic mean and standard errors of the HAI values. (B) Virus-neutralizing antibody activities in mouse sera collected at 2 weeks after the second immunization. Groups and statistics for the HAI and neutralization titers are as described in the legend of Fig. 2.
The neutralization assay, which provides an estimate of the functional antibody levels, demonstrated an impressive 16-fold and 32-fold increase of titers in the PR8+pFlag and PR8+mFliC groups after boost respectively when compared to the PR8 alone group (Fig. 3B).
3.5. Flagellin does not enhance secretory IgA production
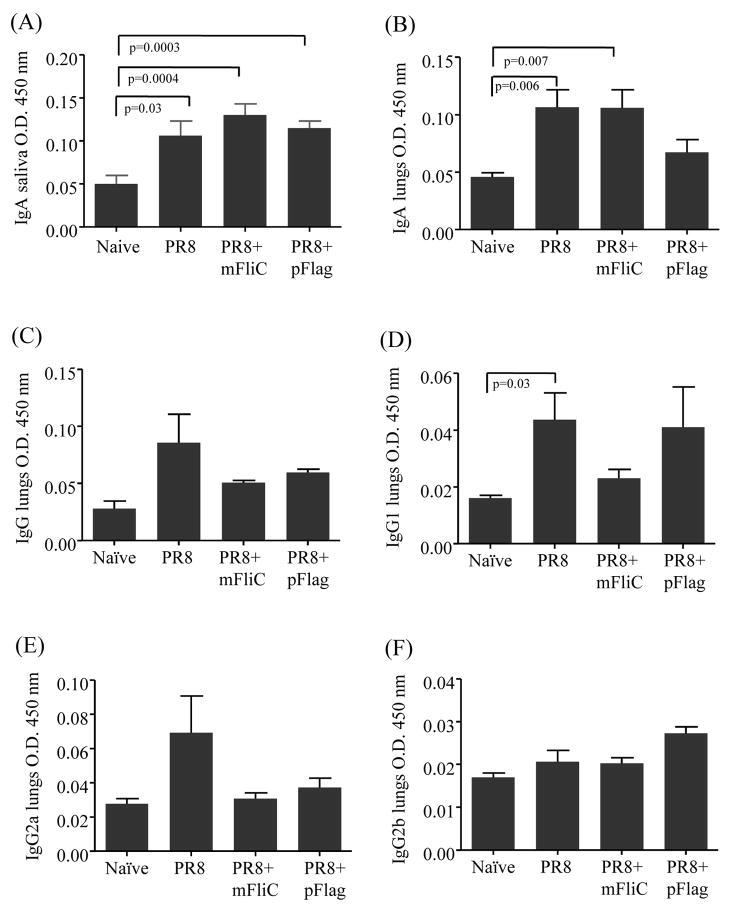
The efficient protection against the invasion of the host by a respiratory pathogen depends heavily on the local immune responses, innate and adaptive, detected in the upper and lower respiratory tract. The IgA titers are mainly indicative of the upper respiratory tract response whereas the IgG levels are important in lower respiratory tract infections. In our experiments the intranasal administration of inactivated influenza virus alone induced measurable mucosal immune responses, which were significantly higher than the naïve group, as seen by the IgA titers in saliva 2 weeks after prime (Fig. 4A) and in lung supernatants on day 4 after boost (Fig. 4B) [39]. Despite the enhanced systemic immune response in the flagellin-treated groups [15] we did not observe any statistically significant increase of secreted immunoglobulin A (sIgA) or the secreted IgG levels and isotypes against PR8 in the mucosal tissue in the presence of the adjuvant after prime or boost (Fig. 4C-F). The latter are likely serum-derived through transudation onto the mucosal surfaces [40].
Fig. 4.
Mucosal responses in saliva and lung supernatants. (A) At 2 weeks after prime saliva was collected after intraperitoneal injection of carbamoylcholine chloride; samples were mixed with a protease inhibitor, phenylmethylsulfonyl fluoride (PMSF), and kept frozen until analysis. IgA titers were determined by ELISA. (B-F) At 4 days after boost bronchoalveolar lavages were collected after flushing the lungs with PBS and stored at −80°C until analyzed. IgA, IgG and isotypes were determined with ELISA. All samples were diluted 2-fold. Bars represent averages and standard errors are from 6 mice per group. Groups are as described in the legend of Fig. 2.
3.6. Intranasal immunization with the adjuvanted influenza vaccine enhances cytokine levels
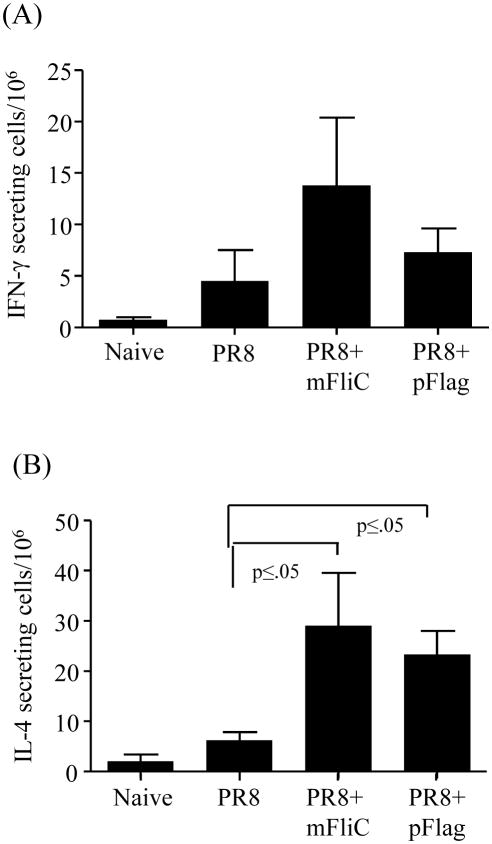
We assessed the T cell responses by measuring cytokine production as an indicator of cellular immune responses. Briefly, we isolated spleen cells from mice immunized with inactivated influenza virus alone or in the presence of either flagellin, 4 days after the last immunization. The cells were re-stimulated with inactivated influenza virus and their ability to produce cytokines IFN-γand IL-4 was quantified by ELISPOT (Fig. 5). Inclusion of flagellins resulted in increased IFN-γ production when compared to the PR8 group although the data were not statistically significant (Fig. 5A). The presence of either flagellin induced significantly higher IL-4 production than PR8 alone (p≤0.05) (Fig. 5B). These results indicate that intranasal immunization with the adjuvanted vaccine is capable to elicit systemic Th2 biased cellular immune responses in addition to the humoral responses observed.
Fig. 5.
IFN-γ and IL-6 secretion by T cells in response to inactivated influenza antigen. The frequency of cellular immune responses is shown in Balb/c mice vaccinated at weeks 0 and 4 and sacrificed 4 days post-boost. Spleens from all groups of immunized mice were individually processed and cells were cultured in the presence of inactivated PR8 virus for stimulation. (A) Splenocytes were analyzed with ELISA for IFN-γ production (pg/ml) 4 days after boost upon re-stimulation with formalin-inactivated PR8 virus. Data are averages and standard errors for six mice per group. (B) IL-4 levels were determined with cytokine ELISA (pg/ml) and bars represent averages and standard errors for six mice per group. mFliC, pFlag, PR8, PR8+mFliC and PR8+pFlag groups are as described in the legend of Fig. 2.
3.7. Body weight changes and survival rates upon challenge with live mouse-adapted virus
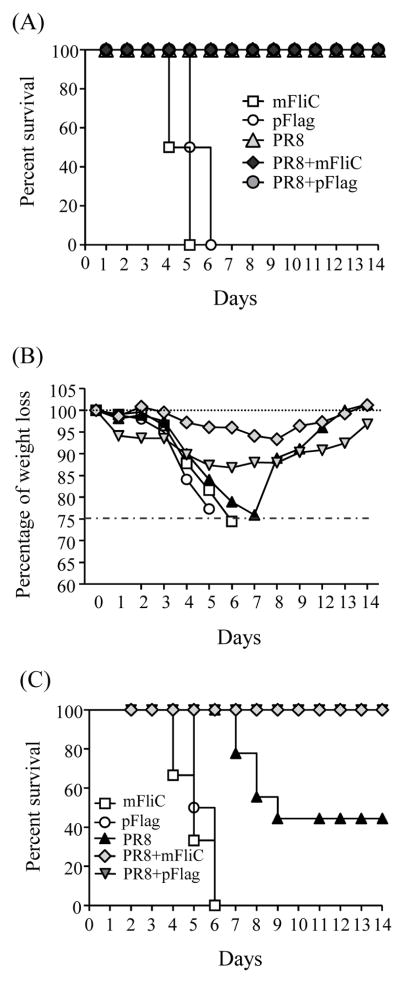
A successful vaccine proves its efficacy by the protection induced against challenge with a high virus dose. One hundred per cent of all vaccinated groups survived after infection with 10×LD50 of mouse-adapted PR8 virus (Fig. 6A) with no weight loss (data not shown). The control groups immunized only with monomeric or polymeric flagellin died between day 5 and 6 (Fig. 6A). When we infected the immunized mice with 40×LD50, we observed minimal changes of the body weights in the mice vaccinated with PR8+mFliC, 7% below their initial body weight, beginning at day 4 post-infection and reaching the lowest levels by day 8. The PR8+pFlag group experienced more severe body weight loss starting at day 2 with the lowest body weight levels on day 6. The group vaccinated with PR8 alone lost almost 25% of the body weight by day 7. Both negative control groups that received monomeric or polymeric flagellin were euthanized by day 7 because they lost more than 25% of their weight (Fig. 6B). By day 9, only 33% of the PR8 group mice survived as compared to 100% observed in the PR8+mFliC and PR8+pFlag groups; all the surviving animals showed a steady recovery with a gradual regain of their weight (Fig. 6C). These results clearly demonstrate the efficacy of either flagellin to enhance protection against lethal viral challenge.
Fig. 6.
Protective efficacy from challenge infection with influenza virus. Post-challenge survival rates of immunized and naïve mice were monitored for 14 days. (A) Challenge with 10×LD50. One hundred per cent of mice immunized with PR8, PR8+mFliC and PR8+pFlag survived upon challenge with 10×LD50 of mouse-adapted PR8 virus. There were no changes in body weights (data not shown). Control mice from the mFliC and pFlag groups died by day 6. (B) Challenge with 40xLD50. Body weights (BW) recorded during the infection period of 14 days. (C) Challenge with 40×LD50. One hundred per cent of mice vaccinated with PR8+mFliC and PR8+pFlag survived the challenge with 40×LD50 of mouse-adapted virus, but only 33% of PR8-vaccinated mice survived. Control mice from the groups of mFliC and pFlag died by day 6. The graphs represent the average data of 6 mice per group.
3.8 The presence of flagellin in the influenza vaccine augments the early inflammatory immune responses
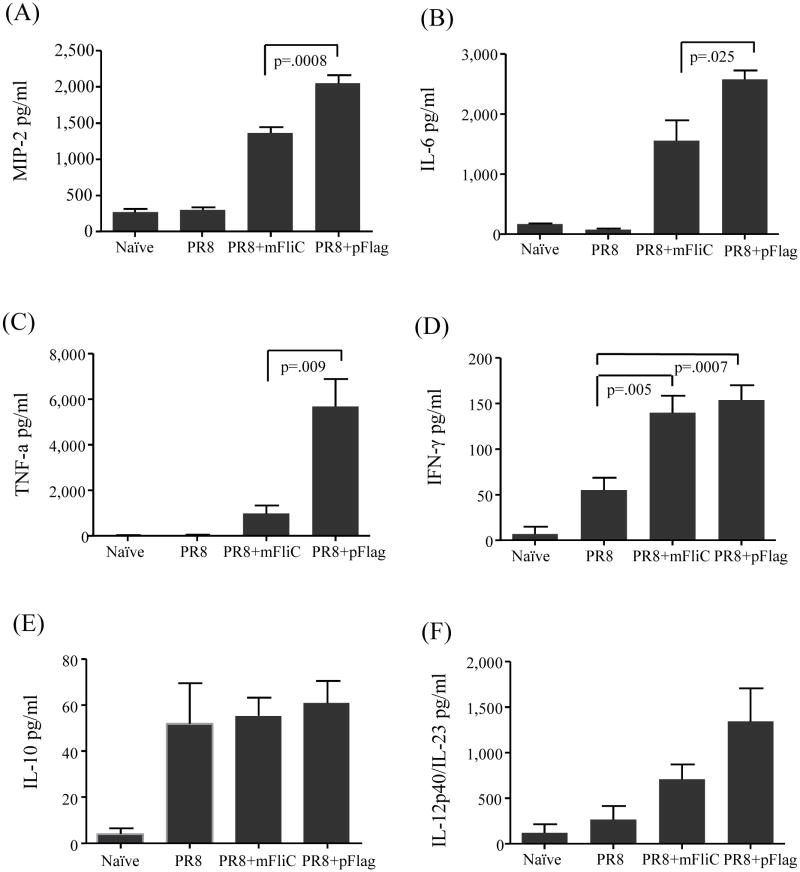
Previous studies of intranasal or systemic administration of flagellin indicated that the ligand plays a very important role in initiating innate immune responses [15, 41]. Based on these observations we compared the early steps of immune responses post-vaccination. We administered the vaccine to Balb/c mice and 16 hours later we collected fluid from BALF and lung suspensions to determine the levels of TNF-α, IFN-γ, IL-6, MIP-2, IL1–10 and IL-12p40/IL-23 (Fig. 7A). We found that both adjuvant preparations enhance the cytokines at similar levels (BALF data not shown). In particular we were interested in the macrophage inflammatory protein 2 (MIP-2), a mouse analogue of α-chemokines [42] secreted by the alveolar epithelial cells [43], that has been reported to be induced by live influenza virus and responds by attracting granulocytes at the site of inflammation [44]. The co-administration of either flagellin type with PR8 enhanced by 3-fold (PR8+mFliC) and 5-fold (PR8+pFlag) the increase of MIP-2, as compared to PR8 alone (Fig. 7A). The polymeric form demonstrated more potent activity than the monomeric recombinant form, as shown by the significantly higher levels of MIP-2 (p=0.0008). Similarly both flagellins exhibited an impressive induction of the pro-inflammatory cytokine IL-6 although the polymeric form induced 60% higher IL-6 production compared to the monomeric counterpart (p=0.025) (Fig. 7B) [45]. The addition of polymeric flagellin demonstrated an even more dramatic effect on TNF-α production, as the levels of the cytokine were 7-fold higher than those induced by the monomeric flagellin (Fig. 7C). In all the aforementioned assays, immunization with inactivated virus alone had no effect on cytokine levels when compared to the naïve mice. The exception to the trend was the IFN-γ production, which was doubled in the presence of flagellins with no significant differences between the PR8+mFliC and PR8+pFlag groups (Fig. 7D). As reported elsewhere, the presence of flagellin did not affect the levels of the anti-inflammatory cytokine IL-10 (Fig. 7E) [46] or the pro-inflammatory cytokine IL-12p70 (data not shown). Although the levels of IL-12p40 were increased in the PR8+mFliC or PR8+pFlag groups, the data variation was quite large (Fig. 7F) [16]. The lack of differences in the levels of IFN-γ in the presence of different forms of flagellins demonstrated an IFN-γ-independent production of TNF-α and IL-12/IL-23 as previously reported [47].
Fig. 7.
Early cellular immune responses in lungs of vaccinated animals. Mice were vaccinated with PR8 alone or co-administered with mFliC or pFlag and 16 hours later lungs were collected. The lung tissues from all groups of immunized mice were individually processed and their suspensions were diluted 10-fold for the determination of cytokines with ELISA. Data were quantified as pg/ml. (A) MIP-2. (B) IL-6, (C) TNF-α. (D) IFN-γ. (E) IL-10 (F) IL-12p40/IL-23. Results represent the average and standard deviations from 6 mice per group. Groups are as described in the legend of Fig. 2.
3.9. Neutrophil recruitment in the lungs of immunized animals is enhanced in the presence of flagellins
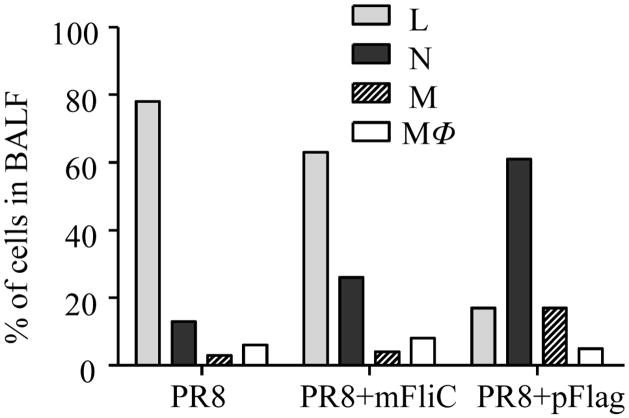
To determine the types of infiltrating cells and their recruitment upon intranasal administration of the vaccine, the lungs of female BALB/c mice vaccinated with PR8 without or with flagellin were examined 16 hours post-immunization. Figure 8 shows the percentage of different cell types recruited in the lungs and detected in the BALF of immunized animals. The broncoalveolar lavage fluid from naïve animals showed only lung epithelial cells and was devoid of any type of cell infiltrate (data not shown). The PR8-treated Balb/c mice had the highest percentage of lymphocytes among all vaccinated groups (78%). The PR8+mFliC group demonstrated a 2-fold increase in the percentage of granulocytes and a moderate decrease of lymphocytes when compared to the PR8-treated animals. Neutrophil and monocyte accumulation were highest in the lungs of PR8-polymeric flagellin-treated Balb/c mice (61 and 17% respectively) whereas the numbers of macrophages were similar among all groups. These data strongly correlate with the MIP-2 results (Fig. 8A) since this chemokine induces white blood cell infiltration in the site of administration.
Fig. 8.
Cell populations recruited in BALF fluid. Mice (n=6) were vaccinated with PR8 alone or with mFliC or pFlag and 16 hours later BALF was collected. The cell pellets from BALF of individual mice were resuspended in PBS and smears of them were prepared on slides, air-dried, stained with a modified Wright-Giemsa stain and counted based on cell morphology. Percentages of cell populations were calculated from at least 500 cells counted per optic field in several optic fields. Naïve mice were used as controls (not shown). L: lymphocytes, N: neutrophils, M: monocytes, MΦ: macrophages.
4. Discussion
The present study demonstrates that the recombinant monomeric (mFliC) or polymeric (pFlag) flagellin derived directly from bacteria are promising adjuvants for influenza vaccines. Intranasal immunization with inactivated virus and either form of flagellin conferred 100% protection against high lethal doses of live, mouse adapted influenza virus under conditions where the immunization with virus alone provided only partial protection.
The presence of either type of TLR5 ligand enhanced the systemic PR8-specific IgG titers and the functional antibodies, including the hemagglutination inhibition and neutralizing antibodies, to similar levels. Previous reports postulated that soluble flagellin induces Th2-biased responses [16, 19]. Based on the IgG1 and IgG2a levels detected with the doses tested, we found that mFliC enhanced both the Th1 and Th2 responses [48] whereas pFlag did not affect the IgG1 or IgG2a titers. We noted that the combination of the antigen with each form of flagellin exerted a different effect on the isotype switching; the presence of recombinant soluble flagellin induced a higher IgG3 and IgM production whereas the polymeric form mainly enhanced IgG2b secretion. It is known that IgG3 and IgM are the major source of cryoglobulins [49] and their combination with IgG2a causes a mixed type II cryoglobulinemia characteristic of Balb/c mice which predominantly activates complement via the alternative pathway [50, 51]. On the other hand the increased IgG2b levels, which are prominent in the PR8+pFlag group, activate complement via the classical pathway [52]. It has been suggested that the classical pathway is the most effective immune defense against microorganisms [50], and the polymeric flagellin may therefore have some advantages as an adjuvant for enhancing the humoral immunity against infection.
Contrary to our expectations, the humoral responses detected in mucosal secretions were not as impressive as in the mouse sera. The PR8-specific sIgA production was not significantly affected by the flagellin-TLR5 activation after prime or boost. As previously reported, flagellins likely belong to the category of cytokine/chemokine adjuvants that can provide effective protective immunity [53] and their adjuvant effects are directed towards cell-mediated responses mediated by CD4+ or CD8+ lymphocytes [48, 54]. Our findings are in agreement with these reports because either type of flagellin affects the chemokine/cytokine secretion in the mucosal surfaces early in the induction of mucosal immune responses. Our data also showed that both flagellins could increase the number of IL-4 secreting cells and that both PR8+mFliC and PR8+pFlag groups presented statistically significant increases compared to the PR8 alone group. Both flagellins also induced higher numbers of IFN-γ secreting cells than PR8 alone. Altogether the systemic cellular immune responses were enhanced but not polarized in the presence of the ligand.
The levels of cytokines in the lungs within the first 24 hours post-vaccination indicated that T cells respond to the influenza vaccine by producing IL-12p40, which is connected to DC activation and maturation in the lungs [55, 56]. The co-administration of either flagellin induced robust increases of IFN-γ. In agreement with previous observations showing that TNF-α production can be independent of IFN-γ levels [18, 57] we found that although both flagellins enhanced the production of IFN-γ at similar concentrations they did not exert the same effect on TNF- αlevels. It was also evident that the presence of TLR5 ligands boosted the levels of the pro-inflammatory cytokine IL-6 and the chemokine MIP-2 in a similar pattern, although the polymeric flagellin was a significantly more potent inducer of lung cytokine production than the monomeric form. The differences in MIP-2 levels may have contributed to the cell types we detected in the lungs because the presence of this chemoattractant is responsible for granulocyte/macrophage recruitment in the airways [18, 57]. We observed that when we administered inactivated influenza virus alone more lymphocytes were found in the lungs whereas the addition of flagellins, in particular polymeric flagellin, caused a massive recruitment of granulocytes and macrophages/monocytes. In contrast, we did not find any of these cell types in naïve mouse lungs. The attraction of white blood cells to the airways of vaccinated animals shortly after the administration of the antigen with flagellins promoted an immediate inflammatory response. These robust innate responses are necessary to initiate strong cellular and humoral responses, which are desirable in vaccines against viruses. Finally the participation of IL-10 as anti-inflammatory cytokine in the local mucosal responses was unaffected by the presence of flagellin, implying that at least in the early responses there is no suppression of inflammation induced by the TLR agonists.
The robust humoral immune responses against influenza virus observed in the presence of the TLR5 ligands lead us to several conclusions: (1) both flagellins when administered intranasally with inactivated influenza virus are promising adjuvants capable to induce protection at very high challenge doses of influenza virus (2) both flagellins enhance the early pro-inflammatory immune responses in the lungs assisting in the immediate protection of the host although the polymeric flagellin is a more potent inducer of certain cytokines detected in the lungs. Several studies have supported the role of monomeric flagellin as an active ligand with higher affinity for TLR5 receptors [14, 30, 58, 59]. It is possible that some of the soluble polymeric flagellin could dissociate into monomers contributing in the observed effect. However it is unlikely that this could be a major contributing effect because we used a 10-fold excess of monomer in our comparative study. It was recently postulated that different post-translational modifications in the bacterial flagellins affect the signaling pathways after binding to TLR5 subsequently leading to different levels of NF-κB activation and induction of the pro-inflammatory gene expression [59]. Therefore, the observed differences may be the result of variation in the secondary modification patterns of the mFliC protein after its expression in a heterologous host. Our findings indicate that both soluble pFlag and mFliC are very promising candidate adjuvants for influenza vaccine as they both induce potent humoral and cellular immune responses. Within 24 hours after co-administration of polymeric flagellin with the influenza vaccine, we observe a rapid and intense inflammatory reaction in the lungs which is more pronounced than with the monomeric flagellin. This results to infiltration of the respiratory tract by several populations of nucleated cells, most of them representatives of innate immunity, which contribute to the amplification of adaptive immune responses.
Acknowledgments
This work was supported by NIH grant R01 AI068003 and NIH grant U01 AI0745579. We thank Dahnide Taylor, Erin-Joi Collins-McNeal and Tanya Cassingham for their valuable assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palese P. Influenza: old and new threats. Nat Med. 2004 Dec;10(12 Suppl):S82–7. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 2.Cox NJ, Subbarao K. Influenza. Lancet. 1999 Oct 9;354(9186):1277–82. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003 Jan 8;289(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 5.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004 Mar 5;303(5663):1522–6. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001 Aug;2(8):675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R, Janeway CA., Jr Innate immune induction of the adaptive immune response. Cold Spring Harb Symp Quant Biol. 1999;64:429–35. doi: 10.1101/sqb.1999.64.429. [DOI] [PubMed] [Google Scholar]
- 8.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004 Jun;199:227–50. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004 Oct;5(10):987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 10.Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999 May;18(3):265–76. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 11.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003 May 15;170(10):5165–75. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 12.Reichhart JM. TLR5 takes aim at bacterial propeller. Nat Immunol. 2003 Dec;4(12):1159–60. doi: 10.1038/ni1203-1159. [DOI] [PubMed] [Google Scholar]
- 13.Hackett J, Attridge S, Rowley D. Oral immunization with live, avirulent fla+ strains of Salmonella protects mice against subsequent oral challenge with Salmonella typhimurium. J Infect Dis. 1988 Jan;157(1):78–84. doi: 10.1093/infdis/157.1.78. [DOI] [PubMed] [Google Scholar]
- 14.McSorley SJ, Cookson BT, Jenkins MK. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J Immunol. 2000 Jan 15;164(2):986–93. doi: 10.4049/jimmunol.164.2.986. [DOI] [PubMed] [Google Scholar]
- 15.Honko AN, Sriranganathan N, Lees CJ, Mizel SB. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect Immun. 2006 Feb;74(2):1113–20. doi: 10.1128/IAI.74.2.1113-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Didierlaurent A, Ferrero I, Otten LA, Dubois B, Reinhardt M, Carlsen H, et al. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J Immunol. 2004 Jun 1;172(11):6922–30. doi: 10.4049/jimmunol.172.11.6922. [DOI] [PubMed] [Google Scholar]
- 17.Lee SE, Kim SY, Jeong BC, Kim YR, Bae SJ, Ahn OS, et al. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect Immun. 2006 Jan;74(1):694–702. doi: 10.1128/IAI.74.1.694-702.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honko AN, Mizel SB. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect Immun. 2004 Nov;72(11):6676–9. doi: 10.1128/IAI.72.11.6676-6679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham AF, Khan M, Ball J, Toellner KM, Serre K, Mohr E, et al. Responses to the soluble flagellar protein FliC are Th2, while those to FliC on Salmonella are Th1. Eur J Immunol. 2004 Nov;34(11):2986–95. doi: 10.1002/eji.200425403. [DOI] [PubMed] [Google Scholar]
- 20.Han S, Yang K, Ozen Z, Peng W, Marinova E, Kelsoe G, et al. Enhanced differentiation of splenic plasma cells but diminished long-lived high-affinity bone marrow plasma cells in aged mice. J Immunol. 2003 Feb 1;170(3):1267–73. doi: 10.4049/jimmunol.170.3.1267. [DOI] [PubMed] [Google Scholar]
- 21.Pino O, Martin M, Michalek SM. Cellular mechanisms of the adjuvant activity of the flagellin component FljB of Salmonella enterica Serovar Typhimurium to potentiate mucosal and systemic responses. Infect Immun. 2005 Oct;73(10):6763–70. doi: 10.1128/IAI.73.10.6763-6770.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Boado YS, Cobb LM, Deora R. Bordetella bronchiseptica flagellin is a proinflammatory determinant for airway epithelial cells. Infect Immun. 2005 Nov;73(11):7525–34. doi: 10.1128/IAI.73.11.7525-7534.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM, et al. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci U S A. 2005 Jun 28;102(26):9247–52. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Yedidia T, Arnon R. Epitope-based vaccine against influenza. Expert Rev Vaccines. 2007 Dec;6(6):939–48. doi: 10.1586/14760584.6.6.939. [DOI] [PubMed] [Google Scholar]
- 25.Mizel SB, Graff AH, Sriranganathan N, Ervin S, Lees CJ, Lively MO, et al. A Fusion Protein, Flagellin/F1/V, is an Effective Plague Vaccine in Mice and Two Species of Nonhuman Primates. Clin Vaccine Immunol. 2008 Nov 5; doi: 10.1128/CVI.00333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bargieri DY, Rosa DS, Braga CJ, Carvalho BO, Costa FT, Espindola NM, et al. New malaria vaccine candidates based on the Plasmodium vivax Merozoite Surface Protein-1 and the TLR-5 agonist Salmonella Typhimurium FliC flagellin. Vaccine. 2008 Nov 11;26(48):6132–42. doi: 10.1016/j.vaccine.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 27.McDonald WF, Huleatt JW, Foellmer HG, Hewitt D, Tang J, Desai P, et al. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J Infect Dis. 2007 Jun 1;195(11):1607–17. doi: 10.1086/517613. [DOI] [PubMed] [Google Scholar]
- 28.Gewirtz AT, Simon PO, Jr, Schmitt CK, Taylor LJ, Hagedorn CH, O’Brien AD, et al. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest. 2001 Jan;107(1):99–109. doi: 10.1172/JCI10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001 Apr 26;410(6832):1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 30.Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003 Dec;4(12):1247–53. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim GF, Fleet GH, Lyons MJ, Walker RA. Method for the isolation of highly purified Salmonella flagellins. J Clin Microbiol. 1985 Dec;22(6):1040–4. doi: 10.1128/jcm.22.6.1040-1044.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogushi K, Wada A, Niidome T, Mori N, Oishi K, Nagatake T, et al. Salmonella enteritidis FliC (flagella filament protein) induces human beta-defensin-2 mRNA production by Caco-2 cells. J Biol Chem. 2001 Aug 10;276(32):30521–6. doi: 10.1074/jbc.M011618200. [DOI] [PubMed] [Google Scholar]
- 33.Skountzou I, Quan FS, Jacob J, Compans RW, Kang SM. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine. 2006 Aug 28;24(35–36):6110–9. doi: 10.1016/j.vaccine.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Compans RW. Hemagglutination-inhibition: rapid assay for neuraminic acid-containing viruses. J Virol. 1974 Nov;14(5):1307–9. doi: 10.1128/jvi.14.5.1307-1309.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sha Z, Compans RW. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000;74(11):4999–5005. doi: 10.1128/jvi.74.11.4999-5005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang BZ, Quan FS, Kang SM, Bozja J, Skountzou I, Compans RW. Membrane-anchored Flagellin Incorporation into Influenza VLPs Enhances the Breadth of Immune Responses. J Virol. 2008 Sep 10; doi: 10.1128/JVI.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skountzou I, Quan FS, Gangadhara S, Ye L, Vzorov A, Selvaraj P, et al. Incorporation of glycosylphosphatidylinositol-anchored granulocyte- macrophage colony-stimulating factor or CD40 ligand enhances immunogenicity of chimeric simian immunodeficiency virus-like particles. J Virol. 2007 Feb;81(3):1083–94. doi: 10.1128/JVI.01692-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang SM, Guo L, Yao Q, Skountzou I, Compans RW. Intranasal immunization with inactivated influenza virus enhances immune responses to coadministered simian-human immunodeficiency virus-like particle antigens. J Virol. 2004 Sep;78(18):9624–32. doi: 10.1128/JVI.78.18.9624-9632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takada A, Matsushita S, Ninomiya A, Kawaoka Y, Kida H. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine. 2003 Jul 4;21(23):3212–8. doi: 10.1016/s0264-410x(03)00234-2. [DOI] [PubMed] [Google Scholar]
- 40.Reading PC, Morey LS, Crouch EC, Anders EM. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol. 1997 Nov;71(11):8204–12. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders CJ, Yu Y, Moore DA, 3rd, Williams IR, Gewirtz AT. Humoral immune response to flagellin requires T cells and activation of innate immunity. J Immunol. 2006 Sep 1;177(5):2810–8. doi: 10.4049/jimmunol.177.5.2810. [DOI] [PubMed] [Google Scholar]
- 42.Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A. 1989 Jan;86(2):612–6. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manzer R, Dinarello CA, McConville G, Mason RJ. Ozone exposure of macrophages induces an alveolar epithelial chemokine response through IL-1alpha. Am J Respir Cell Mol Biol. 2008 Mar;38(3):318–23. doi: 10.1165/rcmb.2007-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirabayashi T, Ochiai H, Sakai S, Nakajima K, Terasawa K. Inhibitory effect of ferulic acid and isoferulic acid on murine interleukin-8 production in response to influenza virus infections in vitro and in vivo. Planta Med. 1995 Jun;61(3):221–6. doi: 10.1055/s-2006-958060. [DOI] [PubMed] [Google Scholar]
- 45.Salazar-Gonzalez RM, Srinivasan A, Griffin A, Muralimohan G, Ertelt JM, Ravindran R, et al. Salmonella flagellin induces bystander activation of splenic dendritic cells and hinders bacterial replication in vivo. J Immunol. 2007 Nov 1;179(9):6169–75. doi: 10.4049/jimmunol.179.9.6169. [DOI] [PubMed] [Google Scholar]
- 46.Vicente-Suarez I, Takahashi Y, Cheng F, Horna P, Wang HW, Wang HG, et al. Identification of a novel negative role of flagellin in regulating IL-10 production. Eur J Immunol. 2007 Nov;37(11):3164–75. doi: 10.1002/eji.200737306. [DOI] [PubMed] [Google Scholar]
- 47.de Boer T, van Dissel JT, Kuijpers TW, Rimmelzwaan GF, Kroon FP, Ottenhoff TH. Influenza virus vaccination induces interleukin-12/23 receptor beta 1 (IL-12/23R beta 1)-independent production of gamma interferon (IFN-gamma) and humoral immunity in patients with genetic deficiencies in IL-12/23R beta 1 or IFN-gamma receptor I. Clin Vaccine Immunol. 2008 Aug;15(8):1171–5. doi: 10.1128/CVI.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McSorley SJ, Ehst BD, Yu Y, Gewirtz AT. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J Immunol. 2002 Oct 1;169(7):3914–9. doi: 10.4049/jimmunol.169.7.3914. [DOI] [PubMed] [Google Scholar]
- 49.Abdelmoula M, Spertini F, Shibata T, Gyotoku Y, Luzuy S, Lambert PH, et al. IgG3 is the major source of cryoglobulins in mice. J Immunol. 1989 Jul 15;143(2):526–32. [PubMed] [Google Scholar]
- 50.Celik I, Stover C, Botto M, Thiel S, Tzima S, Kunkel D, et al. Role of the classical pathway of complement activation in experimentally induced polymicrobial peritonitis. Infect Immun. 2001 Dec;69(12):7304–9. doi: 10.1128/IAI.69.12.7304-7309.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trendelenburg M, Fossati-Jimack L, Cortes-Hernandez J, Turnberg D, Lewis M, Izui S, et al. The role of complement in cryoglobulin-induced immune complex glomerulonephritis. J Immunol. 2005 Nov 15;175(10):6909–14. doi: 10.4049/jimmunol.175.10.6909. [DOI] [PubMed] [Google Scholar]
- 52.Manderson AP, Pickering MC, Botto M, Walport MJ, Parish CR. Continual low-level activation of the classical complement pathway. J Exp Med. 2001 Sep 17;194(6):747–56. doi: 10.1084/jem.194.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuki Y, Kiyono H. New generation of mucosal adjuvants for the induction of protective immunity. Rev Med Virol. 2003 Sep-Oct;13(5):293–310. doi: 10.1002/rmv.398. [DOI] [PubMed] [Google Scholar]
- 54.Bargieri DY, Rosa DS, Braga CJ, Carvalho BO, Costa FT, Espindola NM, et al. New malaria vaccine candidates based on the Plasmodium vivax Merozoite Surface Protein-1 and the TLR-5 agonist Salmonella Typhimurium FliC flagellin. Vaccine. 2008 Nov 11;26(48):6132–42. doi: 10.1016/j.vaccine.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 55.Monteiro JM, Harvey C, Trinchieri G. Role of interleukin-12 in primary influenza virus infection. J Virol. 1998 Jun;72(6):4825–31. doi: 10.1128/jvi.72.6.4825-4831.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klinke DJ., 2nd A multi-scale model of dendritic cell education and trafficking in the lung: implications for T cell polarization. Ann Biomed Eng. 2007 Jun;35(6):937–55. doi: 10.1007/s10439-007-9318-6. [DOI] [PubMed] [Google Scholar]
- 57.Sakai S, Kawamata H, Mantani N, Kogure T, Shimada Y, Terasawa K, et al. Therapeutic effect of anti-macrophage inflammatory protein 2 antibody on influenza virus-induced pneumonia in mice. J Virol. 2000 Mar;74(5):2472–6. doi: 10.1128/jvi.74.5.2472-2476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wyant TL, Tanner MK, Sztein MB. Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect Immun. 1999 Jul;67(7):3619–24. doi: 10.1128/iai.67.7.3619-3624.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon R, Samuel CE. Activation of NF-kappaB-dependent gene expression by Salmonella flagellins FliC and FljB. Biochem Biophys Res Commun. 2007 Mar 30;355(1):280–5. doi: 10.1016/j.bbrc.2007.01.148. [DOI] [PMC free article] [PubMed] [Google Scholar]