Abstract
In chronic heart failure (CHF), arterial baroreflex function is impaired, in part, by activation of the central renin-angiotensin system. A metabolite of Angiotensin II (Ang II), Ang-(1–7), has been shown to exhibit cardiovascular effects that are in opposition to that of Ang II. However, the action of Ang-(1–7) on sympathetic outflow and baroreflex function is not well understood, especially in CHF. The aim of this study was to determine the effect of intracerebroventricular infusion of Ang-(1–7) on baroreflex control of heart rate (HR) and renal sympathetic nerve activity (RSNA) in conscious rabbits with CHF. We hypothesized that central Ang-(1–7) would improve baroreflex function in CHF. Ang-(1–7) (2 nmol/1 μl/hour) or artificial cerebrospinal fluid (1 μl/hour) was infused by an osmotic mini-pump for 4 days in sham and pacing-induced CHF rabbits (n=3–6/group). Ang-(1–7) treatment had no effects in sham rabbits but reduced HR and increased baroreflex gain (7.4±1.5 bpm/mm Hg vs. 2.5±0.4 bpm/mm Hg, P<0.05) in CHF rabbits. The Ang-(1–7) antagonist A779 (8 nmol/1 μl/hr) blocked the improvement in baroreflex gain in CHF. Baroreflex gain increased in CHF+Ang-(1–7) animals when only the vagus was allowed to modulate baroreflex control by acute treatment with the β-1 antagonist metoprolol, indicating increased vagal tone. Baseline RSNA was significantly lower and baroreflex control of RSNA was enhanced in CHF rabbits receiving Ang-(1–7). These data suggest that augmentation of central Ang-(1–7) inhibits sympathetic outflow and increases vagal outflow in CHF thus contributing to enhanced baroreflex gain in this disease state.
Keywords: angiotensin-(1–7), heart failure, sympathetic nervous system, baroreflex, vagus nerve, blood pressure, heart rate
Introduction
Chronic heart failure (CHF) is characterized by heightened sympathetic tone in compensation for reduced cardiac function.1, 2 This compensation is mediated, in part, by activation of the renin-angiotensin system (RAS) and increased production of angiotensin II (Ang II).3 Recent studies in experimental models of CHF and hypertension have suggested that in the brain the responses to Ang II are enhanced by increases in Angiotensin Type 1 receptors (AT1R) and decreases in AT2R, whereas the Ang-(1–7) axis is concurrently diminished.4–6 Our laboratory recently has found in rabbits with CHF that expression of angiotensin converting enzyme 2 (ACE2) decreases in regions of the brain that are involved in sympathetic function.7 However, there have been limited studies examining the effects Ang-(1–7) in the brain on the neural control of cardiovascular function in CHF.
The actions of Ang (1–7) as examined in many tissues generally oppose the actions of Ang II. In the heart, Ang-(1–7) is expressed in cardiac myocytes and appears to have an ionotropic effect and possesses coronary vasodilator activity.8, 9 In contrast to Ang II, Ang-(1–7) appears to inhibit the cardiac remodeling process in several rat models.10, 11 In a study by Benter, et al. oral administration of Ang-(1–7) prevented development of hypertension in spontaneously hypertensive rats treated with L-NAME.12
Ang-(1–7) is produced in many areas of the brain,13 and its actions have been linked to sympathetic regulation.14 Potts et al. showed that in anesthetized rabbits, Ang-(1–7) evoked sympatho-excitation when injected into the rostral ventrolateral medulla (RVLM).15 Similar results were obtained by Fontes et al. and by Silva et al. in rats.16, 17 In a more recent study, Gomes da Silva et al. injected the mas receptor antagonist A-779 in the paraventricular nucleus of anesthetized rats and observed a decrease in renal sympathetic nerve activity (RSNA) suggesting that Ang-(1–7) is sympatho-excitatory.18 On the other hand, other studies suggest that Ang-(1–7) may be sympatho-inhibitory.19 In a recent study by Campagnole-Santos and Guimarães,20 central infusion of Ang-(1–7) reduced blood pressure and heart rate in DOCA-salt hypertensive rats. Thus the central effect of Ang-(1–7) on sympathetic tone is unclear, and to our knowledge, has not been studied in CHF.
Our laboratory has previously shown that baroreflex function is impaired in CHF due to increased Ang II, increased sympathetic tone, and decreased vagal tone.21 Some studies have shown that Ang-(1–7) improves baroreflex function in hypertensive models.22, 23 However, no studies have determined the role of central Ang-(1–7) on baroreflex control in CHF or have determined the sympathetic and vagal components responsible for the improvements in baroreflex function.
Therefore, we tested the hypothesis that central Ang-(1–7) enhances baroreflex control of HR and renal sympathetic nerve activity (RSNA) and favorably modulates sympathovagal balance in the setting of CHF. We determined the effect of central Ang-(1–7) on renal sympathetic outflow and cardiac sympathovagal balance using direct recordings of RSNA and heart rate (HR) in a conscious rabbit model of pacing induced heart failure. We also determined the central effect of Ang-(1–7) on baroreflex control of HR and RSNA.
Materials and Methods
An expanded methods section is available in the Online Data Supplement at http://hyper.ahajournals.org.
Animals
Experiments were carried out on 32 male New Zealand White rabbits weighing between 3.0 and 4.5kg (Charles River Laboratories; Wilmington, MA). These experiments were reviewed and approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee. Experiments were carried out in five experimental groups: a normal sham operated group with central artificial cerebrospinal fluid (aCSF) infusion (Sham+aCSF), a sham group with central Ang-(1–7) infusion (Sham+Ang-(1–7)), a CHF group with aCSF infusion (CHF+aCSF), a CHF group with Ang-(1–7) infusion (CHF+ Ang-(1–7)), and a CHF group with Ang-(1–7) and A779 infusion (CHF+ Ang-(1–7)+A779).
Cardiac Baroreflex and Autonomic Blockade Protocol (13 Rabbits)
A representative timeline of these studies is provided in Figure S1 (please see http://hyper.ahajournals.org). Rabbits were instrumented with left ventricular pacing electrodes and a radiotelemetry transducer (DataSciences, Inc. Minneapolis MN) to monitor mean arterial blood pressure (MAP) and HR. Two weeks after the insertion of telemetry, an ICV brain cannula was inserted and was attached to an osmotic minipump (Alzet 2001, Cupertino, CA) filled with aCSF which infused at a rate of 1 μl/hr. On the day of the first experiment, the rabbit was placed in a box in a dimly lit room. The rabbit was allowed to rest quietly for 20 min before data were collected. Baseline recordings of MAP and HR were taken for five minutes. Following baseline recording the response to nasopharyngeal stimulation with 60 ml of cigarette smoke was measured, which our laboratory has used previously to activate and measure vagal tone.24
The arterial baroreflex control of HR was determined by intravenous infusions of sodium nitroprusside (SNP, 100 μg/kg) and phenylephrine (PE, 80 μg/kg) at a rate of 0.5 ml/min. After SNP lowered MAP to its lowest point (approximately 40 mmHg), the SNP infusion was stopped and replaced immediately with PE which was infused at the same rate until MAP reached ~110 mmHg.
The following day, the aCSF pump was replaced with a pump filled with Ang-(1–7). Ang-(1–7) (Sigma, St. Louis, MO) was dissolved in aCSF (2 nmol/1 μl/hr). Three days following the insertion of this pump, baseline recordings and cardiac baroreflex assessment was repeated. Following the sham (prepace) experiments, the Ang-(1–7) pump was replaced with another aCSF pump and CHF was induced in each rabbit by chronic ventricular pacing as previously described.25 The experimental protocol above was repeated in each rabbit after they reached an ejection fraction below 45%.
In each rabbit, after generation of a control baroreflex curve, a second curve was constructed 10 min after IV administration of 0.2 mg/kg of atropine methylbromide. A new set of baseline recordings, smoke response, and baroreflex function was carried out. On the following day, the same procedures were carried out, after administration of 1 mg/kg IV of metoprolol bitartrate.
RSNA Measurement Protocol (16 Rabbits)
Additional rabbits were randomly divided into four groups (n=4/group) for measurement of baseline RSNA and baroreflex control of RSNA. A representative timeline for these animals is provided in Figure S1. Nerve recording electrodes were implanted as previously described26 along with a ICV cannula and osmotic mini-pump containing aCSF or Ang-(1–7). Recording electrodes and the ICV cannula plus mini-pump were implanted in CHF rabbits when EF fell to below 45% after pacing.
RSNA was digitized at 1000 samples/sec and amplified with an Animal Bio Amp (ADInstruments, Inc.) with the bandwidth set between 25 Hz and 1 KHz. The spike frequency of the raw nerve activity was determined by setting a window discriminator approximately 10% above the noise. Baseline recordings and baroreflex experiments were carried out 3 to 5 days after the implantation of the nerve electrodes. Raw nerve activity and frequency were recorded continuously during the experiments. The data are expressed as a percent of the maximal spike frequency. The maximal activity was determined in each rabbit by observing its response to 60 ml of cigarette smoke administered into the external nares.
Statistical Analysis
Data are expressed as mean±SEM. All statistical analysis was performed with SigmaPlot 11 software. Differences between groups were analyzed with a one-way ANOVA or repeated measures ANOVA where appropriate followed by the Newman Keuls post hoc test. P<0.05 was considered statistically significant.
Results
Cardiac Dimensions and Hemodynamics
Body weight, baseline hemodynamics, and echocardiographic data in the five groups studied are provided in Table 1. The CHF group exhibited a significantly lower EF and fractional shortening (FS) along with higher resting HR and left ventricular systolic diameter and volume compared with the sham group. Further clinical signs of CHF were observed including ascites and pulmonary edema. Central infusion of Ang-(1–7) did not alter cardiac dimensions in Sham nor CHF rabbits. Ang-(1–7) significantly reduced HR in CHF animals. CHF rabbits receiving central Ang-(1–7)+A779 had a significantly higher resting HR than CHF rabbits receiving only Ang-(1–7). Interestingly however, the HR was significantly lower than CHF rabbits receiving aCSF. Similar changes in baseline HR and EF were observed in the subset of rabbits used for recording of RSNA (Online Table S1). All time domain parameters of HRV were depressed in CHF+aCSF rabbits (Online Table S2). Central Ang-(1–7) normalized HRV in the CHF state.
Table 1.
Baseline Hemodynamics in Sham and CHF Rabbits before and after Ang-(1–7) Infusion
| Variable | Sham+aCSF | Sham+Ang-(1–7) | CHF+aCSF | CHF+Ang-(1–7) | CHF+Ang-(1–7)+A779 |
|---|---|---|---|---|---|
| N | 13 | 11 | 11 | 10 | 3 |
| Body Weight, kg | 3.6±0.1 | 3.6±0.1 | 3.7±0.1 | 3.6±0.1 | 3.5±0.1 |
| Heart Rate, bpm | 198.5±4.6 | 186.8±4.5 | 248.0±5.5* | 209.6±4.9† | 221.5±5.5*† |
| MAP, mmHg | 74.3±1.9 | 69.1±3.1 | 73.4±3.0 | 67.3±3.2 | 76.1±6.3 |
| LVEDD, mm | 16.2±0.5 | 16.3±0.4 | 17.1±0.4 | 16.6±0.6 | 16.7±0.1 |
| LVSD, mm | 7.1±1.4 | 7.5±0.3 | 13.6±0.4* | 13.5±0.5* | 13.8±0.2* |
| LVd Vol, ml | 7.6±0.6 | 6.7±0.5 | 8.6±0.5 | 8.2±0.7 | 8.1±0.1 |
| LVs Vol, ml | 2.2±0.2 | 2.4±0.3 | 4.7±0.3* | 4.8±0.5* | 4.8±0.2* |
| FS % | 34.2±1.7 | 35.9±0.8 | 20.5±0.5* | 19.0±0.9* | 18.0±1.0* |
| EF % | 70.3±0.6 | 73.2±1.0 | 45.1±1.0* | 42.3±1.7* | 40.4±1.9* |
Values are mean±SEM. LV indicates left ventricle; MAP, mean arterial pressure; LVEDD, left ventricular end-diastolic diameter; LVSD, left ventricular systolic diameter; LVd Vol, left ventricular diastolic diameter; LVs Vol, left ventricular systolic diameter; FS, fractional shortening; EF, ejection fraction
P<0.05 vs. Sham+aCSF,
P<0.05 vs. CHF+aCSF.
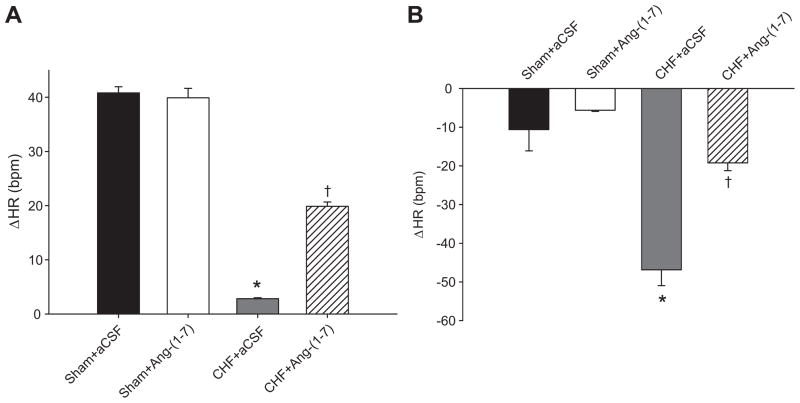
Baseline HR was also recorded before and after intravenous injections of atropine and metoprolol to determine changes in sympathovagal balance. The increase in HR in response to atropine (Figure 1A) seen in Sham+aCSF rabbits was essentially abolished in CHF+aCSF group, indicating a dramatic reduction in vagal activity in CHF. Ang-(1–7) treatment in CHF increased the tachycardic response to atropine, indicating an increase in vagal tone. The bradycardic response to metoprolol (Figure 1B) was significantly lower in CHF+Ang-(1–7) rabbits compared with CHF+aCSF reflecting a decrease in sympathetic tone in response to chronic ICV infusion of Ang-(1–7).
Figure 1.
Change in resting heart rate after autonomic blockade with intravenous infusions of atropine (A) or metoprolol (B). The tachycardic response to atropine was significantly higher in CHF+Ang-(1–7) rabbits compared with CHF+aCSF reflecting increased vagal tone. The HR after autonomic blockade was compared to baseline HR in Table 1 to determine the change in HR. The bradycardic response to metoprolol was significantly lower in CHF+Ang-(1–7) rabbits compared with CHF+aCSF reflecting decreased sympathetic tone. *P<0.05 compared with Sham+aCSF. †P<0.05 compared with CHF+aCSF.
Effect of Ang-(1–7) on the response to smoke
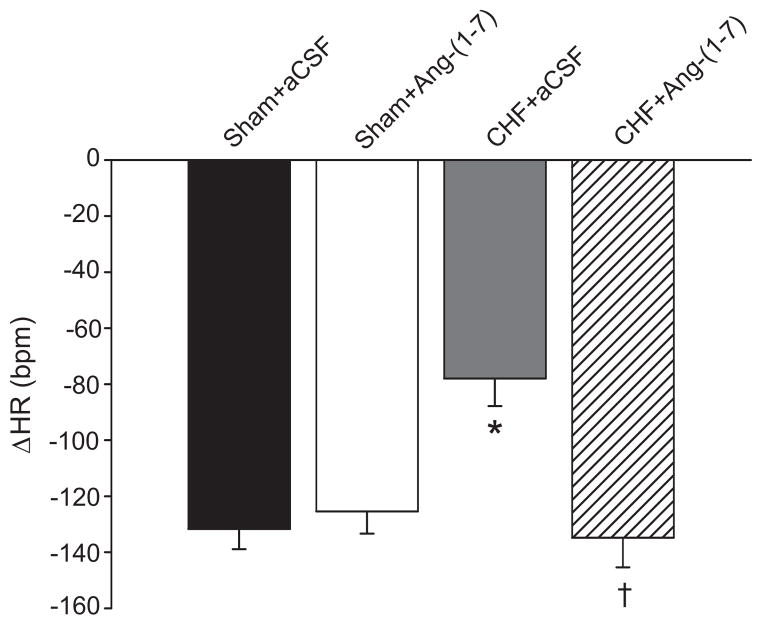
Figure 2 shows the change in HR in response to nasopharyngeal stimulation. An abrupt fall in HR with little or no increase in blood pressure, was seen in sham rabbits. The bradycardia was significantly diminished in CHF+aCSF. Ang-(1–7) normalized this response. In all groups, atropine abolished the change in HR to near zero (data not shown).
Figure 2.
Change in heart rate from baseline due to smoke delivered to the external nares in Sham and CHF rabbits before and after central Ang-(1–7) infusion or with Ang-(1–7) and A779. The HR after smoke was compared to baseline HR in Table 1 to determine the change in HR. The bradycardic response from nasopharyngeal stimulation was blunted in CHF but normalized with Ang-(1–7). *P<0.05 compared with Sham+aCSF. †P<0.05 compared with CHF+aCSF.
Resting RSNA following Ang-(1–7)
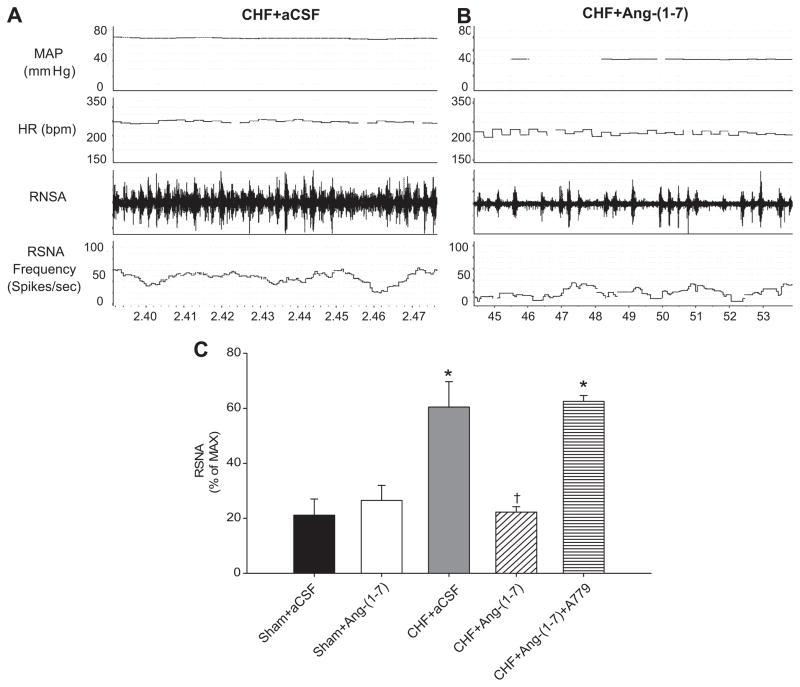
Figure 3 shows an original recording of baseline MAP, HR, and RSNA in a CHF rabbit given aCSF (Figure 3A) and a CHF rabbit given ICV Ang-(1–7) (Figure 3B). The frequency of sympathetic bursts in CHF+Ang-(1–7) appears to be lower than that in CHF+aCSF. The mean data for all groups (Figure 3C) shows that CHF+aCSF rabbits had significantly higher RSNA than sham rabbits. Ang-(1–7) lowered resting RSNA in CHF and A779 treatment returned RSNA to CHF+aCSF levels.
Figure 3.
Baseline RSNA in rabbits with pacing-induced CHF with and without ICV Ang-(1–7) infusion. A: An original recording of MAP, HR, raw RSNA, and RSNA frequency from a CHF rabbit receiving aCSF. B: A recording from a different CHF rabbit receiving ICV Ang-(1–7). C: Mean data for baseline RSNA expressed as a percent of maximum nerve activity in each group of rabbits. Ang-(1–7) significantly reduced RSNA in CHF rabbits. This effect was abolished with A779 infusion. *P<0.05 compared with Sham+aCSF. †P<0.05 compared with CHF+aCSF.
Cardiac and Sympathetic Baroreflex Function in Heart Failure
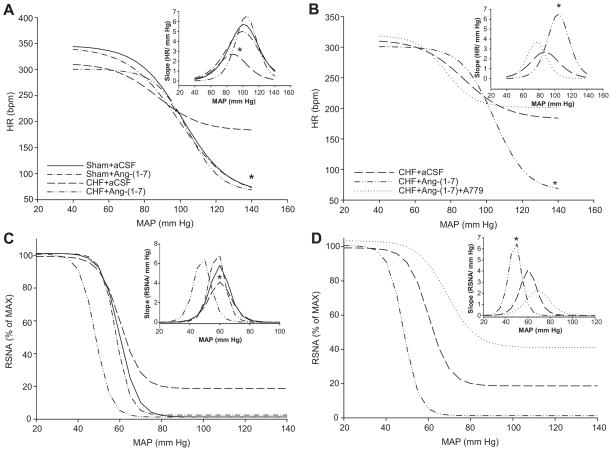
Representative recordings of baroreflex changes are show in Figure S2 (please see http://hyper.ahajournals.org). Figure 4 shows composite baroreflex curves of HR and RSNA for all groups before autonomic blockade. In the unblocked conscious state, baroreflex sensitivity was reduced in CHF+aCSF rabbits (Sham+aCSF: 5.6±0.5 bpm/mm Hg; CHF+aCSF: 2.6±0.3 bpm/mm Hg; P<0.05). Ang-(1–7) infusion had no effect on BRS in sham animals but significantly improved baroreflex sensitivity in CHF animals (CHF+aCSF: 2.6±0.3 bpm/mm Hg; CHF+Ang-(1–7): 6.6±1.0 bpm/mm Hg; P<0.05). HR range was also significantly increased in CHF rabbits given central Ang-(1–7) (CHF+aCSF: 129.9±15.1 bpm; CHF+Ang-(1–7): 236.7±4.7 bpm; P<0.05). This improvement in baroreflex function was largely due to an enhancement of the minimum HR achieved after administration of PE (Sham+aCSF: 62.1±7.3 bpm; Sham+Ang-(1–7): 57.3±5.3 bpm; CHF+aCSF: 182.4±12.8 bpm; CHF+Ang-(1–7): 64.2±7.2 bpm; P<0.05). There were no differences in the BP50 between groups.
Figure 4.
Composite arterial baroreflex curves for control of HR and RSNA in sham and CHF rabbits infused with ICV aCSF or Ang-(1–7) and the first derivative of the baroreflex curves representing the baroreflex gain (inset). A: Composite baroreflex curves for control of HR. * Minimum HR and maximal gain are significantly lower in all groups compared with CHF+aCSF (P<0.05). B: Composite baroreflex curves of HR and baroreflex gain in CHF following Ang-(1–7)+A779 infusion. CHF+aCSF and CHF+Ang-(1–7) curves are the same curves from panel A. * Minimum HR is significantly lower and peak baroreflex slope is significantly higher in CHF+Ang-(1–7) compared with other groups (P<0.05). C: Composite baroreflex curves for control of RSNA. Curves represent the same groups as in panel A. * Maximal gain is significantly lower in CHF+aCSF compared to all other groups (P<0.05). D: Composite baroreflex curves of RSNA and baroreflex gain in CHF following Ang-(1–7)+A779 infusion. Curves represent the same groups as in panel B. CHF+aCSF and CHF+Ang-(1–7) curves are the same curves from panel C. * Peak baroreflex slope is significantly higher in CHF+Ang-(1–7) compared with other groups (P<0.05).
For baroreflex control of RSNA, CHF significantly depressed baroreflex gain which was normalized with Ang-(1–7), but no significant changes in minimum or maximum RSNA were observed (Figure 4C). A779 abolished the improvement in baroreflex gain seen after Ang-(1–7) infusion (Figure 4D). The mean curve parameters for baroreflex control of RSNA are shown in Table S4 (please see http://hyper.ahajournals.org).
A779 abolished the improvement in baroreflex gain (CHF+Ang-(1–7): 6.6±1.0 bpm/mmHg; CHF+Ang-(1–7)+A779: 3.6±0.4 bpm/mmHg; P<0.05) and the improvement in the minimum heart rate (CHF+Ang-(1–7): 129.9±15.1 bpm; CHF+Ang(1–7)+A779: 236.7±4.7 bpm; P<0.05) seen from Ang-(1–7).
Cardiac Baroreflex Function after Autonomic Blockade
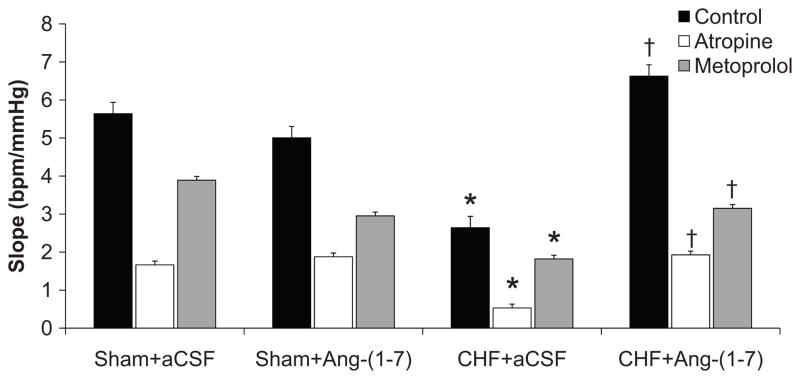
Figure 5 shows mean data for the peak baroreflex slopes in the four groups of rabbits before and after intravenous administration of atropine or metoprolol. The peak slope was significantly decreased in the CHF+aCSF group compared to Sham+aCSF in control, atropine, and metoprolol states. Ang-(1–7) had no effect on baroreflex slope in sham animals after autonomic blockade. CHF+Ang-(1–7) animals showed increased baroreflex slope in the control, atropine, and metoprolol states compared to the same state in the CHF+aCSF group. Additional baroreflex curve parameters following autonomic blockade are presented in Table S3 (please see http://hyper.ahajournals.org).
Figure 5.
Maximal baroreflex gain (peak slope) from baroreflex response in Sham and CHF rabbits before and after central Ang-(1–7) infusion in the unblocked (control) state and following atropine or metoprolol. *P<0.05 compared with same pharmacological group in Sham+aCSF. †P<0.05 compared with same pharmacological treatment in CHF+aCSF.
Discussion
The present study was carried out to determine the effects of chronic central infusion of Ang-(1–7) on baroreflex control of sympathetic outflow and cardiac sympathovagal balance in CHF. The most important findings of this study are that, 1) central Ang-(1–7) enhances arterial baroreflex control of RSNA in CHF, 2) Ang-(1–7) enhances baroreflex control of HR by a profound effect on vagal outflow, and 3) these effects were blocked by central administration of a mas receptor antagonist.
Chronic ICV infusion of Ang-(1–7) resulted in a decrease in resting HR and RSNA and an increase in baroreflex gain in animals with CHF. HRV was significantly increased in CHF rabbits treated with Ang-(1–7). The HR response to cigarette smoke (a vagally mediated bradycardia)24 was blunted in CHF animals and restored to control following central Ang-(1–7) infusion. The change in baseline HR following administration of atropine was augmented by central Ang-(1–7) infusion. An enhancement of vagal outflow in CHF rabbits receiving ICV Ang-(1–7) is also supported by the increased baroreflex slope following metoprolol. The change in HR following administration of the β-1 blocker metoprolol was also significantly reduced in CHF animals receiving central infusion of Ang-(1–7) indicating decreased cardiac sympathetic outflow. The fact that both cardiac sympathetic outflow and RSNA were decreased following ICV Ang-(1–7) in CHF suggests a global reduction in sympathetic outflow by Ang-(1–7). Thus, Ang-(1–7) exerts both a cardiac vagotonic effect as well as a sympathoinhibitory effect in the setting of CHF thus restoring sympathovagal balance.
The central effects of Ang-(1–7) are not uniformly agreed upon. Gomes da Silva et al. injected the mas receptor antagonist, A-779 in the paraventricular nucleus of anesthetized rats and observed a decrease in RSNA suggesting that Ang-(1–7) is sympatho-excitatory.18 However, this effect was transient compared to Ang II. On the other hand, other studies suggest that Ang-(1–7) may have a sympathoinhibitory role. Gironacci et al. showed that Ang-(1–7) decreased norepinephrine release from the hypothalamus of SHR.27 The current study supports the view that Ang-(1–7) opposes the actions of Ang II with a sympathoinhibitory effect in CHF.
The mechanism(s) by which Ang-(1–7) enhances arterial baroreflex function is not completely understood. There is substantial data showing that Ang II reduces baroreflex sensitivity by actions at several sites in the medulla.28 For instance, Ang II acting through the AT1R in the NTS inhibits baroreflex function.29 Sakima et al. showed that in rats with low levels of brain angiotensinogen, inhibition of Ang-(1–7) reduced baroreflex sensitivity.30 Data from this same laboratory have convincingly shown that blockade of Ang-(1–7) receptors reduced baroreflex function in Sprague-Dawley rats when the Ang-(1–7) antagonist, (D-Ala7)-Ang-(1–7) was injected into the NTS.23 This maneuver most likely shifted the balance between Ang II and Ang-(1–7) towards the baroreflex inhibitory effects of Ang II.
A unique finding in the present study is that chronic Ang-(1–7) infusion into the brain activates the cardiac vagus in animals with CHF, that have low resting vagal tone. While these studies cannot determine the site of Ang-(1–7) activation of central nuclei that may contribute to this vagal effect it is likely that at least one area is the NTS. A study by Campagnole-Santos et al. showed that microinjection of Ang-(1–7) into the NTS in anesthetized rats resulted in bradycardia.31 Importantly, Barnes et al. showed that Ang-(1–7) stimulated neurons in the dorsal motor nucleus of the vagus in a canine brain slice preparation.32 Furthermore, Becker et al. have shown intense binding of Ang-(1–7) to mas receptors in neurons in the NTS.33 Thus, it is likely that the bradycardic effect of central Ang-(1–7) along with the enhancement in baroreflex function is mediated by activation of the NTS. This notion would also explain the sympatho-inhibition and improvement in baroreflex function following Ang-(1–7) infusion. Since mas receptors have also been identified in other nuclei known to be rich in Ang II receptors such as the paraventricular nucleus and rostral ventrolateral medulla,33 it is also possible that other structures are responsible for the central effects of Ang-(1–7).
The cellular actions of Ang-(1–7) are still being defined. However it is clear that in neurons, working through the G-protein coupled mas receptor, Ang-(1–7) activates a nNOS dependent mechanism that regulates neuronal excitability. In catecholaminergic neurons in culture (CATH.a) Yang et al. showed that Ang-(1–7) increased nitric oxide (NO) formation.34 This effect was inhibited by both the mas receptor antagonist, A779 and by selective nNOS inhibition but not by eNOS or iNOS inhibition. More importantly, patch clamp recordings of outward potassium current showed that Ang-(1–7) significantly increased this current. The increase in current was also blocked by A779 or specific nNOS inhibition. These data would suggest that Ang-(1–7) contributes to a reduction in neuronal excitability. This is pertinent to the CHF state and sympathoexcitation. We have recently shown a reduction in the potassium channel protein Kv4.3 in the medulla of rats with CHF.35 Furthermore, Ang II working through the AT1R reduces potassium channel current thus contributing to an increase in neuronal excitability.35 Therefore, based on the current study and those cited above we propose that one function of Ang-(1–7) is to limit neuronal discharge in opposition to the actions of Ang II.
This effect may be mediated by enhanced NO formation. The effects of Ang-(1–7) on baroreflex function in our study were blocked by the mas receptor antagonist A779. This suggests that the effects of Ang-(1–7) are mediated by the mas receptor. However, some studies suggest that Ang-(1–7) also has affinity for the Ang II receptors. 36, 37 It is also possible that the mas receptor itself may functionally interact with AT1 and AT2.38 It is also unknown whether endogenous mas receptor expression is altered in CHF. Thus, studies investigating the physiological changes and interactions of the mas receptor are necessary to determine its role in the two axes of the RAS.
While previous studies show central administration of Ang-(1–7) evokes hypotensive effects in both normal and disease states,12, 17 the current study only shows significant effects of Ang-(1–7) in the CHF state and no change in MAP in either state. We did however observe a trend for a decrease in MAP and HR in the Sham+Ang-(1–7) group. It is also possible that higher doses of Ang-(1–7) may show significant effects in Sham animals. In addition, previous studies have been performed only in the anesthetized state or in rodent models. The lack of an effect in Sham animals in the present study may also be due to the use of the conscious rabbit model. Finally, it is possible that the physiological effects of Ang-(1–7) are primarily seen in disease states following an imbalance of Ang II and Ang-(1–7).
The balance between Ang II and Ang-(1–7) is critically dependent on the production and activity of ACE and ACE2. In a previous study we showed differential expression of ACE and ACE2 in the medulla and hypothalamus from CHF rabbits.7 In CHF animals the balance between these enzymes is tipped towards ACE and the generation of Ang II thereby promoting sympathoexcitation. These data therefore suggest that overexpression of ACE2 and concurrent increased levels of Ang-(1–7) would provide protection from excessive sympathetic outflow.
Perspectives
In summary, these data provide strong evidence that Ang-(1–7) given centrally can reduce sympathetic function and enhance baroreflex sensitivity in a sympathoexcitatory state such as CHF. The fact that all the components of the renin-Ang II system have been found in the central nervous system39 suggests that a therapy could be developed to enhance the sympathoinhibitory effects of Ang-(1–7). Precedence for this concept has been provided in other tissues. It has been shown that upregulation of ACE2 in the lung can ameliorate the pathology associated with pulmonary hypertension.40 Systemic infusion of ACE2 has been shown to reduce oxidative stress associated with Ang II41 and reduce cardiac fibrosis in response to Ang II.42 Thus, this apparent “natural inhibitor” of Ang II and its deleterious effects should and most likely will be exploited for its therapeutic potential.
Supplementary Material
Acknowledgments
We acknowledge the expert technical assistance of Johnnie F. Hackley, Sarah C. Clayton, Richard Robinson and Kaye Talbitzer.
Source of Funding:
This study was supported by National Institutes of Health Grant PO1 HL-62222.
Footnotes
Conflicts
None
References
- 1.Floras JS. Clinical aspects of sympathetic activation and parasympathetic withdrawal in heart failure. J Am Coll Cardiol. 1993;22:72A–84A. doi: 10.1016/0735-1097(93)90466-e. [DOI] [PubMed] [Google Scholar]
- 2.Francis GS. Neurohumoral mechanisms involved in congestive heart failure. Am J Cardiol. 1985;55:15A–21A. doi: 10.1016/0002-9149(85)90791-x. [DOI] [PubMed] [Google Scholar]
- 3.Francis GS. The relationship of the sympathetic nervous system and the renin-angiotensin system in congestive heart failure. Am Heart J. 1989;118:642–648. doi: 10.1016/0002-8703(89)90291-3. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Gao L, Wang H, Zucker IH, Wang W. Interaction between cardiac sympathetic afferent reflex and chemoreflex is mediated by the NTS AT1 receptors in heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H1216–H1226. doi: 10.1152/ajpheart.00557.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiBona GF, Jones SY, Brooks VL. ANG II receptor blockade and arterial baroreflex regulation of renal nerve activity in cardiac failure. Am J Physiol Regul Integr Comp Physiol. 1995;269:R1189–R1196. doi: 10.1152/ajpregu.1995.269.5.R1189. [DOI] [PubMed] [Google Scholar]
- 6.Heringer-Walther S, Batista EN, Walther T, Khosla MC, Santos RAS, Campagnole-Santos MJ. Baroreflex improvement in SHR after ACE inhibition involves angiotensin-(1–7) Hypertension. 2001;37:1309–1314. doi: 10.1161/01.hyp.37.5.1309. [DOI] [PubMed] [Google Scholar]
- 7.Kar S, Gao L, Zucker IH. Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J Appl Physiol. 2010;108:923–932. doi: 10.1152/japplphysiol.00840.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brosnihan KB, Li P, Ferrario CM. Angiotensin-(1–7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27:523–528. doi: 10.1161/01.hyp.27.3.523. [DOI] [PubMed] [Google Scholar]
- 9.Averill DB, Ishiyama Y, Chappell MC, Ferrario CM. Cardiac angiotensin-(1–7) in ischemic cardiomyopathy. Circulation. 2003;108:2141–2146. doi: 10.1161/01.CIR.0000092888.63239.54. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Qian C, Roks AJM, Westermann D, Schumacher S, Escher F, Schoemaker RG, Reudelhuber TL, van Gilst WH, Schultheiss H, Tschöpe C, Walther T. Circulating rather than cardiac angiotensin-(1–7) stimulates cardioprotection after myocardial infarction. Circulation: Heart Failure. 2010;3:286–293. doi: 10.1161/CIRCHEARTFAILURE.109.905968. [DOI] [PubMed] [Google Scholar]
- 11.Giani JF, Muñoz MC, Mayer MA, Veiras LC, Arranz C, Taira CA, Turyn D, Toblli JE, Dominici FP. Angiotensin-(1–7) improves cardiac remodeling and inhibits growth-promoting pathways in the heart of fructose-fed rats. Am J Physiol Heart Circ Physiol March. 2010;298:H1003–H1013. doi: 10.1152/ajpheart.00803.2009. [DOI] [PubMed] [Google Scholar]
- 12.Benter IF, Yousif MH, Anim JT, Cojocel C, Diz DI. Angiotensin-(1–7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am J Physiol Heart Circ Physiol. 2006;290:H684–H691. doi: 10.1152/ajpheart.00632.2005. [DOI] [PubMed] [Google Scholar]
- 13.Chappell MC, Brosnihan KB, Diz DI, Ferrario CM. Identification of angiotensin-(1–7) in rat brain. evidence for differential processing of angiotensin peptides. J Biol Chem. 1989;264:16518–16523. [PubMed] [Google Scholar]
- 14.Schiavone MT, Santos RA, Brosnihan KB, Khosla MC, Ferrario CM. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1–7) heptapeptide. Proceedings of the National Academy of Sciences. 1988;85:4095–4098. doi: 10.1073/pnas.85.11.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potts PD, Horiuchi J, Coleman MJ, Dampney RAL. The cardiovascular effects of angiotensin-(1–7) in the rostral and caudal ventrolateral medulla of the rabbit. Brain Res. 2000;877:58–64. doi: 10.1016/s0006-8993(00)02626-3. [DOI] [PubMed] [Google Scholar]
- 16.Gomes da Silva AQ, Sousa dos Santos RA, Peliky Fontes MA. Blockade of endogenous angiotensin-(1–7) in the hypothalamic paraventricular nucleus reduces renal sympathetic tone. Hypertension. 2005;46:341–348. doi: 10.1161/01.HYP.0000179216.04357.49. [DOI] [PubMed] [Google Scholar]
- 17.Silva LCS, Fontes MAP, Campagnole-Santos MJ, Khosla MC, Campos JRR, Guertzenstein PG, Santos RAS. Cardiovascular effects produced by micro-injection of angiotensin-(1–7) on vasopressor and vasodepressor sites of the ventrolateral medulla. Brain Res. 1993;613:321–325. doi: 10.1016/0006-8993(93)90920-i. [DOI] [PubMed] [Google Scholar]
- 18.Gomes da Silva AQ, Sousa dos Santos RA, Peliky Fontes MA. Blockade of endogenous angiotensin-(1–7) in the hypothalamic paraventricular nucleus reduces renal sympathetic tone. Hypertension. 2005;46:341–348. doi: 10.1161/01.HYP.0000179216.04357.49. [DOI] [PubMed] [Google Scholar]
- 19.Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1–7) Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 20.Campagnole-Santos Maria J, Guimarães Priscila. Angiotensin-(1–7) ICV chronic infusion attenuates DOCA-salt hypertension. FASEB J. 2008;22:953.12. [Google Scholar]
- 21.Liu JL, Kulakofsky J, Zucker IH. Exercise training enhances baroreflex control of heart rate by a vagal mechanism in rabbits with heart failure. J Appl Physiol. 2002;92:2403–2408. doi: 10.1152/japplphysiol.00039.2002. [DOI] [PubMed] [Google Scholar]
- 22.Campagnole-Santos MJ, Heringer SB, Batista EN, Khosla MC, Santos RA. Differential baroreceptor reflex modulation by centrally infused angiotensin peptides. Am J Physiol Regul Integr Comp Physiol. 1992;263:R89–R94. doi: 10.1152/ajpregu.1992.263.1.R89. [DOI] [PubMed] [Google Scholar]
- 23.Diz DI, Garcia-Espinosa MA, Gallagher PE, Ganten D, Ferrario CM, Averill DB. Angiotensin-(1–7) and baroreflex function in nucleus tractus solitarii of (mRen2)27 transgenic rats. J Cardiovasc Pharmacol. 2008;51:542–548. doi: 10.1097/FJC.0b013e3181734a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mousa TM, Gao L, Cornish KG, Zucker IH. Effects of angiotensin II on autonomic components of nasopharyngeal stimulation in male conscious rabbits. J Appl Physiol. 2005;98:1607–1611. doi: 10.1152/japplphysiol.01322.2004. [DOI] [PubMed] [Google Scholar]
- 25.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: A role for angiotensin II. Circulation. 2000;102:1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Zucker IH. Regulation of sympathetic nerve activity in heart failure: A role for nitric oxide and angiotensin II. Circ Res. 1999;84:417–423. doi: 10.1161/01.res.84.4.417. [DOI] [PubMed] [Google Scholar]
- 27.Gironacci MM, Valera MS, Yujnovsky I, Pena C. Angiotensin-(1–7) inhibitory mechanism of norepinephrine release in hypertensive rats. Hypertension. 2004;44:783–787. doi: 10.1161/01.HYP.0000143850.73831.9d. [DOI] [PubMed] [Google Scholar]
- 28.Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: Pathways and mechanisms of the medulla oblongata. Brain Res Bull. 2000;51:119–128. doi: 10.1016/s0361-9230(99)00237-3. [DOI] [PubMed] [Google Scholar]
- 29.Matsumura K, Averill DB, Ferrario CM. Angiotensin II acts at AT1receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1611–R1619. doi: 10.1152/ajpregu.1998.275.5.R1611. [DOI] [PubMed] [Google Scholar]
- 30.Sakima A, Averill DB, Kasper SO, Jackson L, Ganten D, Ferrario CM, Gallagher PE, Diz DI. Baroreceptor reflex regulation in anesthetized transgenic rats with low glia-derived angiotensinogen. Am J Physiol Heart Circ Physiol. 2007;292:H1412–H1419. doi: 10.1152/ajpheart.00984.2006. [DOI] [PubMed] [Google Scholar]
- 31.Campagnole-Santos MJ, Diz DI, Santos RA, Khosla MC, Brosnihan KB, Ferrario CM. Cardiovascular effects of angiotensin-(1–7) injected into the dorsal medulla of rats. Am J Physiol Heart Circ Physiol. 1989;257:H324–H329. doi: 10.1152/ajpheart.1989.257.1.H324. [DOI] [PubMed] [Google Scholar]
- 32.Barnes KL, Knowles WD, Ferrario CM. Angiotensin II and angiotensin (1–7) excite neurons in the canine medulla in vitro. Brain Res Bull. 1990;24:275–280. doi: 10.1016/0361-9230(90)90215-l. [DOI] [PubMed] [Google Scholar]
- 33.Becker LK, Etelvino GM, Walther T, Santos RAS, Campagnole-Santos MJ. Immunofluorescence localization of the receptor mas in cardiovascular-related areas of the rat brain. Am J Physiol Heart Circ Physiol. 2007;293:H1416–H1424. doi: 10.1152/ajpheart.00141.2007. [DOI] [PubMed] [Google Scholar]
- 34.Yang R, Yin J, Li Y, Zimmerman MC, Schultz HD. Angiotensin-(1–7) increases neuronal potassium current via a nitric oxide-dependent mechanism. Am J Physiol Cell Physiol. 2011;300:C58–C64. doi: 10.1152/ajpcell.00369.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao L, Li Y, Schultz HD, Wang W, Wang W, Finch M, Smith LM, Zucker IH. Downregulated Kv4.3 expression in the RVLM as a potential mechanism for sympathoexcitation in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2010 March;298:H945–H955. doi: 10.1152/ajpheart.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1–7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension. 2005;45:960–966. doi: 10.1161/01.HYP.0000160325.59323.b8. [DOI] [PubMed] [Google Scholar]
- 37.Rowe BP, Saylor DL, Speth RC, Absher DR. Angiotensin-(1–7) binding at angiotensin II receptors in the rat brain. Regul Pept. 1995;56:139–146. doi: 10.1016/0167-0115(95)00010-9. [DOI] [PubMed] [Google Scholar]
- 38.de Castro CH, Souza dos Santos RA, Ferreira AJ, Bader M, Alenina N, Pinto de Almeida A. Evidence for a functional interaction of the angiotensin-(1–7) receptor mas with AT1 and AT2 receptors in the mouse heart. Hypertension. 2005;46:937–942. doi: 10.1161/01.HYP.0000175813.04375.8a. [DOI] [PubMed] [Google Scholar]
- 39.Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: Fact, hypothesis, or fantasy. Physiology. 2008;23:187–193. doi: 10.1152/physiol.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shenoy V, Qi Y, Katovich MJ, Raizada MK. ACE2, a promising therapeutic target for pulmonary hypertension. Current Opinion in Pharmacology. 2011;11:150–155. doi: 10.1016/j.coph.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong J, Guo D, Chen CB, Wang W, Schuster M, Loibner H, Penninger JM, Scholey JW, Kassiri Z, Oudit GY. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension. 2011;57:314–322. doi: 10.1161/HYPERTENSIONAHA.110.164244. [DOI] [PubMed] [Google Scholar]
- 42.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang X, Penninger JM, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–728. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.