Abstract
Calibrated blood oxygenation level dependent (BOLD) imaging, a technique used to measure changes in cerebral O2 metabolism, depends on an accurate model of how the BOLD signal is affected by the mismatch between cerebral blood flow (CBF) and cerebral metabolic rate of O2 (CMRO2). However, other factors such as the cerebral blood volume (CBV) distribution at rest and with activation also affect the BOLD signal. The Davis model originally proposed for calibrated BOLD studies (Davis et al., 1998) is widely used because of its simplicity, but it assumes CBV changes are uniformly distributed across vascular compartments, neglects intravascular signal changes, and ignores blood-tissue signal exchange effects as CBV increases and supplants tissue volume. More recent studies suggest that venous CBV changes are smaller than arterial changes, and that intravascular signal changes and CBV exchange effects can bias estimated CMRO2. In this paper, recent experimental results for the relationship between deoxyhemoglobin and BOLD signal changes are integrated in order to simulate the BOLD signal in detail by expanding a previous model to include a tissue compartment and three blood compartments rather than only the venous blood compartment. The simulated data were then used to test the accuracy of the Davis model of calibrated BOLD, demonstrating that the errors in estimated CMRO2 responses across the typical CBF-CMRO2 coupling range are modest despite the simplicity of the assumptions underlying the original derivation of the model. Nevertheless, the accuracy of the model can be improved by abandoning the original physical meaning of the two parameters α and β and treating them as adjustable parameters that capture several physical effects. For a 3 Tesla field and a dominant arterial volume change with activation, the accuracy of the Davis model is improved with new values of α=0.14 and β=0.91.
Keywords: Blood oxygenation level dependent, cerebral blood flow, cerebral metabolic rate of oxygen, mathematical modeling, functional MRI
1. Introduction
Functional magnetic resonance imaging (fMRI) is widely used for mapping spatial and temporal patterns of brain activity. The most common measurement is the blood oxygenation level dependent (BOLD) signal, which results from changes in local deoxyhemoglobin content (Ogawa et al., 1993). When the brain is activated, an unexpected physiological phenomenon occurs: local cerebral blood flow (CBF) increases much more than the cerebral metabolic rate of oxygen (CMRO2) (Fox and Raichle, 1986), decreasing both local oxygen extraction fraction and local deoxyhemoglobin content. Deoxyhemoglobin is paramagnetic and creates magnetic field distortions within and around blood vessels, thereby decreasing the MR signal. Thus decreased blood deoxyhemoglobin content results in the basic BOLD effect of increased MR signal with neural activation. However, interpreting relative levels of BOLD signal change in terms of neural activity is difficult due to the BOLD signal’s complex dependence on changes in cerebral blood volume (CBV) in addition to CBF and CMRO2. Although this sensitivity of the BOLD signal to multiple factors creates some difficulty in interpreting the signal, it also offers the possibility of estimating changes in CMRO2 when BOLD and CBF measurements are combined.
The calibrated-BOLD approach proposed by Davis et al. (1998) does just this; it combines measurements of CBF and BOLD responses to neural activation and hypercapnia stimulation with the goal of estimating the CMRO2 change during brain activation. Changes in O2 metabolism are important as they are expected to reflect the underlying energy requirement of evoked neural activity (Lin et al., 2010). This multimodal imaging approach combining BOLD and CBF measurements requires a theoretical framework that accurately describes how the BOLD signal depends on changes in CBF, CMRO2 and CBV. The Davis model is one such framework and has been used in nearly all subsequent applications of calibrated BOLD. As originally derived, this nonlinear model essentially describes the extravascular signal change with the assumption that it depends on both the change in venous oxygenation and the change in venous CBV. These changes in turn are related to alterations in CBF and CMRO2 in an equation involving two parameters, α and β. The parameter β was originally meant to describe a nonlinear MR signal dependence on venous oxygenation, reflecting the idea that deoxyhemoglobin has a weaker effect on the signal change in the smallest vessels compared to larger veins because of the effects of diffusion (Ogawa et al., 1993). Similarly, the parameter α was meant to be the exponent in a power law relationship between blood flow and venous blood volume. Finally, there is an overall scaling parameter M, typically determined by a separate hypercapnia experiment, that depends on the amount of deoxyhemoglobin present in the baseline state and details of the image acquisition. The calibration step with hypercapnia uses the same model to determine M, and requires two assumptions: hypercapnia changes CBF but not CMRO2 (Chen and Pike, 2010; Jones et al., 2005; Sicard and Duong, 2005) and CBV has the same dependence on CBF in the activation and hypercapnia experiments.
However, the Davis model is potentially oversimplified: it only considers extravascular signal changes and leaves out intravascular contributions to the BOLD signal (Boxerman et al., 1995); it neglects the possibility that changes in deoxyhemoglobin containing CBV (capillary and venous) may be proportionally smaller than the arterial CBV changes (Chen and Pike, 2009a; Hillman et al., 2007; Kim et al., 2007; Kim and Kim, 2006); and volume exchange effects were neglected, ignoring the possibility that differences in the intrinsic signal between blood and tissue may change the overall signal upon neural activation (Buxton et al., 2004; Leontiev and Buxton, 2007; Obata et al., 2004). To clarify the assumptions involved, we retain α as a parameter in the Davis model, but define the exponent representing the relationship between CBV and CBF as φ. The original assumption setting α=φ=0.38 based on experiments in non-human primates (Grubb et al., 1974) assumes that CBV changes are proportionally equal for all blood compartments. The second parameter was assumed to be β=1.5, based on Monte Carlo simulations of only the extravascular signal dependence on deoxyhemoglobin at 1.5T (Davis et al., 1998).
Previous models of the BOLD effect have sought to address these deficiencies. Buxton et al. (1998), with corrections and improvements by Obata et al. (2004), incorporated intravascular signal changes and volume exchange effects by modeling the BOLD signal change as a volume-weighted sum of signal from two compartments: intravascular (venous) and extravascular. More recent models have directly addressed the issue of relating CBV changes to CBF by incorporating direct measurements of CBV in calculating CMRO2 (Donahue et al., 2009; Lin et al., 2008; Lu et al., 2004). These studies used the VASO technique to measure changes in total CBV, but this approach requires two additional assumptions: (1) an absolute baseline CBV value must be assumed to derive the fractional change in CBV necessary for use in the Davis model, and (2) the venous CBV change is assumed to be proportional to the total CBV change measured with VASO. Moving beyond steady-state modeling of BOLD, other approaches have sought to incorporate transient dynamics of CBV (Kim and Kim, 2010b), deoxyhemoglobin (Mandeville et al., 1999), and CMRO2 (Buxton, 2010; Hyder et al., 2010) or explain the coupling and uncoupling of CBF and CMRO2 (Friston et al., 2000; Zheng et al., 2010) that underlies the BOLD signal.
The focus of this work was to model the steady-state BOLD signal and examine how it can be combined with measurements of CBF in order to calculate CMRO2 more accurately. To do so, we followed the approach taken by Uludag et al. (2009) in which the Obata-Buxton model (Buxton et al., 1998; Obata et al., 2004) was expanded to not only include intravascular signal changes but also volume exchange effects. We did not linearize the model, because we were concerned that intravascular signal changes are actually quite large and would therefore violate the linearization assumption. Based on the knowledge that the BOLD signal is primarily a result of changes in the local deoxyhemoglobin content, we modeled the BOLD response as the volume-weighted sum of signals from four compartments: an extravascular tissue compartment and three blood compartments separated into arteries, capillaries and veins to allow for differences in the blood volume distribution between the three blood compartments at baseline (ωa,c,v) and the fractional changes with activation (φa,c,v). This detailed analytic steady-state BOLD prediction model (the “detailed model”) allows for arbitrary changes in CBF and CMRO2 as well as arbitrary coupling of CBV and CBF. We do not explicitly examine transient aspects of the BOLD signal, and instead focus on approximate steady-state conditions, corresponding to typical calibrated BOLD applications using block design stimulus presentations. Furthermore, we make no assumptions about the relationship between CBF and CMRO2 responses. Instead, CBF and CMRO2 are varied independently allowing examination of the full range of BOLD effects. Finally within our model, we include the ability to alter hematocrit (Hct), baseline O2 extraction fraction (OEF0), echo time (TE), the intrinsic intravascular to extravascular signal ratio (λ), the intrinsic extravascular signal decay rate (), and arterial O2 saturation (SAO2) in order to more accurately model the BOLD effect and increase our understanding of the relationship between BOLD, CBF and CMRO2.
We addressed the following questions using the detailed model:
By allowing α and β to be free parameters, can the Davis model be optimized to improve its performance in calculating changes in oxygen metabolism?
How large are the errors in oxygen metabolism calculations associated with using the Davis model (with either the original or with optimized values for the parameters α and β), and do these errors vary for different coupling ratios of CBF and CMRO2 changes?
How sensitive are CMRO2 calculations to variations of the physiological state, particularly the blood volume distribution at baseline and the balance of arterial and venous CBV changes with activation?
If the assumptions underlying the hypercapnia calibration experiment (no effect on CMRO2 and the same relationship between CBF and CBV as during neural activation) are incorrect, how will this impact calculations using the Davis model?
Our primary finding is that despite the oversimplifications of the Davis model, the same mathematical form of the Davis model with corrections to α and β adequately captures the behavior of the much more complex physiology described by the detailed model. The classic Davis model is reasonably accurate for most activation experiments in which both CBF and CMRO2 increase in parallel, without optimization of α and β provided that M is accurately measured. However, studies of the response to drugs such as caffeine have shown more divergent effects, lowering CBF and possibly increasing CMRO2, which leads to inaccuracies in calculations by the original Davis model. Parameter optimization improves the accuracy of the Davis model across the full range of potential CBF and CMRO2 responses. By permitting α and β to be free parameters, though, their physical interpretation becomes complicated in that they no longer correspond to their original meaning. While variation of many of the physiological parameters has a strong effect on the magnitude of the BOLD response, this effect is adequately captured in the scaling parameter M, so that Davis model estimates of CMRO2 change remain relatively accurate even with fixed α and β. The primary physiological parameters for which this is not true are related to CBV, both the baseline distribution between compartments and the balance between arterial and venous changes. Additional errors due to assumptions about the hypercapnia calibration are largest when the CBF change is much larger than the CMRO2 change. These results emphasize the necessity of accurately determining how CBV distributes with activation and also the importance of measuring M values accurately for each subject. In this paper, our focus is on the Davis model, and its ability to calculate CMRO2 changes from BOLD and CBF data, because the Davis model is particularly well-suited for the calibrated BOLD experiment. In future studies, we will examine the effects of hypoxia and the accuracy of other models in predicting the BOLD signal.
2. Theory
The Davis model for calibrated-BOLD is presented and the associated assumptions are analyzed. A detailed four-compartment model for the BOLD response is developed and implemented using Matlab. Note that upper case variables describe absolute quantities (e.g., F is CBF, the blood inflow to the arteriolar compartment in units of ml blood/ml tissue-sec, and Va is the arteriolar blood volume fraction in units of ml blood/ml tissue), while lower case variables represent the same quantity normalized to its baseline value (e.g., f and va are dimensionless and equal to 1 at rest). Similarly, Δ denotes an absolute change while δ specifies a percent change from the baseline state.
2.1 The Davis model
With this nomenclature in mind, the Davis model relates f and normalized CMRO2, r, to the percent change in the BOLD signal (δS) using the following equation:
| [1] |
As originally derived, the mathematical form in the brackets represents the idea that the signal is modulated both by changes in the volume of blood containing deoxyhemoglobin (modeled with the first term in f relating CBV to CBF by a power law) and also by the magnetic susceptibility of the blood (modeled by the second term in r and f).
The r/f term models the susceptibility effect due to a change of deoxyhemoglobin concentration in the venous blood. The parameter β was introduced based on Monte Carlo simulations of water molecule diffusion around magnetized vessels to describe how the signal depends on the susceptibility. This dependence is expected to differ between large and small vessels because of the effects of diffusion on the extravascular signal change near the smaller vessels. The typical value used is β=1.5 (Davis et al., 1998), although it has been argued that the value should be reduced at higher magnetic field strengths (Boxerman et al., 1995; Chiarelli et al., 2007b; Ogawa et al., 1993).
The third parameter, M, essentially lumps together a number of effects that modulate the magnitude of the BOLD response, including aspects of local brain physiology and image acquisition parameters. In particular, M varies with the amount of deoxyhemoglobin present in the baseline state. It is typically calculated from an additional experiment using combined BOLD and CBF measurements obtained in response to hypercapnic stimulation. This assumes there is no change in CMRO2 (r=1) and that CBV with activation (AC) and with hypercapnia (HC) changes in the same way in response to CBF changes (φHC=φAC) as noted above.
2.2 Development of a Detailed Model of the BOLD Response
The calibrated BOLD methodology depends on an accurate model of how the BOLD signal is related to the mismatch between CBF and CMRO2, which is often expressed as the coupling parameter, n, defined as the ratio of the fractional changes (e.g., a 50% increase in CBF accompanied by a 20% increase in CMRO2 would be n=2.5). The model presented here, following the approach by Uludag et al. (2009), separates the signal into four compartments: one extravascular and three intravascular. Previous models have included multiple compartments to varying degrees (Blockley et al., 2009; Donahue et al., 2009; Obata et al., 2004). The Davis model considers only the extravascular compartment, neglects blood volume exchange effects, and assumes CBV changes are uniformly distributed across vascular compartments. The Obata model (Obata et al., 2004) explicitly includes both intravascular and extravascular components, however it only includes exchange effects with venous CBV thereby neglecting significant changes occurring in the arterial vasculature. More recent models examined effects of both arterial and venous compartments on the BOLD signal, but neglected effects of the capillary compartment (Donahue et al., 2009; Lin et al., 2008). These models also do not account for differing dependencies of arterial and venous CBV on CBF, variable baseline distribution of CBV, differences in the intravascular to extravascular proton density, and baseline extravascular signal decay rate constant ().
The basic signal equation for the detailed model is derived in the Appendix with the result shown in equations [2] and [3]:
| [2] |
where
| [3] |
Here, V denotes the volume fractions of the individual compartments (E=extravascular, I=intravascular, A=arterial, C=capillary, and V=venous). Baseline is denoted by the subscript “0” (V0). The parameter ε is the signal ratio at baseline of an intravascular volume to extravascular volume (e.g. εv is the signal ratio in the experiment between equal volumes of venous blood and extravascular tissue). TE is the echo time of the MR measurement, and is the change in the MR signal relaxation rate with the stimulus for each of the four compartments, three intravascular and one extravascular.
Volume fractions were determined by examining a range of total baseline intravascular volume fractions (VI,0) and fractional distributions (ω) as shown in Table 1. Changes in total CBV were modeled as in Grubb et al. (1974) by assuming a power law relationship to CBF with exponent φ. Distributions of ΔCBV to the compartments containing deoxyhemoglobin were calculated separately using different exponents (φc and φv) for capillary and venous ΔCBV respectively. Ranges of φ and φv were examined while φc was assumed to be half of φv reflecting small changes in capillary CBV (Stefanovic et al., 2008). Arterial ΔCBV was calculated as the remainder of total ΔCBV after subtraction of capillary and venous ΔCBV. Next, a range of hemoglobin saturations (SO2) was studied for each vascular compartment: venous O2 saturation (SVO2) was calculated from SAO2 across a range of f, r, and OEF0; capillary O2 saturation (SCO2) was calculated as a weighted sum of SAO2 and SVO2. Values of for the blood and tissue compartments were calculated based on relationships from the literature, which incorporate the effects of Hct, SO2, baseline OEF and CBF. Equations (2) and (3) thereby allow us to simulate the BOLD signal across a wide range of physiological states.
Table 1. Parameters defining the standard physiology.
Values without a marker are assumed. Values marked with † are calculated using the assumed values and the detailed model.
| Variable | Standard value (range tested) |
Description |
|---|---|---|
| TE | 32 ms (20-40 ms) | Echo time |
| V I,0 | 0.05 (0.01-0.15) | Total blood volume fraction at baseline (Roland et al., 1987) |
| ω a | 0.2 (0-0.4) | Arterial fraction of baseline total blood volume (Weber et al., 2008) |
| ω c | 0.4 (0.6-0.2) | Capillary fraction of baseline total blood volume (Weber et al., 2008) |
| ω v | 0.4 (0.2-0.6) | Venous fraction of baseline total blood volume (Weber et al., 2008) |
| φ | 0.38 (0.3-0.65) | Grubb’s constant relating CBF to total CBV (Grubb et al., 1974) |
| φ c | 0.1 (0-0.33) | Exponent relating CBF to capillary CBV (Stefanovic et al., 2008) |
| φ v | 0.2 (0-0.65) | Exponent relating CBF to venous CBV (Chen and Pike, 2009a) |
| Va(t) | 0.016 | †Arterial blood volume with activation (Section 3.1) |
| Vc(t) | 0.021 | †Capillary blood volume with activation from Grubb relation and φc |
| Vv(t) | 0.022 | †Venous blood volume with activation from Grubb relation and φc |
| OEF0 | 0.4 (0.25-0.65) | Resting oxygen extraction fraction (Marchal et al., 1992) |
| κ | 0.4 | Fraction of capillary blood considered to be “arterial” (Tsai et al., 2003) |
| SAO2 | 0.98 | Arterial oxygen saturation (Schutz, 2001) |
| SCO2 | 0.74 | †Capillary oxygen saturation at baseline (Eqn. A8) |
| SVO2 | 0.59 | †Venous oxygen saturation at baseline (Eqn. A6 and A7) |
| Hct | 0.44 (0.25-0.65) | Resting hematocrit of arteries and vein (Gustard et al., 2003) |
| Hctc | 0.33 (0.19-0.49) | †Resting hematocrit of capillaries calculated from Hct (Sakai et al., 1985) |
| A* | 21.2 s−1 | †Constant term in quadratic dependence of intravascular on Hct for arteries and vein (Zhao et al., 2007) |
| 19.7 s−1 |
†Constant term in quadratic dependence of intravascular on Hct for capillaries (Zhao et al., 2007) |
|
| C* | 174.7 s−1 |
†Quadratic term in quadratic dependence of intravascular on Hct for arteries and vein (Zhao et al., 2007) |
| 142.7 s−1 |
†Quadratic term in quadratic dependence of intravascular on Hct for capillaries (Zhao et al., 2007) |
|
| 21.3 s−1 | †Resting arterial rate of signal decay (Eqn. A5) (Zhao et al., 2007) | |
| 28.9 s−1 | †Resting capillary rate of signal decay (Eqn. A5) (Zhao et al., 2007) | |
| 50.9 s−1 | †Resting venous rate of signal decay (Eqn. A5) (Zhao et al., 2007) | |
| 25.1 s−1 | Resting extravascular rate of signal decay (Perthen et al., 2008) | |
| 0 s−1 | †Change in arterial signal decay rate with activation (Eqn. A9) | |
| −3.1 s−1 | †Change in capillary signal decay rate with activation (Eqn. A9) | |
| −10.2 s−1 | †Change in venous signal decay rate with activation (Eqn. A9) | |
| −0.4 s−1 | †Change in extravascular signal decay rate (Eqn. A10: (Ogawa et al., 1993) | |
| λ | 1.15 (0.9-1.3) | Intravascular to extravascular spin density ratio determined experimentally |
| ε a | 1.30 | †Ratio of baseline intravascular arterial to extravascular signal (Eqn. A2) |
| ε c | 1.02 | †Ratio of baseline intravascular capillary to extravascular signal (Eqn. A2) |
| ε v | 0.50 | †Ratio of baseline intravascular venous to extravascular signal (Eqn. A2) |
| γ | 2.68 × 108 | Gyromagnetic ratio of protons |
| Δ χ | 2.64 × 10-7 | Susceptibility of fully deoxygenated blood (Spees et al., 2001) |
| B0 | 3 T | Magnetic field strength |
| SO2,off | 0.95 | Blood saturation for equal tissue-blood susceptibility (Spees et al., 2001) |
denotes calculated parameters at n=2.5 (δCBF=50%, δCMRO2=20%)
3. Simulations and Results
Following the outline presented in Figure 1, we determined a set of typical physiological values for the detailed model inputs (Table 1), and used this simulation to optimize the Davis model parameters α and β. Next, we used the detailed model to simulate changes to the BOLD activation and hypercapnia responses as the physiological parameters vary. The focus of the simulations was to test the accuracy of CMRO2 estimates from the calibrated BOLD experiment using the Davis model, although the effect of different physiological parameters (e.g., hematocrit) on the BOLD response is interesting in itself. Here, we are essentially asking the question of whether the simple calibration step accurately captures the variability of the BOLD response due to variation in particular physiological parameters.
Figure 1. Work flow diagram showing development and implementation of the detailed model.
(a) A standard physiological set was defined including blood volume fractions, hemoglobin content and saturation, tissue properties and signal acquisition parameters (Table 1). (b) Following the approach used by Uludag et al. (2009), a detailed four-compartment model was developed (Appendix). (c) BOLD data were generated using this detailed model. (d) The BOLD signal simulated for the standard reference physiology was normalized to the signal at fHC=1.6 and rHC=1.0 (δSHC). This is the simulated hypercapnia (HC) signal. The Davis model equation was similarly normalized in order to reduce the number of free parameters from three including M down to two (α and β). (e) Non-linear parameter optimization was performed using the Matlab function fmincon. The BOLD signal produced by the optimized Davis model is now shown. (f) By analyzing BOLD data generated using the detailed model, the efficacy of the Davis model was determined by its ability to calculate CMRO2 changes accurately.
3.1 Producing Simulated BOLD Data for a Standard Subject
For imaging parameters, we assumed a 3T system with TE=32 ms. For the standard subject, the total intravascular volume fraction at rest was assumed to be VI,0=0.05 (Roland et al., 1987). The fractional distribution of CBV at baseline (ω) between arteries, capillaries and veins was assumed to be ωa=0.2, ωc=0.4 and ωv=0.4 consistent with intra-cortical measurements of CBV distribution (Weber et al., 2008). The exponent relating f to total v was assumed to be φ=0.38 (f=vφ) as in Grubb et al. (1974). The venous CBV was assumed to change by a smaller fraction than the total CBV change, with the exponent φv=0.2 relating normalized venous volume (vv) to f (Chen and Pike, 2009a). The capillary volume change exponent was assumed to be half of φv (φv=0.1) (Stefanovic et al., 2008). Arterial ΔCBV was then calculated to be the remainder of total ΔCBV resulting in an effective φa=1.1 at f=1.5.
Venous oxygenation at baseline was determined from equation A7 (Appendix) assuming SAO2=98% (Schutz, 2001) and OEF0=0.4 (Marchal et al., 1992). Activation SVO2 was determined across a range of f and r using equations A6 and A7 (Appendix). Capillary oxygen saturation was calculated from SAO2 and SVO2 using equation (A8) with κ=0.4 effectively giving a slightly greater weight to venous blood (Tsai et al., 2003). Hct was set to 0.44, and the baseline tissue relaxation rate constant () was assumed to be 25.1 s−1 based on previous 3T studies from our group (Perthen et al., 2008). Changes in were calculated using equations A9 and A10. Table 1 includes the full list of standard parameter values and resulting intermediaries such as values of associated with each compartment.
The intravascular to extravascular intrinsic signal ratio was experimentally determined using a GE Signa Excite 3-T whole-body system with a body transmit coil and an 8-channel receive-only head coil. Two subjects were imaged using a high-resolution flow-compensated spoiled-GRASS protocol with TR/TE/α=4000 ms/5.4 ms/10°. Images were viewed using AFNI (Cox, 1996). Intravascular measurements were taken from the sagittal sinus while extravascular measurements were taken from the visual cortex gray matter. The spin density ratio was then calculated from the signal ratio to be 1.15. This fraction allows calculation of the intrinsic intravascular to extravascular signal ratio using equation A2 (Appendix). For arteries, capillaries and veins, this resulted in intrinsic signal ratios of εA=1.30, εC=1.02, and εV=0.50 (Table 1). With these values, volume exchange effects will add to the BOLD response for arterial changes, subtract from the BOLD response for venous changes, and have little effect for capillary changes.
3.2 Optimization of the Davis Model
Numerical simulations
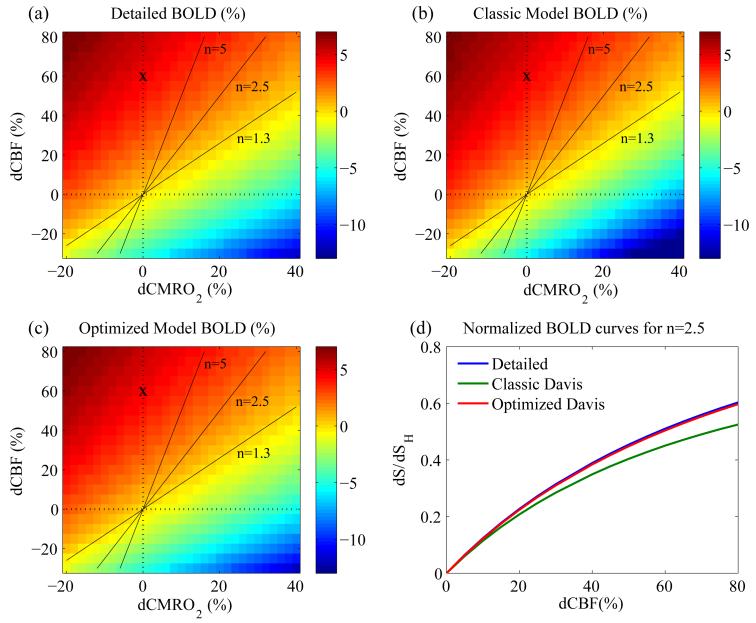
For this standard set of parameters, the detailed 4-compartment model was used to calculate the BOLD signal for arbitrary changes in CBF (f) and CMRO2 (r) (Fig. 2a). In the current simulations, f was varied from 0.7 to 1.8 and r was varied from 0.8 to 1.4. BOLD hypercapnic calibration was simulated for f=1.6 and r=1 (marked by ‘x’ on Fig. 2); this is a typical CBF change measured for 5% CO2 inhalation (Ances et al., 2009; Perthen et al., 2008). Optimization of α and β of the Davis model was performed by normalizing the simulated BOLD data to the simulated hypercapnic BOLD signal. The Davis model equation was similarly normalized to this hypercapnia (subscript HC) value of δSHC and fHC=1.6 thereby eliminating the scaling parameter, M (Fig. 1d). Using the Matlab function fmincon, non-linear parameter optimization of α and β was performed using this simulated and normalized BOLD signal associated with the standard subject. Fitting was performed across the entire δCBF-δCMRO2 plane thereby making α and β insensitive to specific values and coupling ratios of blood flow and oxygen metabolism.
Figure 2. Simulated BOLD response using the three models (detailed, classic Davis and optimized Davis).
Dashed lines correspond to δCBF=0% and δCMRO2=0%. Solid lines represent the coupling parameters n=1.3, n=2.5, and n=5. The line for n=1.3 is approximately where the BOLD signal is 0. The ‘x’ marks the assumed response for an ideal hypercapnia calibration experiment. (a) BOLD response simulated by the detailed model. (b) BOLD response simulated by the classic Davis model. The contours are similar, although there is a very slight difference in the slope, and a general underestimation of the BOLD signal most apparent in the bottom right corner where flow is decreased and O2 metabolism is increased. (c) BOLD response simulated by the optimized Davis model closely matches the simulation by the detailed model. (d) Normalized BOLD signals at n=2.5 for the three models. Normalizing the BOLD signal to the simulated hypercapnia BOLD signal removes dependence on M in the Davis models and shows that the classic Davis model (green) underestimates the BOLD signal. In contrast, the optimized Davis model (red) fits the simulated data from the detailed model (blue) very well.
Simulation results
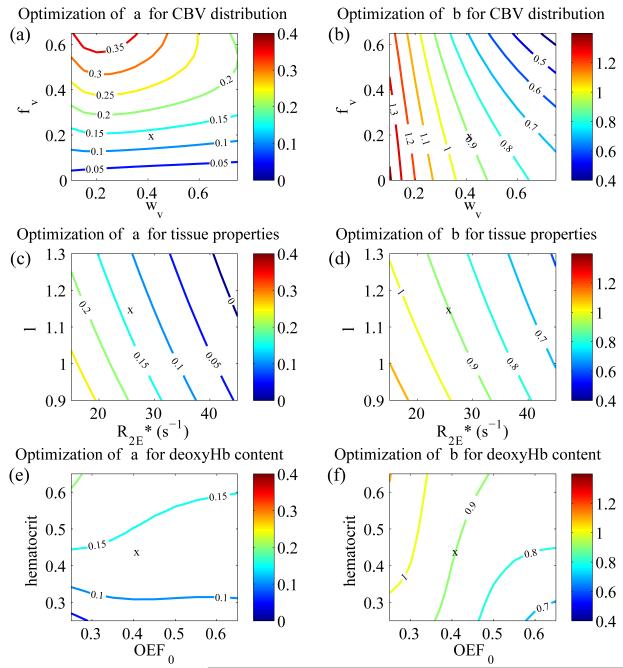
The optimized values of α=0.14 and β=0.91 are designated the “optimized” parameters while the original values of α=0.38 and β=1.5 are designated the “classic” parameters. The Davis models associated with these parameters are similarly notated. The BOLD surfaces predicted with the Davis model using the optimized and classic parameter values are shown in Figure 2b and c. The classic Davis model predicts a modest systematic difference in the BOLD signal while the optimized Davis model produces a signal nearly identical to that of the detailed model. We also performed this optimization of α and β for large ranges of the detailed model inputs: φv=0-0.65, ωv=0.2-0.6, , λ=0.9-1.3, Hct=0.25-0.65, and OEF0=0.25-0.65 (Fig. 3). Of these physiologic variables, the best-fit value of α shows the strongest dependence on φv (Fig. 3a) while β shows the strongest dependence on ωv (Fig. 3b). The fitted α and β showed somewhat weaker dependence on tissue properties and baseline deoxyhemoglobin content (Fig. 3c-f). Optimized α is approximately linearly dependent on these parameters. Simultaneously, β shows quadratic dependence on baseline CBV distribution (ωv) and linear dependence on φv, the tissue parameters ( and λ), and the baseline deoxyhemoglobin content parameters (Hct and OEF0). α and β are much less dependent on the other inputs to the detailed model (see supplementary data, Fig. S1).
Figure 3. Dependence of Davis model α and β on CBV distribution, tissue properties, and hemoglobin content and saturation.
‘x’ marks the location defined by the standard parameter set where α=0.14 and β=0.91 are the best-fit values. Physiological inputs to the detailed model with the highest impact on the calibrated BOLD experiment were varied in pairs to determine their effect on optimization of the Davis model α and β. (a) CBV distribution at baseline (ωv) has only a small impact on optimization of α, while CBV distribution with activation (φv) has the biggest effect on optimized α. The relationship between α and φv is mostly linear based on the spacing of contour lines especially for φv<0.4 and ωv<0.4. (b) Variation in ωv has the largest effect on the optimized β. This relationship is more nonlinear since the spacing of the contour lines grows as ωv increases. ωv has a much smaller impact on β. (c-d) Tissue properties and λ have a smaller impact on optimization of α and β. The relationship between these tissue properties and the parameters appears to be mostly linear. (e-f) Hematocrit and baseline oxygen extraction fraction are shown to have an even smaller effect on α and β.
3.3 Error in δCMRO2 Calculations Associated with the Davis Model α and β
Numerical simulations
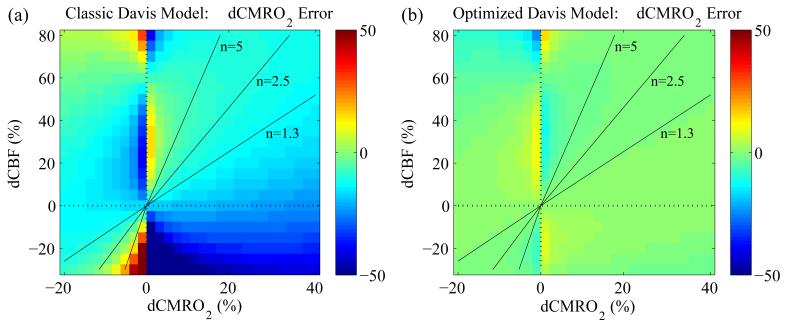
Given the classic and optimized values for α and β, we then tested the primary question related to the calibrated BOLD experiment: how large are the errors in estimation of CMRO2 change when using the Davis model, and does this depend on the particular combination of CBF and CMRO2 changes? For the second part of the question, we were particularly interested in three combinations of CBF/CMRO2 coupling, which we can characterize by the coupling ratio n: δCBF/δCMRO2. The first is in the region n=2.5 (δCBF=50%, δCMRO2=20%), corresponding to activation results in a number of calibrated BOLD studies using the classic Davis model (Perthen et al., 2008; Stefanovic et al., 2004). The second is n=5 (δCBF=50%, δCMRO2=10%), a higher ratio that some PET studies have found (Ito et al., 2005). The third was motivated by studies of the effects of caffeine, which lowers CBF and raises CMRO2 (δCBF=-25%, δCMRO2=30%, n=−0.8) (Griffeth et al., 2011). In these simulations we assumed that hypercapnia changes only CBF, with no effect on CMRO2. To simulate the calibrated BOLD experiment, M values for both the classic and optimized Davis models were determined using eqn. [1] and the BOLD response from the detailed model at fHC=1.6 and rHC=1.0 (marked with an ‘x’ in Figure 2a). This results in values of M=11.1 and M=14.9 for the classic and optimized Davis models respectfully. Using these values for M, The two Davis models were then compared in their ability to accurately predict δCMRO2 from the simulated BOLD and CBF data by comparing the percent error from actual δCMRO2. Errors in δCMRO2 estimates by both models were determined using eqn. [5]:
| [5] |
Simulation results
Errors in estimated δCMRO2 are displayed across the δCBF-δCMRO2 plane in Figure 4. Note that both δCMRO2 and ζ are percentages, although δCMRO2 is the percent change in absolute CMRO2 while ζ is the percent error relative to the actual percent change (e.g., if true δCMRO2 is 10% and the estimated δCMRO2 is 15%, the percent error is ζ=50%). Errors for specific combinations of δCBF and δCMRO2 are listed in Table 2. It is clear that the classic Davis model using α=0.38 and β=1.5 systematically underestimates δCMRO2 (Figure 4a and Table 2, column 1). Along the coupling parameter contours of n=2.5 and n=5, this underestimation is close to −10%. Specifically for n=2.5 (δCBF=50% and δCMRO2=20%), the classic Davis model underestimates δCMRO2 by −9.8% such that it reports a change of 18.0% when it is actually 20%. Even for a much larger value of the coupling parameter such as n=5.0 (δCBF=50% and δCMRO2=10%), the Davis model is only −6.9% in error. Yet, the optimized model improves the accuracy of CMRO2 calculations. For δCBF=50% and δCMRO2=20%, the Davis model with optimized parameters predicts δCMRO2=19.7% (ζ=−1.3%) while for a higher coupling of δCBF=50% and δCMRO2=10%, the optimized model returns δCMRO2=9.8% (ζ=−2.5%). For the region with decreased CBF (−25%) but increased CMRO2 (30%) the error using the classic model was ζ=−38.0% while with the optimized model it was reduced to −1.2%.
Figure 4. Percent error (ζ ) in δCMRO2 calculations using the classic and optimized Davis model parameter sets.
(a) Using the classic Davis model, δCMRO2 is consistently underestimated as indicated by the turquoise areas along the n=2.5 and n=5 coupling parameter lines. (b) The optimized parameters of α=0.14 and β=0.91 produce much more accurate estimates of δCMRO2 across the entire expanse examined. This is apparent from the green coloring across the majority of the δCBF-δCMRO2 plane corresponding to zero error. Errors around δCMRO2=0 are artifacts of division by a small number.
Table 2. Error in δCMRO2 and n as calculated by the classic and optimized Davis models for the standard subject as defined in Table 1.
While the optimized Davis model is best for all couplings of δCBF and δCMRO2, the classic Davis model with the true value of M also produces reasonable estimates of δCMRO2 around n=2.5 and n=5.0. Calculations using the classic Davis model parameters with a biased M (as might be determined from a hypercapnia calibration) are also given, and at n=2.5 this model is also quite accurate. However, the classic Davis model with the biased M is not accurate for n=5.0 or n=−0.8. Even the classic model with the true M is not accurate for n=−0.8.
| Classic α and β | Optimized α and β | Classic α and β Biased M (rHC=0.9) |
|
|---|---|---|---|
| Typical functional activation (MRI): (n=2.5) δCBF = +50% δCMRO2 = +20% |
δCMRO2 = 18.0% ξ = −9.8% n = 2.8 |
δCMRO2 = 19.7% ξ = −1.3% n = 2.5 |
δCMRO2 = 21.0% ξ = 5.1% n = 2.4 |
| Typical functional activation (PET): (n=5.0) δCBF = +50% δCMRO2 = +10% |
δCMRO2 = 9.3% ξ = −6.9% n = 5.4 |
δCMRO2 = 9.8% ξ = −2.5% n = 5.1 |
δCMRO2 = 13.9% ξ = 38.9% n = 3.6 |
| Typical response to caffeine: (n=−0.8) δCBF = −25% δCMRO2 = +30% |
δCMRO2 = 18.6% ξ = −38.0% n = −1.3 |
δCMRO2 = 29.6% ξ = −1.2% n = −0.8 |
δCMRO2 = 12.7% ξ = −57.7% n = −2.0 |
3.4 Error in δCMRO2 Calculations Due to Physiological Variation
Numerical simulations
The previous simulations showed that the Davis model with optimized α and β works well for estimating δCMRO2 for the physiological parameter set defined in Table 1. In practice there is likely to be substantial variation of these parameters across subjects even for a healthy population. As described in section 3.2, we found that the optimal values of α and β do depend on these physiological parameters, and in a typical experiment there will not be sufficient information available to adjust the values of α and β. The important question for the calibrated BOLD method is then: if we adopt the values of α and β optimized for our “best guess” at the underlying physiology (Table 1), how large are the errors in the estimate of δCMRO2 if these physiological parameters vary? To assess the errors in the estimation of δCMRO2 we varied the value of each physiological parameter in the detailed model and then simulated the calibrated BOLD experiment to measure δCMRO2 with either the classic or optimized Davis models. The physiological parameters were varied over the following ranges: φv=0-0.65, ωv=0.2-0.6, , λ=0.9-1.3, Hct=0.25-0.65, OEF0=0.25-0.65, VI,0 (0.01-0.1), φ (0.3-0.65), TE (20-40 ms), and λ (0.9-1.3). A range for φc (0-0.3) was also examined but found to have little effect on calculations of δCMRO2. Therefore, φc was assumed to be equal to half of φv consistent with small changes to capillary volume during activation. Simulated hypercapnia calibration was repeated for each variation in order to calculate M; note the classic value of M is similar to what is typically calculated for experimental data using the Davis model (Fig. 5). The impact of the physiological variations was assessed for two scenarios of combined CBF and CMRO2 change: n=2.5 and n=−0.8 simulating the effects of caffeine. Both of these combinations were analyzed with the two Davis models to determine how accurate the models’ estimates of δCMRO2 would be, i.e. how close to δCMRO2=20% or 30% (Table 2).
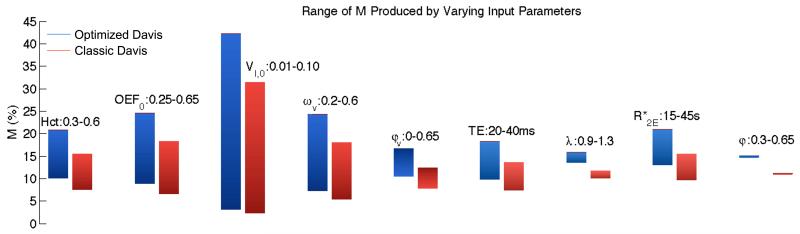
Figure 5. Range of M for classic and optimized Davis models as input parameters vary.
(The parameters are: Hct=hematocrit, OEF0=baseline O2 extraction fraction, VI,0=baseline CBV fraction, ωv=baseline venous CBV fraction, φv=exponent relating venous CBV changes to CBF, TE=echo time, λ=intravascular to extravascular spin density ratio, =intrinsic extravascular signal decay rate, and φ=exponent relating total CBV changes to CBF.) Values for M associated with the standard physiology for the two models are classic M=11.1 and optimized M=14.9. Note the gradient of the bars corresponds to the ranges of the input parameters; the lowest values of the inputs are associated with the dark end of the bars and the highest values are associated with the light end of the bars. Specifically note the large variation in M due to VI,0. Also as expected doubling TE approximately doubles calculated M. There is much smaller variation in M for φv, but sizeable variation in M due to Hct, OEF0, and ωv, consistent with the dependence of M on baseline deoxyhemoglobin-containing blood volume and deoxyhemoglobin concentration. Finally, the tissue relaxation rate constant, , also has a noticeable impact on M as expected.
Simulation results
For n=2.5, varying φv in the range of 0-0.65 has the largest impact on δCMRO2 estimates with the classic model estimating δCMRO2=15.0-30.0% and the optimized model estimating δCMRO2=16.2-34.2% (Fig. 6a). Variations in δCMRO2 calculations produced by Hct, ωv, and were secondary, while the smallest impacts on δCMRO2 calculations were produced by altering VI,0 and φ (see supplementary data, Table S1 for exact ranges). This was despite very large ranges of VI,0 (0.01 to 0.1 while literature values are typically about 0.05) and φ (0-0.65). At n=−0.8, φv again had a significant influence. However at this combination of δCBF and δCMRO2, ωv in the range of 0.2-0.6 produced the largest variance while (15-45 s−1) also had a sizeable effect (Fig. 6b). Meanwhile, the impact of λ, OEF0 and to a lesser extent VI,0 also increased above that of Hct and TE. φ still had very little impact.
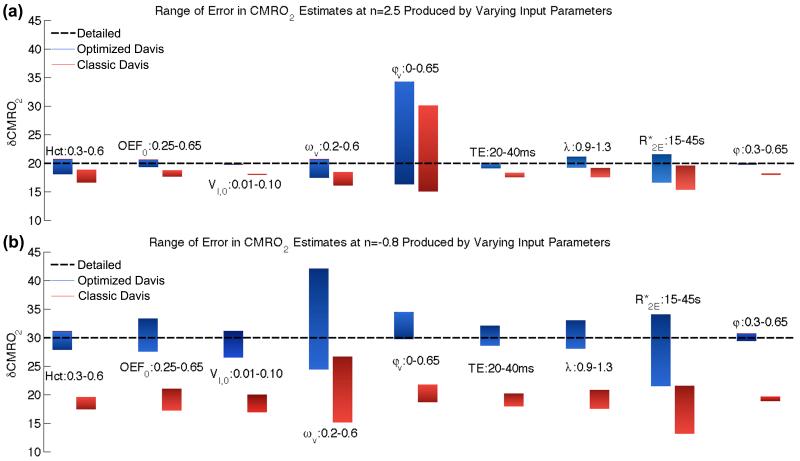
Figure 6. Range of error in estimated δCMRO2 due to single parameter variation.
The nine input parameters to the detailed model again were altered around the standard values in Table 1 (see Introduction or Fig. 5 caption for parameter definitions). (a) At n=2.5 (δCBF=50%) the classic Davis model shows a systematic underestimation of δCMRO2 from the true value of 20.0% to 18.0% (ζ=−9.8%). The optimized Davis model yields a more accurate estimate of δCMRO2. Altering φv has the largest impact on the Davis model estimation (classic: 15.0-30.0%, max ζ=50.1% and optimized: 16.2-34.2%, max ζ=71.0%). Variations in Hct, ωv, and also have sizeable although much smaller effects on both models (see Table S1 for exact ranges). Variations due to VI,0, TE, and φ are exceptionally small and are accounted for by the scaling parameter. The pattern of error at n=5.0 is similar to the pattern of error for n=2.5. (b) In the case of n=−0.8, ωv and baseline have the largest effect on δCMRO2 calculations followed by OEFM0, λ, and φv. The other parameters all also have a larger impact on δCMRO2 calculations around this coupling of CBF and CMRO2 suggesting that the mathematical form of the Davis model is not as reliable in this area of CBF-CMRO2 coupling.
As it was determined that the venous CBV change (φv) had the biggest impact on the accuracy of the Davis model, we more closely examined how variations in φv affected the Davis model for the cases of φv=0, 0.2, 0.38, and 0.65. Again, the hypercapnia experiment was simulated using BOLD data from the detailed model to calculate M for each case. We then analyzed the simulated BOLD data associated with the standard subject across the δCBF-δCMRO2 plane for each value of φv using the two Davis models. Using eqn. 5, we calculated the percent error associated with each point in the f-r plane (Fig. 7). As expected, the optimized Davis model fits best at φv=0.2, but overestimates changes in CMRO2 if φv=0.38 or 0.65, and underestimates changes in CMRO2 when φv=0. Although not shown, the classic Davis model fits the simulated data for φv=0.38 somewhat better but is still not as accurate as the optimized model at φv=0.2.
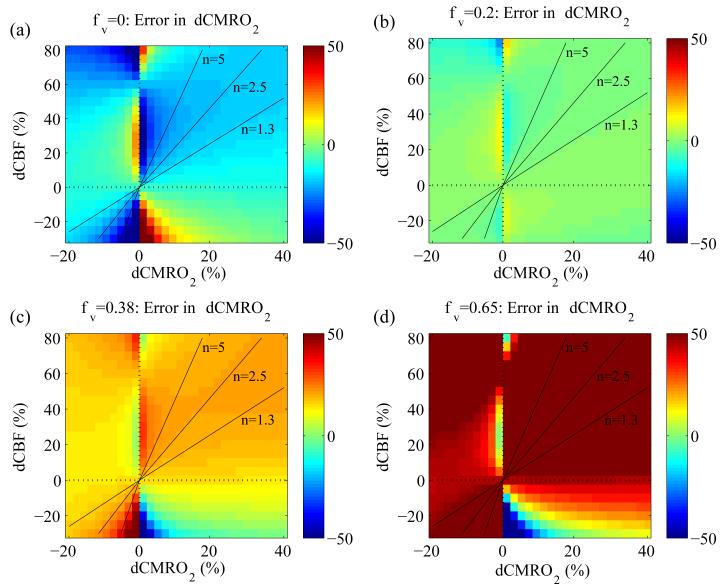
Figure 7. Percent error in δCMRO2 calculations using the optimized Davis model parameters (α=0.14 and β=0.91) as the venous CBV change with activation (φv) is varied.
(a) For φv=0, none of the total ΔCBV is distributed to the veins or capillaries. In this case the optimized Davis model underestimates CMRO2. (b) As noted, recent research suggests φv is lower than total φ such that some ΔCBV distributes to the capillaries and veins, but most goes to the arteries. This plot is identical to Fig. 2b, and as expected due to the parameter optimization it shows that the modified Davis model provides a more accurate estimate of δCMRO2 when most CBV distributes to the arteries. (c) When φv=0.38, ΔCBV distributes equally to all compartments, and in this case the optimized Davis model overestimates CMRO2. (d) If venous CBV changes are much larger, such that φv=0.65, the optimized Davis model grossly overestimates δCMRO2. This is consistent with the findings of Lin et al. (2008).
To address the finding by Lin et al. (2008) that φ=0.65 with activation, we examined in more detail how this would affect δCMRO2 calculations. We repeated the process used to produce Figure 7 for both the classic and optimized Davis models but set φ=0.65 rather than φ=0.38 and combined this with the same four values of φv. The images for φ=0.65 are not shown, since they are practically identical to those in Figure 7 (φ=0.38). This was expected due to the minimal impact φ had on δCMRO2 calculations as shown in Figure 6. This suggests that the venous volume change, rather than the overall volume change, is the key physiological parameter that determines the accuracy of the Davis model estimates of δCMRO2.
3.5 Potential Error in δCMRO2 Calculations Associated with Hypercapnia Calibration
Numerical simulations
The hypercapnia calibration process assumes that inhalation of low levels of CO2 (typically ~5%) does not affect CMRO2 in the brain. In calculations up to this point, we assumed our method of calibration was accurate, but more recent experiments on hypercapnia calibration suggest that inhalation of 5% CO2 decreases CMRO2, with recent estimates giving this decrease between −13% and −26% (Bolar et al., 2010; Xu et al., 2011) for CBF changes of 55% and 32% respectively. To simulate this, we calculated the hypercapnic BOLD signal from the detailed model simulation for fHC=1.6 and rHC varied from 0.8 to 1.1, but we calculated M from equation [1] assuming rHC=1. Additionally, the dependence of CBV on CBF may differ between neural activation and hypercapnia (Chen and Pike, 2010). To simulate this error, we took the hypercapnic BOLD signal from the detailed model simulation for fHC=1.6 and rHC=1.0 but varied φvHC from 0 to 0.4. Again, we analyzed this signal using equation [1] to calculate M but still using α=0.14 and β=0.91, which were optimized for φvHC=0.2.
Simulation results
For the standard subject, the true M for the classic Davis model was determined to be 11.1%, but if hypercapnia calibration decreases CMRO2 by −10% (rHC=0.9) the scaling parameter would be overestimated (M=13.3%). We repeated the calculation of δCMRO2 for this biased M using the classic Davis model at the three values of n (2.5, 5.0 and −0.8). We found the error in δCMRO2 to be remarkably small at n=2.5 and δCMRO2=20% (21.0%, ζ=5.1%) such that the systematic error in M tends to correct systematic error in the classic α and β. At n=5.0 and δCMRO2=10%, this is not the case. This value of M leads to large error in δCMRO2 (13.9%, ζ=38.9%) and underestimate of n=3.6 (Table 3). At n=−0.8 and δCMRO2=30% instead of correcting the error associated with the classic α and β, the hypercapnia M magnified the error and grossly underestimated δCMRO2 (12.7%, ζ=−57.7%) (Table 2, column 3). Errors associated with biases due to both non-iso-metabolic hypercapnia calibration and concurrent variation of all other input parameters as in section 3.4 followed a similar systematic pattern of error (supplementary data, Table S1). The errors in δCMRO2 for different degrees of CMRO2 change with hypercapnia are shown in Figure 8a.
Figure 8. Systematic error in δCMRO2 calculations using the optimized Davis model due to errors in assumptions related to the effects of hypercapnia.
Dashed lines represent true δCMRO2. rHC is the normalized change in δCMRO2 due to hypercapnia (HC), normally assumed to be zero. φvHC is the exponent relating venous CBV to CBF for HC, normally assumed to be equal to the corresponding value for activation (0.2 in these calculations). (a) Deviation from the assumption that hypercapnia is iso-metabolic produces large errors in δCMRO2 calculations. A decrease in cerebral O2 metabolism with hypercapnia as some studies suggest will result in M being overestimated. This results in CMRO2 being overestimated for n=2.5 (green) and n=5.0 (blue) and underestimated for n=−0.8 (red). Interestingly when using the classic Davis model, Table 2 (column 3) shows that this bias in M allows for some correction due to the bias introduced by the classic parameters. (b) If the relationship between CBV and CBF between activation and hypercapnia experiments changes, this also leads to bias in the estimation of CMRO2. One study suggested that φvHC is less than φv due to activation (0.15 versus 0.23 respectively) (Chen and Pike, 2010). Again, this would overestimate δCMRO2 calculations associated with n=2.5 and n=5.0 and underestimate δCMRO2 for n=−0.8.
If the relationship between CBV and CBF between activation and hypercapnia experiments changes, this also leads to bias in M. One study suggested that φvHC is less than φv due to activation (0.15 versus 0.23 respectively) (Chen and Pike, 2010). If this is the case, then this would again lead to M being overestimated (M=11.4%). This error is much lower than the error associated with rHC=0.9, although it would result in δCMRO2 calculations associated with n=2.5 and n=5.0 also being overestimated while for n=−0.8 δCMRO2 will be underestimated (Fig. 8b).
4. Discussion
The calibrated BOLD method introduced by Davis and colleagues (Davis et al., 1998) offers one of the best noninvasive tools currently available for measuring changes in oxygen metabolism in the human brain with good spatial and temporal resolution. Estimating the fractional CMRO2 change between two states (e.g., baseline/activation or pre/post drug administration) requires three things: measurement of both the CBF and BOLD signal differences; a mathematical model of how the BOLD response depends on δCBF and δCMRO2; and a calibration experiment to determine an overall scaling factor for the BOLD response (M). Here, we examined the accuracy of these latter two requirements as they relate to the Davis model. This simple model describes the BOLD response as a function of δCBF and δCMRO2 with three parameters: M, α and β. It has become the standard model for the calibrated BOLD experiment, with α and β assumed to have fixed values of α=0.38 and β=1.5. M is determined from a hypercapnia experiment, with the assumption that mild hypercapnia alters CBF but not CMRO2. In this paper we have analyzed the accuracy of the Davis model with a specific focus on the expected errors in the estimate of δCMRO2 in the calibrated BOLD experiment. We developed a detailed analytical four-compartment model of the BOLD signal for a magnetic field strength of 3T that includes a number of physiological parameters that could potentially affect the BOLD response, including hematocrit, oxygen extraction fraction, cerebral blood volume, and the distribution of blood volume changes between arterial and venous vessels as CBF changes.
Our detailed model of the BOLD response is an expanded version of other modeling approaches, which have sought to improve upon our physiologic understanding of the dependence on changes in blood volume and deoxyhemoglobin (Blockley et al., 2009; Buxton, 2009; Lin et al., 2008; Obata et al., 2004; Stephan et al., 2007; Uludag et al., 2009). However, the way we have used the model in the current study is somewhat different. Although the detailed model is much more complete than the simple Davis model, models such as this are difficult to adapt directly to the calibrated BOLD experiment because there are too many unknown variables. The power of the Davis model is its simplicity, with many potential physiologic sources of variability lumped into a single scaling parameter M that is measured individually. Despite the number of physiological variables that affect the BOLD signal, the surface of BOLD signal as a function of δCBF and δCMRO2 is relatively smooth, so it is plausible that a simpler mathematical form such as the Davis model can provide a reasonably accurate approximation. To that end, we improved the Davis model by allowing α and β to be free parameters and performing non-linear parameter optimization to find the values that best fit the BOLD signal generated by the detailed model. By generating simulated data for a wide range of physiological conditions, we were able to test the ability of the Davis model, with either the classic or optimized values of α and β, to accurately calculate changes in δCMRO2. We examined the impact of both variations in the underlying physiology and systematic errors related to the assumptions about the effects of hypercapnia in the calibration experiment. In analyzing such errors it is important to note that they are not uniform for all combinations of δCBF and δCMRO2, and here we looked in detail at three values of the coupling ratio n (δCBF/δCMRO2): n=2.5 and n=5, corresponding to a range of values reported for stimulus responses, and n~−0.8, a value we recently found for the effects of caffeine (lowered CBF but raised CMRO2).
The key results are as follows:
The simulated data from the detailed model predict an M value close to what is typically observed, supporting the assumptions and parameter values underlying the model.
The Davis model fits this simulated data very well if α and β are allowed to be free parameters, although this requires that one abandon the physical meaning of these parameters as they were originally derived.
If there is physiological variation from the standard physiology used to optimize α and β, then the error expected in δCMRO2 calculations is largest for variation in the venous CBV at baseline and the venous CBV change with activation.
If hypercapnia reduces CMRO2, or produces smaller the venous volume changes for the same CBF relative to activation, then δCMRO2 will be overestimated for the activation response but underestimated for the caffeine response.
These results are discussed in detail below.
4.1 The Davis model scaling parameter is simulated well by the detailed model
For the values of α and β optimized to the standard physiology defined in Table 1, the classic Davis model analysis of the simulated data results in M=11.1%. In comparison, using the classic parameters and correcting for TE, a sampling of published hypercapnia M values range from 7.0 to 14.1 (Chiarelli et al., 2007b; Hoge et al., 1999; Mark et al., 2011; Perthen et al., 2008; Stefanovic et al., 2006). This demonstrates that the detailed model reasonably simulates the BOLD signal. The simulations also show that the exact value of M varies significantly with variation of the baseline physiological parameters, particularly those parameters affecting total baseline deoxyhemoglobin including Hct, OEF0, VI,0 and ωv. (see Figure 5), emphasizing the importance of experimentally measuring M in the calibrated-BOLD experiment. In fact, variation in published M values may be due to physiological variation between subjects and regions of interest. Particularly, regional variation in baseline CBV fraction (VI,0) (Chugh et al., 2009) may account for differences in M between the motor cortex and visual cortex in the above referenced papers. For this reason, using a theoretical estimate of M for a standard set of physiological conditions is likely to introduce significant error in the estimation of CMRO2 (Lin et al., 2008).
4.2 Optimization of Davis Model Parameters, α and β, Improves Fit to Simulated Data
Despite the restrictive assumptions that went into its derivation, the errors in the estimate of δCMRO2 using the classic Davis model are modest for most activation experiments. The classic Davis model with an accurately determined M systematically underestimates δCMRO2 by about −10% (see Fig. 4). Even for a much higher value n=5.0, the Davis model with the true M value underestimates δCMRO2 by only −6.9%. However, for more unusual combinations of CBF and CMRO2 change the errors can be quite a bit larger. For example, for n=−0.8 (increased CMRO2 but decreased CBF) the error balloons to −38.0%. Using the parameter values α=0.14 and β=0.91, optimized for the standard set of physiological parameters defined in Table 1, these errors are reduced, particularly for n=−0.8 where the optimized Davis model returns an error of only −1.2%.
A CBF/CMRO2 coupling ratio described by n=−0.8 may at first glance seem physiologically unreasonable, but in fact such changes may occur in response to a drug, as we recently found for caffeine. In that study, δCBF was found to decrease by −27% (Perthen et al., 2008). Combined with analysis of the BOLD signal using the classic parameters and a hypercapnia calibrated M, a trend toward increased CMRO2 was found (δCMRO2=13.3%, p=0.067). Yet further analysis of this data using the optimized model parameters and hypercapnia calibrated M revealed a significant metabolism increase due to caffeine (δCMRO2=21.8%, p=0.030) (Griffeth et al., 2011). If the hypercapnia calibration is in error (see discussion below), with hypercapnia actually lowering CMRO2, the true CMRO2 change with caffeine may be as high as 30%.
As a consequence of this approach of optimizing α and β, we can neither identify α as simply the exponent relating CBV to CBF, nor can we strictly relate to deoxyhemoglobin concentration using β as in the original derivation of the Davis model. Instead, α and β are effectively capturing several sources of variation arising in the detailed model, and they should just be regarded as fitting parameters. Keeping this in mind, optimal values of α and β depend on the physiologic parameters chosen for the standard state. For example, we included in our standard state the finding that relative CBV changes are larger for arteries than for capillaries and veins (Hillman et al., 2007, Kim et al., 2007, Chen and Pike, 2009). Our finding of α=0.14 after parameter optimization is consistent with this smaller venous CBV change (smaller φv), but α is still not equal to φv implying that it is dependent on other parameters as well. Indeed, baseline CBV distribution and tissue properties also have large impacts on α and β (Fig. 3). As more data becomes available on the true nature of the physiology, this figure will allow for α and β to be appropriately adjusted. For example, this figure shows that α is most affected by and tracks linearly with φv such that when the assumed value of this parameter is increased, the optimized α determined by this process of non-linear parameter optimization also increases (e.g. φv=0.38 produces an optimized α=0.24 and β=0.84).
Similarly, in the original Davis model derivation, β was used to relate extravascular to the concentration of deoxyhemoglobin, which is proportional to the ratio of O2 fractions in activation and baseline. This ratio in turn is equal to the ratio r/f. Classically, the exponent β depended on the diameter of the vessels involved in signal creation, with β=1 for large vessels and β=2 for small vessels (Ogawa et al., 1993). In the Monte Carlo simulations performed by Davis et al. (1998), β was determined to be 1.5 for B0=1.5T. At 3T, the large vessel component of the susceptibility difference will dominate such that β at 3T should be smaller (Boxerman et al., 1995; Chiarelli et al., 2007b). Figure 3 shows that the optimized value of β is affected by a variety of CBV and tissue parameters. Combined with the finding that α tracks with but is not equal to φv, this confirms that the Davis model is an oversimplification that does not model influences of the BOLD signal in a straightforward manner. Instead, the two parameters of the Davis model effectively capture multiple factors affecting the BOLD signal beyond what they were originally intended to model. Therefore, one should avoid using experimentally determined values of φv and β within the Davis model and instead perform more detailed parameter optimizations that capture the full range of physical and physiological factors that affect the BOLD signal.
4.3 Variation in CBV Distribution Has Largest Effect on the Accuracy of the Davis Model
Given the optimized values of α and β for the state defined in Table 1, it is important to determine the errors in δCMRO2 that will result when this model is applied but the underlying physiology differs from this standard state. Despite the limitations of the Davis model, the fact that it relies on a calibration step to measure the variable scaling parameter, M, gives it a distinct advantage as this potentially allows for the absorption of many variable baseline factors, such as VI,0, TE, Hct and OEF0. That is, variation of a physiological variable that only scales the magnitude of the BOLD response, regardless of the exact values of δCBF and δCMRO2, will not affect the estimate of δCMRO2 provided M is accurately measured. For example, Figure 6 shows that VI,0, Hct, TE and φ do not have a large impact on δCMRO2 calculations. Even large variations in VI,0 cause only small deviations in the range of calculated δCMRO2 (Supplementary data, Table S1).
The physiological parameters that are not captured well by M, and so are likely to produce the largest errors in the estimation of CMRO2 changes, are the venous CBV at baseline, the venous CBV change, , and to a lesser extent OEF0. These parameters may vary with brain region, stimulus type or duration, between subjects, and in health and disease. For example although Rostrup et al. (2005) found the relationship between changes in CBF and CBV to be constant across the brain, other groups have found variation in regional baseline CBV (Chugh et al., 2009) and in the temporal dynamics of CBV distribution in vessels as they dilate in response to a stimulus (Kim and Kim, 2010b). There is also evidence of CBV changing with disease (Guckel et al., 2007). In addition to differences in CBV, regional differences have been found in OEF0 and (He and Yablonskiy, 2007), and inter-subject differences have been found in baseline CBF (Ances et al., 2009).
To address this physiologic variation, we varied the basic parameter values over wide ranges and calculated the error in estimated δCMRO2. The change in venous CBV with activation (φv) is the most important factor in reliably estimating δCMRO2 around n=2.5. Recent research has suggested that arterial changes dominate venous change (Chen and Pike, 2009a; Hillman et al., 2007; Kim et al., 2007; Kim and Kim, 2006), but additional studies are needed to precisely quantify the relationship between venous CBV and CBF. Particularly, it is important to define how CBV changes are distributed dynamically, whether this changes with stimulus duration and type, and whether the relationship between CBV and CBF depends on brain region (Kim and Kim, 2010a, b). Any of these considerations could alter φ and/or φv thereby affecting optimization of the Davis model parameters and CMRO2 calculations.
For n=2.5, baseline venous volume fraction (ωv) is secondarily important while for n=−0.8 accurate knowledge of ωv and becomes much more significant, even more so than φv. In this regime of strong uncoupling (decreased CBF with increased CMRO2), baseline CBV, intravascular to extravascular spin density ratio, and baseline oxygen extraction also have sizeable although smaller effects on δCMRO2 calculations (Fig. 6b). As with φv, additional research is needed to confirm the normal range of OEF0, VI,0, and λ. However, it is possible to measure and λ in individual subjects and may soon be possible to reliably measure OEF0 using MR techniques (Chen and Pike, 2009b; Lu and Ge, 2008; Qin et al., 2011). As our knowledge of these values increases, we will be able to increase the accuracy of our model.
The importance of an accurate knowledge of φv is emphasized in Figure 7, which shows how accurate the optimized model is as φv increases from 0 to 0.2, 0.38 and 0.65. The Davis model optimized for φv=0.2 estimates δCMRO2 very well at this value but overestimates δCMRO2 if φv=0.38 or 0.65, and underestimates δCMRO2 for φv=0. We also examined the effect of setting total φ=0.65 as found by Lin et al. using VASO (2008) and found it has little effect compared to φ=0.38 if φv=0.2 remains unchanged. However if this volume change is distributed equally to the compartments such that φv=0.65, then the optimized Davis model will overestimate δCMRO2 in agreement with the results of Lin et al. (2008) and mirroring Figure 7d. Yet, the VASO technique used in the study of Lin et al. specifically measures changes in total CBV rather than venous CBV. Therefore to apply VASO data to the Davis model, it must be assumed that venous CBV changes in the same way as total CBV. Furthermore, a value for baseline CBV must be assumed in order to translate absolute volume changes into fractional changes, which are necessary for calculating the power-law relationship between CBF and CBV. Both assumptions may lead to φv being overestimated. Specifically, experimental findings of larger changes on the arterial side do not support the large venous CBV changes associated with φv=0.65, although more experiments are needed to confirm this. A previous study also examined whether the assumption that venous CBV changes are similar to total CBV changes would affect CMRO2 calculations (Kim et al., 1999). This study reported that the effects are minimal as long as a multiplicative term involving deoxyhemoglobin and volume changes is small. However, this may not be the case in all activation experiments, and furthermore this model assumes that changes in are small and only includes venous blood volume, neglecting the effects of arterial and capillary blood volume.
4.4 Violations of Hypercapnia Calibration Assumptions Lead to Errors in M and δCMRO2
In our initial calculations, we assumed that we could accurately measure M in order to explore the effects of physiologic variation. Recent research has suggested methods other than hypercapnia for more reliably measuring M. This is an active area of research, although one method gaining popularity is hyperoxia (Chiarelli et al., 2007b; Mark et al., 2011). To this point, though, most calibrated BOLD experiments have used hypercapnia to measure the scaling parameter M by assuming CMRO2 is not changed by CO2 inhalation (Chen and Pike, 2010; Jones et al., 2005; Sicard and Duong, 2005), but there is some controversy about this assumption (Kliefoth et al., 1979; Xu et al., 2011; Zappe et al., 2008). If hypercapnia in fact decreases CMRO2 then this process would tend to overestimate M. At larger values of CBF-CMRO2 coupling, this produces large errors in δCMRO2 calculations and underestimates n. Interestingly, this underestimation of n is larger for bigger values of n. This may account for some of the discrepancies between fMRI and PET data in studies reporting larger values of n. For example, one PET study found values of n between 5 and 6 in the somatosensory cortex (Fox and Raichle, 1986) while an fMRI experiment found values closer to n=3-4 (Chiarelli et al., 2007a). One area of CBF-CMRO2 coupling particularly sensitive to M is decreased CBF and increased CMRO2 as produced by caffeine. Here, overestimates of M will tend to underestimate δCMRO2, and it is in this area that error in both M and the parameters α and β have the largest impact on δCMRO2 calculations (Figure 4).
Another assumption of hypercapnia calibration is that CBV changes in different compartments are the same for activation and hypercapnia. As Figure 8 shows, error due to differences in relative CBV changes between activation and hypercapnia followed a pattern similar to violations of the iso-metabolic assumption. If φvHC is less than φv (Chen and Pike, 2010), then M and δCMRO2 will be overestimated. If φvHC is more than φv, then the opposite pattern of errors is apparent. Even with the systematic error in M due to changes in CBV distribution and δCMRO2 with hypercapnia, the Davis model is surprisingly accurate, especially close to n=2.5. This is likely due to the calibration process. Even with the inaccuracies of calibration, some self-correction of errors occurs when the same, albeit incomplete, model is used to analyze both the hypercapnia and activation data.
4.5 Future work
In this paper we have used the detailed model of the BOLD effect to assess and improve the accuracy of the calibrated BOLD method. The detailed model can also be used to address more basic questions of which physiological changes dominate the BOLD effect. Total deoxyhemoglobin, influenced by blood volume and concentration, is the most important factor. Previous models have included to different degrees separate terms for CBV (to model exchange effects) and deoxyhemoglobin concentration in blood (to model the intrinsic signal change of blood). The detailed model includes these effects and others as above, and it can be used to evaluate additional models (Buxton, 2009; Obata et al., 2004; Stephan et al., 2007).
Finally, an area of future application of the detailed model is to analyze potential errors in CMRO2 determination when hyperoxia is used for calibration. Hyperoxia was introduced as an alternative to hypercapnia as a way to avoid uncertainties due to the iso-metabolic assumption of the hypercapnia calibration, and because hyperoxia is more tolerable than hypercapnia (Chiarelli et al., 2007b). However, the hyperoxia calibration makes some additional assumptions such as baseline OEF, and the impact of these assumptions can be tested with the current model. The hyperoxia approach also does not have the same self-correcting behavior as hypercapnia. The difference is that hypercapnia and activation both involve CBF changes and associated assumptions about how CBV is altered. In contrast when using hyperoxia calibration, only the activation part of the experiment depends on the CBF change, and hyperoxia just manipulates deoxyhemoglobin content. For this reason, calibrated BOLD using hyperoxia is likely to be more sensitive to inaccuracies in the modeling of how CBV depends on CBF. All of these effects will be evaluated with the detailed model in future work.
5. Conclusions
The detailed model of the BOLD response developed here provides a theoretical framework for analyzing a number of questions related to the interpretation of the BOLD response. Here, we focused on improving the accuracy of the calibrated BOLD method to estimate changes in CMRO2. Despite the simplicity of the Davis model, and the restrictive assumptions of the original derivation, the mathematical form of the model nevertheless provides a reasonably accurate approximation for this complex phenomenon. The accuracy of the model is improved by using optimized values of the parameters α and β calculated from the detailed model. It is important to recognize, though, that the optimized parameter values no longer correspond to the physiological effects they were originally introduced to model and should be treated simply as fitting parameters. As new experimental data becomes available, particularly an accurate understanding of the venous CBV change with activation, these optimized values can be revised based on the calculations presented here for the detailed model.
Supplementary Material
Figure S1 | Dependence of Davis model α and β on VI,0, λ, TE, φ. Optimization of Davis model parameters α and β was performed using the Matlab fmincon function. (a-d) Davis model α has a negative correlation to λ and a weak positive correlation to φ while not showing a strong dependence on VI,0 or TE. (e-h) Davis model β has a negative correlation to VI,0, λ and TE and a positive correlation to φ.
Figure S2 | Percent error (ζ) in δCMRO2 calculations using the classic and optimized Davis model parameter sets but with biased M. The hypercapnia calibration is often criticized for its assumption that 5% CO2 inhalation does not change oxygen metabolism. Recent experiments have shown that this calibration method may in fact decrease CMRO2 by 10%. If this is the case, M will be biased resulting in biased calculations of δCMRO2 as shown. (a) δCMRO2 appears to be correctly determined close to n=2.5 by the classic Davis model with M biased as noted. (b) The optimized parameters of α=0.14 and β=0.91 combined with a biased M produce accurate estimates of δCMRO2 around n=1.3.
Table S1 | Range of variation and error due to variation in input parameters. The first three rows of errors are for a typical activation experiment with ΔCBF=50% and ΔCMRO2=25% (n=2). The second three rows of errors are for a simulated caffeine experiment with ΔCBF=−25% and ΔCMRO2=30% (n=−0.8). ζ is the fractional error while the ranges listed are associated with the minimum and maximum values respectively of each input parameter.
Research Highlights.
We developed a detailed model of BOLD fMRI including recent experimental measurements.
In particular, the model incorporates volume changes of three vascular compartments.
New parameters for the Davis model improve estimates of oxygen metabolism
Knowledge of blood volume will have the biggest impact on future improvements.
Accurate measurement of the scaling parameter is a confounding factor.
Acknowledgements
We thank Farshad Moradi, Aaron Simon, and David Dubowitz for discussion, and Nic Blockley for confirmation of results as well as comments on the approach. This research was supported by funding from NIH grants NS-36722, NRSA grant HL-7089, NIGMS MSTP training grant GM7198 and HHMI-NIBIB Interfaces grant EB-9380.
Abbreviations
- BOLD
blood oxygenation level dependent
- CBF
cerebral blood flow
- CMRO2
cerebral metabolic rate of oxygen
- CBV
cerebral blood volume
- fMRI
functional magnetic resonance imaging
- OEF
oxygen extraction fraction
- Hct
hematocrit
- VI
intravascular volume
intrinsic extravascular signal decay rate
- SAO2
arterial O2 saturation
- ωv
baseline venous CBV fraction
- φv
exponent relating venous CBV changes to CBF
- TE
echo time
- λ
intravascular to extravascular spin density ratio
Appendix
The MR signal is a volume-weighted average of signals from extravascular and intravascular compartments (Buxton et al., 1998; Obata et al., 2004), and we assume that contributions from the different compartments combine linearly. The intravascular component can be further segmented into arterial, capillary and venous compartments denoted by the subscripts A, C and V respectively. The subscript I denotes the sum of all intravascular compartments. The subscript E denotes the extravascular compartment. We use the subscript 0 to denote the baseline state. Here we are assuming a static model, but ultimately we will apply this model dynamically, requiring a more detailed knowledge of how the different compartments change over time. S is the total signal while SE,A,C,V is the signal intensity of a voxel containing only the designated component, and VE,A,C,V is the corresponding volume fraction of that component. The net signal is then:
| (A1) |
The intrinsic signal for each compartment can be modeled as an exponential decay dependent on the transverse relaxation rate, , and the signal at the theoretical TE=0, which in general will depend on the spin density and the longitudinal relaxation time constant T1. Assuming T1 does not change with activation, such that the signal at TE=0 does not change, then the following equality is formed describing the intrinsic signal ratio of blood:
| (A2) |
λ is the effective intravascular to extravascular spin density ratio, or the signal ratio when TE=0, and this was assumed to have the same value for each of the intravascular compartments. The value of λ was determined experimentally to be 1.15 (Section 3.1). Since BOLD is a fractional signal change, it can be expressed as . Noting VE = 1−VA−VC−VV = 1−VI, it follows that:
| (A3) |
Where
| (A4) |
The intravascular transverse relaxation rate constants were approximated for each compartment using a quadratic model dependent on Hct and SO2 (Silvennoinen et al., 2003; Zhao et al., 2007):
| (A5) |
The dependence of A* and C* on Hct was approximated using a linear fit of the experimental data for 3T reported in Zhao et al. (2007).
The oxygen extraction fraction with activation was calculated from the normalized CBF and CMRO2, which are variable inputs to the detailed model:
| (A6) |
Oxygen saturation for the venous compartment was determined from the arterial saturation and the oxygen extraction fraction:
| (A7) |
Capillary oxygen saturation (SCO2) was calculated as an average of arterial and venous values with weighting κ:
| (A8) |
In calculating values, the A* terms cancel leaving dependence on Hct through C* as well as SO2 from both the baseline and activation states. The second term denoted by the subscript 0 is associated with the baseline state.
| (A9) |
Note that SO2 decreases from arteries to capillaries to veins, while capillary Hct is also 76% of the arterial value (Sakai et al., 1989).
Extravascular change was treated as a linear combination of the contributions from each of the vascular compartments calculated separately as determined by Ogawa et al. (1993). In general, is the sum of two components: the transverse relaxation rate in the presence of fully oxygenated blood ( R2 ) and the signal relaxation rate due to local field inhomogeneities including the effects of deoxygenated blood (R2′). When calculating , R2 remains constant and therefore cancels leaving only the difference in which was previously estimated using Monte Carlo simulations of water proton intravoxel phase dispersion. These experiments demonstrated that depending on vessel size, has either a linear or quadratic dependence on main magnetic field strength B0, Hct, and SO2 as well as purely linear dependence on blood volume fraction (V) (Ogawa et al., 1993):
| (A10) |
Note that Δχis the gyromagnetic ratio of protons, and SO2,off is the O2 saturation that produces no magnetic susceptibility difference between blood and tissue. Total extravascular is then the sum of these three blood compartment contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum Brain Mapp. 2009;30:1120–1132. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blockley NP, Francis ST, Gowland PA. Perturbation of the BOLD response by a contrast agent and interpretation through a modified balloon model. Neuroimage. 2009;48:84–93. doi: 10.1016/j.neuroimage.2009.06.038. [DOI] [PubMed] [Google Scholar]
- Bolar DS, Rosen BR, Evans KC, Sorensen AG, Adalsteinsson E. Depression of cortical gray matter CMRO2 in awake humans during hypercapnia; Proceedings of the ISMRM; Stockholm. 2010.2010. p. 516. [Google Scholar]
- Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magn. Reson. Med. 1995;34:555–566. doi: 10.1002/mrm.1910340412. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Modeling the effects of changes in arterial blood volume on the BOLD signal; Proceedings of the ISMRM; Honolulu. 2009.2009. [Google Scholar]
- Buxton RB. Interpreting oxygenation-based neuroimaging signals: the importance and the challenge of understanding brain oxygen metabolism. Front Neuroenergetics. 2010;2:8. doi: 10.3389/fnene.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23(Suppl 1):S220–233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn. Reson. Med. 1998;39:855–864. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Pike GB. BOLD-specific cerebral blood volume and blood flow changes during neuronal activation in humans. NMR Biomed. 2009a;22:1054–1062. doi: 10.1002/nbm.1411. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Pike GB. Human whole blood T2 relaxometry at 3 Tesla. Magn Reson Med. 2009b;61:249–254. doi: 10.1002/mrm.21858. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Pike GB. MRI measurement of the BOLD-specific flow-volume relationship during hypercapnia and hypocapnia in humans. Neuroimage. 2010;53:383–391. doi: 10.1016/j.neuroimage.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Gallichan D, Piechnik SK, Wise R, Jezzard P. Flow-metabolism coupling in human visual, motor, and supplementary motor areas assessed by magnetic resonance imaging. Magn Reson Med. 2007a;57:538–547. doi: 10.1002/mrm.21171. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Wise R, Gallichan D, Jezzard P. A calibration method for quantitative BOLD fMRI based on hyperoxia. Neuroimage. 2007b;37:808–820. doi: 10.1016/j.neuroimage.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Chugh BP, Lerch JP, Yu LX, Pienkowski M, Harrison RV, Henkelman RM, Sled JG. Measurement of cerebral blood volume in mouse brain regions using micro-computed tomography. Neuroimage. 2009;47:1312–1318. doi: 10.1016/j.neuroimage.2009.03.083. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc. Natl. Acad. Sci. USA. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, Blicher JU, Ostergaard L, Feinberg DA, MacIntosh BJ, Miller KL, Gunther M, Jezzard P. Cerebral blood flow, blood volume, and oxygen metabolism dynamics in human visual and motor cortex as measured by whole-brain multi-modal magnetic resonance imaging. J Cereb Blood Flow Metab. 2009;29:1856–1866. doi: 10.1038/jcbfm.2009.107. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Mechelli A, Turner R, Price CJ. Nonlinear responses in fMRI: the Balloon model, Volterra kernels, and other hemodynamics. Neuroimage. 2000;12:466–477. doi: 10.1006/nimg.2000.0630. [DOI] [PubMed] [Google Scholar]
- Griffeth VEM, Perthen JE, Buxton RB. Prospects for Quantitative fMRI: Investigating the Effects of Caffeine on Baseline Oxygen Metabolism and the Response to a Visual Stimulus in Humans. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb RL, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5:630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- Guckel FJ, Brix G, Hennerici M, Lucht R, Ueltzhoffer C, Neff W. Regional cerebral blood flow and blood volume in patients with subcortical arteriosclerotic encephalopathy (SAE) Eur Radiol. 2007;17:2483–2490. doi: 10.1007/s00330-007-0617-y. [DOI] [PubMed] [Google Scholar]
- Gustard S, Williams EJ, Hall LD, Pickard JD, Carpenter TA. Influence of baseline hematocrit on between-subject BOLD signal change using gradient echo and asymmetric spin echo EPI. Magn Reson Imaging. 2003;21:599–607. doi: 10.1016/s0730-725x(03)00083-3. [DOI] [PubMed] [Google Scholar]
- He X, Yablonskiy DA. Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn Reson Med. 2007;57:115–126. doi: 10.1002/mrm.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman EM, Devor A, Bouchard MB, Dunn AK, Krauss GW, Skoch J, Bacskai BJ, Dale AM, Boas DA. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. Neuroimage. 2007;35:89–104. doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn. Reson. Med. 1999;42:849–863. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hyder F, Sanganahalli BG, Herman P, Coman D, Maandag NJ, Behar KL, Blumenfeld H, Rothman DL. Neurovascular and Neurometabolic Couplings in Dynamic Calibrated fMRI: Transient Oxidative Neuroenergetics for Block-Design and Event-Related Paradigms. Front Neuroenergetics. 2010:2. doi: 10.3389/fnene.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Ibaraki M, Kanno I, Fukuda H, Miura S. Changes in cerebral blood flow and cerebral oxygen metabolism during neural activation measured by positron emission tomography: comparison with blood oxygenation level-dependent contrast measured by functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2005;25:371–377. doi: 10.1038/sj.jcbfm.9600030. [DOI] [PubMed] [Google Scholar]
- Jones M, Berwick J, Hewson-Stoate N, Gias C, Mayhew J. The effect of hypercapnia on the neural and hemodynamic responses to somatosensory stimulation. Neuroimage. 2005;27:609–623. doi: 10.1016/j.neuroimage.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Kim SG, Rostrup E, Larsson HBW, Ogawa S, Paulson OB. Determination of relative CMRO2 from CBF and BOLD changes: significant increase of oxygen consumption rate during visual stimulation. Magn. Reson. Med. 1999;41:1152–1161. doi: 10.1002/(sici)1522-2594(199906)41:6<1152::aid-mrm11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Kim T, Hendrich KS, Masamoto K, Kim SG. Arterial versus total blood volume changes during neural activity-induced cerebral blood flow change: implication for BOLD fMRI. J Cereb Blood Flow Metab. 2007;27:1235–1247. doi: 10.1038/sj.jcbfm.9600429. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim SG. Quantification of cerebral arterial blood volume using arterial spin labeling with intravoxel incoherent motion-sensitive gradients. Magn Reson Med. 2006;55:1047–1057. doi: 10.1002/mrm.20867. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim SG. Cortical layer-dependent arterial blood volume changes: improved spatial specificity relative to BOLD fMRI. Neuroimage. 2010a;49:1340–1349. doi: 10.1016/j.neuroimage.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Kim SG. Temporal dynamics and spatial specificity of arterial and venous blood volume changes during visual stimulation: implication for BOLD quantification. J Cereb Blood Flow Metab. 2010b doi: 10.1038/jcbfm.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliefoth AB, Grubb RL, Jr., Raichle ME. Depression of cerebral oxygen utilization by hypercapnia in the rhesus monkey. J Neurochem. 1979;32:661–663. doi: 10.1111/j.1471-4159.1979.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Leontiev O, Buxton RB. Reproducibility of BOLD, perfusion, and CMRO(2) measurements with calibrated-BOLD fMRI. Neuroimage. 2007;35:175–184. doi: 10.1016/j.neuroimage.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Fox PT, Hardies J, Duong TQ, Gao JH. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc Natl Acad Sci U S A. 2010;107:8446–8451. doi: 10.1073/pnas.0909711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Fox PT, Yang Y, Lu H, Tan LH, Gao JH. Evaluation of MRI models in the measurement of CMRO2 and its relationship with CBF. Magn Reson Med. 2008;60:380–389. doi: 10.1002/mrm.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med. 2008;60:357–363. doi: 10.1002/mrm.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Golay X, Pekar JJ, Van Zijl PC. Sustained poststimulus elevation in cerebral oxygen utilization after vascular recovery. J Cereb Blood Flow Metab. 2004;24:764–770. doi: 10.1097/01.WCB.0000124322.60992.5C. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJA, Ayata C, Zaharchuk G, Moskowitz MA, Rosen BR, Weisskoff RM. Evidence of a cerebrovascular post-arteriole Windkessel with delayed compliance. J. Cereb. Blood Flow and Metabol. 1999;19:679–689. doi: 10.1097/00004647-199906000-00012. [DOI] [PubMed] [Google Scholar]
- Marchal G, Rioux P, Petit-Taboue M-C, Sette G, Travere J-M, LePoec C, Courtheoux P, Derlon J-M, Baron J-C. Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol. 1992;49:1013–1020. doi: 10.1001/archneur.1992.00530340029014. [DOI] [PubMed] [Google Scholar]
- Mark CI, Fisher JA, Pike GB. Improved fMRI calibration: precisely controlled hyperoxic versus hypercapnic stimuli. Neuroimage. 2011;54:1102–1111. doi: 10.1016/j.neuroimage.2010.08.070. [DOI] [PubMed] [Google Scholar]
- Obata T, Liu TT, Miller KL, Luh WM, Wong EC, Frank LR, Buxton RB. Discrepancies between BOLD and flow dynamics in primary and supplementary motor areas: application of the balloon model to the interpretation of BOLD transients. Neuroimage. 2004;21:144–153. doi: 10.1016/j.neuroimage.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim S-G, Merkle H, Ellerman JM, Ugurbil K. Functional brain mapping by blood oxygenation level - dependent contrast magnetic resonance imaging: a comparison of signal characteristics with a biophysical model. Biophysical J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perthen JE, Lansing AE, Liau J, Liu TT, Buxton RB. Caffeine-induced uncoupling of cerebral blood flow and oxygen metabolism: a calibrated BOLD fMRI study. Neuroimage. 2008;40:237–247. doi: 10.1016/j.neuroimage.2007.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q, Grgac K, van Zijl PC. Determination of whole-brain oxygen extraction fractions by fast measurement of blood T(2) in the jugular vein. Magn Reson Med. 2011;65:471–479. doi: 10.1002/mrm.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PE, Eriksson L, Stone-Elander S, Widen L. Does mental activity change the oxidative metabolism of the brain? J. Neuroc. 1987;7:2373–2389. [PMC free article] [PubMed] [Google Scholar]
- Rostrup E, Knudsen GM, Law I, Holm S, Larsson HB, Paulson OB. The relationship between cerebral blood flow and volume in humans. Neuroimage. 2005;24:1–11. doi: 10.1016/j.neuroimage.2004.09.043. [DOI] [PubMed] [Google Scholar]
- Sakai F, Igarashi H, Suzuki S, Tazaki Y. Cerebral blood flow and cerebral hematocrit in patients with cerebral ischemia measured by single-photon emission computed tomography. Acta Neurol Scand Suppl. 1989;127:9–13. doi: 10.1111/j.1600-0404.1989.tb01805.x. [DOI] [PubMed] [Google Scholar]
- Sakai F, Nakazawa K, Tazaki Y, Ishii K, Hino H, Igarashi H, Kanda T. Regional cerebral blood volume and hematocrit measured in normal human volunteers by single-photon emission computed tomography. J. Cereb. Blood Flow and Metabol. 1985;5:207–213. doi: 10.1038/jcbfm.1985.27. [DOI] [PubMed] [Google Scholar]
- Schutz SL. Oxygen saturation monitoring by pulse oximetry. In: Lynn-McHale DJ, Carlson KK, editors. AACN Procedure Manual for Critical Care. 4th ed. Elsevier Saunders; St. Louis, Mo: 2001. [Google Scholar]
- Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage. 2005;25:850–858. doi: 10.1016/j.neuroimage.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvennoinen MJ, Clingman CS, Golay X, Kauppinen RA, van Zijl PC. Comparison of the dependence of blood R2 and R2* on oxygen saturation at 1.5 and 4.7 Tesla. Magn Reson Med. 2003;49:47–60. doi: 10.1002/mrm.10355. [DOI] [PubMed] [Google Scholar]
- Spees WM, Yablonskiy DA, Oswood MC, Ackerman JJ. Water proton MR properties of human blood at 1.5 Tesla: magnetic susceptibility, T(1), T(2), T*(2), and non-Lorentzian signal behavior. Magn Reson Med. 2001;45:533–542. doi: 10.1002/mrm.1072. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Hutchinson E, Yakovleva V, Schram V, Russell JT, Belluscio L, Koretsky AP, Silva AC. Functional reactivity of cerebral capillaries. J Cereb Blood Flow Metab. 2008;28:961–972. doi: 10.1038/sj.jcbfm.9600590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Pike GB. Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage. 2004;22:771–778. doi: 10.1016/j.neuroimage.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Rylander KM, Pike GB. The effect of global cerebral vasodilation on focal activation hemodynamics. Neuroimage. 2006;30:726–734. doi: 10.1016/j.neuroimage.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Weiskopf N, Drysdale PM, Robinson PA, Friston KJ. Comparing hemodynamic models with DCM. Neuroimage. 2007;38:387–401. doi: 10.1016/j.neuroimage.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AG, Johnson PC, Intaglietta M. Oxygen gradients in the microcirculation. Physiol Rev. 2003;83:933–963. doi: 10.1152/physrev.00034.2002. [DOI] [PubMed] [Google Scholar]
- Uludag K, Muller-Bierl B, Ugurbil K. An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage. 2009;48:150–165. doi: 10.1016/j.neuroimage.2009.05.051. [DOI] [PubMed] [Google Scholar]
- Weber B, Keller AL, Reichold J, Logothetis NK. The microvascular system of the striate and extrastriate visual cortex of the macaque. Cereb Cortex. 2008;18:2318–2330. doi: 10.1093/cercor/bhm259. [DOI] [PubMed] [Google Scholar]
- Xu F, Uh J, Brier MR, Hart J, Jr., Yezhuvath US, Gu H, Yang Y, Lu H. The influence of carbon dioxide on brain activity and metabolism in conscious humans. J Cereb Blood Flow Metab. 2011;31:58–67. doi: 10.1038/jcbfm.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappe AC, Uludag K, Logothetis NK. Direct measurement of oxygen extraction with fMRI using 6% CO2 inhalation. Magn Reson Imaging. 2008;26:961–967. doi: 10.1016/j.mri.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Zhao JM, Clingman CS, Narvainen MJ, Kauppinen RA, van Zijl PC. Oxygenation and hematocrit dependence of transverse relaxation rates of blood at 3T. Magn Reson Med. 2007;58:592–597. doi: 10.1002/mrm.21342. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Pan Y, Harris S, Billings S, Coca D, Berwick J, Jones M, Kennerley A, Johnston D, Martin C, Devonshire IM, Mayhew J. A dynamic model of neurovascular coupling: Implications for blood vessel dilation and constriction. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.01.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Dependence of Davis model α and β on VI,0, λ, TE, φ. Optimization of Davis model parameters α and β was performed using the Matlab fmincon function. (a-d) Davis model α has a negative correlation to λ and a weak positive correlation to φ while not showing a strong dependence on VI,0 or TE. (e-h) Davis model β has a negative correlation to VI,0, λ and TE and a positive correlation to φ.
Figure S2 | Percent error (ζ) in δCMRO2 calculations using the classic and optimized Davis model parameter sets but with biased M. The hypercapnia calibration is often criticized for its assumption that 5% CO2 inhalation does not change oxygen metabolism. Recent experiments have shown that this calibration method may in fact decrease CMRO2 by 10%. If this is the case, M will be biased resulting in biased calculations of δCMRO2 as shown. (a) δCMRO2 appears to be correctly determined close to n=2.5 by the classic Davis model with M biased as noted. (b) The optimized parameters of α=0.14 and β=0.91 combined with a biased M produce accurate estimates of δCMRO2 around n=1.3.
Table S1 | Range of variation and error due to variation in input parameters. The first three rows of errors are for a typical activation experiment with ΔCBF=50% and ΔCMRO2=25% (n=2). The second three rows of errors are for a simulated caffeine experiment with ΔCBF=−25% and ΔCMRO2=30% (n=−0.8). ζ is the fractional error while the ranges listed are associated with the minimum and maximum values respectively of each input parameter.