Abstract
The intervertebral disc is a soft tissue, positioned between each of the vertebrae, that accommodates applied biomechanical forces to the spine. The central compartment of the disc contains the nucleus pulposus (NP), which is enclosed by the annulus fibrosus and the endplate cartilage. The NP is derived from the notochord, a rodlike structure of mesodermal origin. Development of the notochord is tightly regulated by interactive transcription factors and target genes. Since a number of these molecules are unique, they have been used for cell lineage and fate mapping studies of tissues of the intervertebral disc. These studies have shown that in a number of species including human, NP tissue retains notochordal cells throughout life. In the adult NP, there are present both large and small notochordal cells, as well as a progenitor cell population which can differentiate along the mesengenic pathway. Since tissue renewal in the intervertebral disc is dependent on the ability of these cells to commit to the NP lineage and undergo terminal differentiation, studies have been performed to assess which signaling pathways may regulate these activities. The notch signaling pathway is active in the intervertebral disc and is responsive to hypoxia, probably through HIF-1α. From a disease viewpoint, it is hypothesized that an oxemic shift, possibly mediated by alterations in the vascular supply to the tissues of the disc, would be expected to lead to a failure in notochordal progenitor cell activation and a decrease in the number of differentiated cells. In turn, this would lead to decrements in function and enhancement of the effect of agents that are known to promote disc degeneration.
Keywords: notochord, nucleus pulposus, intervertebral disc, axial skeleton, brachyury, Sonic hedgehog, notch, disc degeneration
I. INTRODUCTION
The incidence of low back pain is extraordinarily high and is a cause for societal and fiscal concern. Most often, it is due to degenerative changes in the disc that contribute to intervertebral osteochondrosis as well as osteophytosis; patients also suffer from associated arthritic changes of the zygapophyseal joints. Compromised disc function leads to a loss of stability of the spine, while limitations in flexion and extension interfere with posture and mobility. Surgeons have developed an armamentarium of approaches for repairing the damaged disc. While the more common strategies provide pain relief, they cannot prevent further degeneration, nor completely restore disc function. Viewed from this perspective, these procedures are less than optimal, as they fail to prevent further deterioration of the health of the compromised spine.
Discussions concerning the function of the nucleus pulposus have been ongoing since the sixteenth century. Although anatomical features of the discs were described by Vesalius in 1543, and by Winslow in 1776, it was not until the 19th century that von Luschka provided the first detailed account of the disc structure.
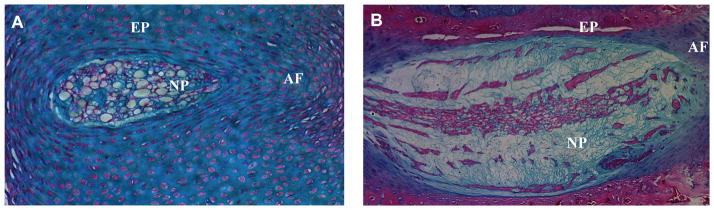
The disc is a soft tissue positioned between each of the vertebrae of the spine. It permits a range of motions between vertebrae and accommodates biomechanical forces applied to the spine. The disc structure is complex, including a peripheral annulus fibrosus that composed of tightly packed parallel collagen type I fibrils. Sharpey fibers from the annulus are inserted into contiguous superior and inferior vertebral bodies and cartilaginous endplates. The inner aspect of the annulus fibrosus is poorly organized and contains both collagen type I and II and aggregating proteoglycans. The annulus and the cartilaginous endplates enclose the nucleus pulposus, an aggrecan-rich gel-like tissue. In the neonate, the nucleus pulposus is highly cellular with relatively little extracellular proteoglycan. In contrast, in the adult, the proportion of cells to matrix is low (Fig. 1). As the disc matures, the composition of the nucleus pulposus changes: the large vacuolated cells that have been assumed to be of notochordal origin decrease in number, whereas smaller chondrocyte-like cells increase. These cellular events are also accompanied by the changes in the extracellular microenvironment.
FIGURE 1.
A saggital section through neonatal (A) and mature (B) rat intervertebral disc showing the nucleus pulposus (NP), annulus fibrosus (AF), and endplate cartilage (EP). Note that in the neonate, the NP is highly cellular containing large vacuolated notochordal cells and there is little deposition of extracellular proteoglycan matrix. At this stage the sclerotomal-derived EP and AF are not completely organized and endochondral ossification of the vertebrae is yet to occur. Original Mag. X10. In the adult NP, the ratio of proteoglycan matrix to cells is high. The AF exhibits a characteristic lamellar structure in the adult. Original Mag. X4.
Since vascularity of the disc is limited, the oxygen tension within the nucleus pulposus is low, causing the cells to tune their metabolism to the available oxygen supply. The hypoxic nucleus pulposus cells evidence almost complete reliance on the glycolytic pathway to generate metabolic energy, which is controlled by the transcription factor HIF-1α.1 HIF-1 regulates expression of a number of genes involved in glycolysis as well as mitochondrial energy metabolism.2,3 Not surprisingly, the disc cells have very few mitochondria, an extensive ER, and a large number of vacuoles filled with an osmotically active material.4,5 Measurement of the osmotic pressure of the disc shows that it is hypertonic, almost 200 mOsm/kg above the norm. Our previous work showed that adaptation to this osmotic environment is mediated through the activities of a TonEBP/NFAT5.6,7 Upon activation, TonEBP binds to the TonE element of genes that control osmolarity by regulating levels of non-ionic osmolytes, eg, betaine, taurine, myoinositol, and sorbitol. Thus, this transcription factor regulates the expression of osmoregulatory genes that are required to maintain the survival and function of the notochordal nucleus pulposus.6
There has been considerable debate concerning the embryological origin of the nucleus pulposus. For example, while Gegenbauer and Hertig considered that the nucleus pulposus was derived from the notochord, luminaries such as Virchow, Heildberg, and Weiss opined that the nucleus pulposus was derived from peri- or extra-notochordal tissues. While the evidence has mounted that the nucleus pulposus is notochordal in origin, surprisingly, these arguments have continued to the present day, especially those that relate to the postnatal fate of the notochordal cells. As a result, this debate has obfuscated our understanding of the fate of cells of the nucleus pulposus in the adult, especially in relationship to degenerative disc disease.8 The focus of the current debate is whether the pathogenesis of disc disease in adults is due to the loss and/or the replacement of the original notochordal cells by other cell types. The goal of this review is to address these competing views and elaborate on the role of the notch signaling system on the homeostasis of notochordal cells of the nucleus pulposus.
II. NOTOCHORD FUNCTION DURING EMBRYOGENESIS AND INTERVERTEBRAL DISC FORMATION
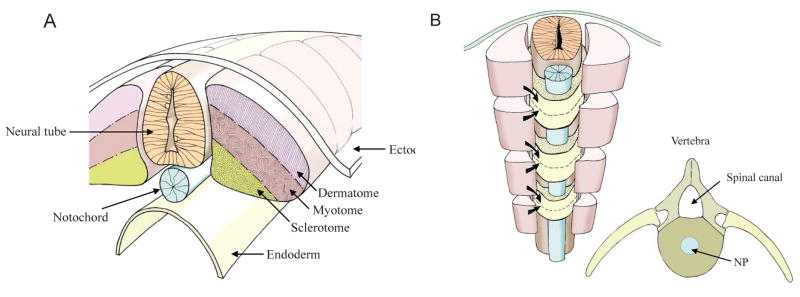
The body axis of vertebrates, established during gastrulation, is characterized by the formation of the notochord. This rod-like midline structure is of mesodermal origin and serves as a primitive axial skeleton.9–11 Flanked by two rows of somites, it causes patterning of the paraxial mesoderm to generate the metameric array of vertebrae. Each somite gives rise to cell populations that are progenitors of the dermis/muscle (dermatomyotome) and axial skeleton (sclerotome)12,13 (Fig. 2). The notochord may also specify other tissues such as the asymmetry of the developing heart.14,15
FIGURE 2.
(A) During segmentation the paraxial mesoderm forms pairs of somites along the neural tube (light orange) and notochord (blue). Each somite is composed of a dermatome (light purple), myotome (light brown), and sclerotome (dark yellow). The ectoderm lies above and the endoderm below. (B) Sclerotomal cells migrate from adjacent somites above and below each future vertebra. Dermatomal cells stream beneath the ectoderm to form the dermis, while the myotomal cells form muscle. Insert B shows the architecture of the vertebrae with the spinal canal, spinal processes, and the nucleus pulposus in blue. Courtesy of Dr. Richard Dryden.
During the segmentation period of development, the notochord acquires a thick three-layered connective tissue sheath consisting of large diameter collagenous fibers. The mechanical strength imparted by this firm sheath together with the turgor pressure generated by the vacuolated notochordal cells provides stiffness to the combined structure that permits elongation of the developing embryo.9 From week 5 to week 12 of development, the notochord in the human embryo gradually breaks down and cellular remnants, enclosed within a primitive annulus fibrosus, form the presumptive nucleus pulposus16,17 (Fig. 2). However, there remains some uncertainty whether the notochordal cells, located in the developing vertebral bodies, either disintegrate, or migrate to the intervertebral regions and contribute to the formation of the nucleus pulposus.18,19 Possibly, a few dormant notochordal cells persist in the adult vertebral body and, if activated, may give rise to chordomas.20 Thus, it would not be unreasonable to assume that in chordate embryos, the nucleus pulposus is the only tissue that is completely derived from the notochord.20 Based on these earlier investigations, considerable information has accrued concerning the relationship between the notochord and the nucleus pulposus during development. In contrast, the fate of the notochordal cells as well as their involvement in functional maintenance of the intervertebral disc in postnatal life is less well understood. Nonetheless, there is now overwhelming evidence that the notochordal nature of the nucleus pulposus is preserved in the adult state.20–23
III. MOLECULAR REGULATION OF NOTOCHORD AND DISC DEVELOPMENT
The development of the notochord from the axial mesenchyme is specified by a number of signaling molecules and transcription factors.24,25 Following neurulation, the notochord expresses transcription factors encoded by Foxa2/HNF-3β, the secreted patterning factor, Sonic Hedgehog (Shh), as well as brachyury, a T-box transcription factor that determines cell differentiation and survival. During early vertebrate development, brachyury is first expressed in the mesoderm, and only later, after separation of axial and paraxial lineages, is its expression restricted to the notochord and tailbud.26 In homozygotes, Dobrovolskaïa-Zavadskaïa reported that these mutations are associated with developmental defects in tail and vertebrae.27 Another important transcription factor required for the development of the node, notochord, and floor plate of the neural tube is Foxa2/HNF-3β.28,29 Foxa2 mutants do not generate a node and a definitive endoderm, and lack all notochordal cells.
Sonic Hedgehog is involved in notochord development and along with noggin mediates inductive actions of the notochord.30–32 However, little is know about Shh expression and function in the postnatal nucleus pulposus. Not surprisingly, Shh has been used for lineage studies of the notochord, while brachyury has been utilized as both an indicator of the notochord as well as a molecular marker of the cells of the nucleus pulposus. Since the expression of these important molecules provides insights into the ontology of the cells that contribute to, and are retained in, the mature nucleus pulposus, their expression will be discussed in a later section of this review.
It is noteworthy that notochord development is influenced indirectly by the abnormal formation of vertebrae. Thus, mutants that lack collagen type II,18,33 the homeobox transcription factors Bapx1,34,35 and the paired box transcription factors Pax1 and Pax936,37 that are expressed in the sclerotome, display abnormal development of the vertebral bodies and notochord. In these mutants, the notochord forms normally, but it is not removed from the vertebral bodies, thereby compromising nucleus pulposus morphogenesis. In addition, two other genes, Sox5 and Sox6 encode highly homologous transcription factors, L-Sox5 and Sox6, respectively, which are expressed in sclerotome-derived cells and in the notochord.38 These proteins are required for peri-notochordal sheath formation, cell survival, and development of the nucleus pulposus.39 Not surprisingly, Sox5−/−/Sox6−/− embryos exhibit extreme defects in notochord development and formation of the axial skeleton.39
Among small molecules regulating notochord function are the retinoids. These molecules are secreted by the paraxial mesoderm and known to influence both the overlying neural plate as well as the development of vertebrae and the intervertebral disc. Retinoids are also known to regulate expression of hox genes, which are important in specifying the anterior-posterior axis and the formation of vertebral elements.40 Imbalance of retinoic acid levels leads to abnormal development of the axial skeleton, disrupted notochord segmentation, and formation of oversized vertebrae and vertebral fusion.41,42 It is, thus, likely that retinoids may have a role in the establishment of somites.43 If so, this would explain why a disturbance in retinoid synthesis leads to vertebral fusions and misshaped/sized vertebrae.
One final group of molecules that needs to be briefly mentioned are components of the notch signaling system. While they will be described in more detail later in this review, it is important to comment that this group of proteins appear to play a critical role at both the pre- and post-gastrulation stages of development. There is evidence to suggest that notch regulates proliferation of floor plate cells.44–46 Later, jagged and other notch ligands are expressed in the notochord.47–49 Interestingly, activation of notch signaling through jagged results in preferential development of non-vacuolated notochordal cells that are responsible for the formation of the peri-notochordal sheath.50 The notch target jagged also regulates notochordal patterning activities and the levels of hedgehog, required for speciation of muscle cells. Since the notochord serves as an inducer of a multitude of critical activities, the following question is raised: is this signaling system active in the adult nucleus pulposus and, if indeed, what is its role in maintaining and organizing the annulus and endplate cartilage in the post-embryonic state?
In summary, the development of the notochord and paraxial mesoderm is tightly regulated by a series of interactive transcription factors and target genes. Since a number of these molecules are unique they can be used successfully for cell lineage and fate mapping studies of tissues of the intervertebral disc. Moreover, since the notochord is extraordinarily active in terms of organizing and inducing changes in the paraxial mesoderm, floor plate, and neural tube, the possibility exists that cells of the nucleus may well retain at least a part of this activity in the adult. Indeed, it has been speculated that loss of these activities may coincide with the induction of the degenerative state.
IV. THE CELLULAR COMPOSITION OF THE ADULT NUCLEUS PULPOSUS
Discussion of the cellular composition of the nucleus pulposus in the post-embryonic and adult states has markedly influenced current thinking concerning the functional importance of this tissue. That a heterogeneous population of cells may exist in the nucleus pulposus was supported by the observation from Hunter and his colleagues who showed the presence of small chondrocyte-like and large “actin-filled’ notochordal cells.5,51 Subsequently, two groups of cells were isolated from the nucleus pulposus by flow cytometric analysis, lending support to the argument that the nucleus contained small chondrocyte-like and large notochordal cells.52 Gene expression profile of these two populations showed differences in levels of expression of select markers. Moreover, cell mixing studies indicated that notochordal cells stimulated glycosaminoglycan and aggrecan core protein synthesis by nucleus pulposus cells.53 Henriksson et al. and Risbud et al. raised the possibility that a subpopulation of notochordal cells may serve as “signaling centers” and/or progenitor cells that influence the synthetic activities of neighboring cells and preserve cell type and number.54,55
A number of authorities contend that the number of notochordal cells in the nucleus pulposus declines after birth due to their slow transformation and/or replacement by chondrocyte-like cells.52,56–59 Some argue that a “centripetal sequential replacement mechanism” serves to replace notochordal remnants with chondrocytes, possibly facilitated by chemotactic signals generated by extant notochordal cells.60,61
The observation that cells of the nucleus pulposus appear to be heterogeneous in size has fueled the argument that the cell composition of the nucleus pulposus is species-specific.62–64 For example, in rodents, it is thought that notochordal cells are the dominant cell population in the immature tissue, but few notochordal cells are present after one year.19,65 The decline in notochordal-like cells from the nucleus pulposus is thought to be linked to disc degeneration in certain breeds of dogs.5,8,51,53 Likewise, it has been surmised that notochordal cells are rarely present after adolescence in humans.17,66,67 This prediction has fueled the debate that the disappearance of notochordal cells and their replacement by chondrocytic cells may initiate or contribute to degenerative disc disease. Histological studies using needle punctured mouse discs show sequential transformation of large notochordal cells first into chondrocyte-like cells and then into cells with a fibro-cartilagenous phenotype.68 Recent studies by Gilson et al. and Minogue et al. support this viewpoint.21,22
In contrast to the studies mentioned above, there are a number of investigations in which definitive markers of the notochord have been used to delineate the ontology of cells of the intervertebral disc. Choi et al. created fate maps of the disc cells by knocking into the Shh gene the recombinase gene cre, which is inducible by tamoxifen (ShhCre-ERT2)20. As was discussed earlier, Shh is involved in notochord development and along with noggin mediates inductive actions of the notochord and thus, as expected, Shh-Cre is highly expressed in the notochord.69 When ShhCreERT mice were mated with R26R Cre reporter mice, all cells in the nucleus pulposus were labeled, leading to the conclusion that the entire nucleus pulposus was descended from the notochord. While this finding confirms many earlier studies of notochordal development, it said little about the adult disc. To address this issue, these workers evaluated the expression of the reporter in 19-month-old animals. In these skeletally mature animals, all of the cells of the nucleus pulposus were labeled. This finding strengthened the view that cells in the adult disc were descended from “a homogeneous population of Shhcre” expressing notochordal cells. Not surprisingly, the annulus fibrosus and the cartilaginous endplates were devoid of Shhcre descendant cells, indicating a non-notochordal lineage. Thus, it is evident that even the chondrocyte-like cells of the adult nucleus pulposus are derived from Shh-expressing notochord cells, and not from cells of the surrounding Shh-negative mesenchyme.
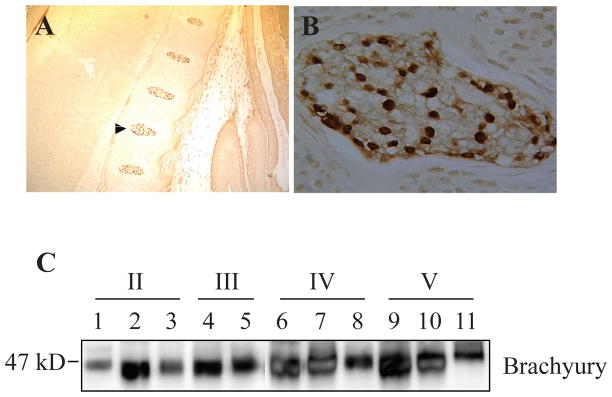
Aside from Shh, in both the notochord and the disc considerable attention has been directed at brachyury. As indicated earlier, brachyury is required for differentiation of axial midline mesoderm into notochord,14,70,71 and misexpression of brachyury gives rise to a fully differentiated ectopic notochord.72 Moreover, duplication of the brachyury gene results in chordomas that are derived from notochordal remnants.73 Thus, the expression of brachyury should serve as a useful guide to the presence of notochordal cells in the adult nucleus pulposus. Genome-wide microarray analysis indicates that adult bovine, as well as human nucleus pulposus tissues, expressed brachyury and different cytokeratins (CK8, 18 and 19).21,22 The latter genes are usually expressed by epithelial-derived tissues, as well as the notochord.74,75 If it is assumed that in these species the notochordal cells are lost from the disc early in life, then these results are unexpected. Moreover, Minogue et al. showed that both the large notochordal and small chondrocyte-like nucleus pulposus cells have substantially overlapping expression patterns.22 Moreover, the differences in expression of marker genes between the two cells types were never greater than ~10-fold, suggesting that both the cell types may have been derived from a common lineage. This inference is in accord with a recent observation that the rabbit notochordal cells can differentiate into cells of different morphologies in vitro.76 Importantly, these studies question the much debated notion that notochordal cells are lost during the postnatal life.21,22;77,78 We have measured brachyury protein expression in embryonic mouse as well as in adult human nucleus pulposus tissue samples.23 Our data show that there is a robust expression of brachyury in the nucleus pulposus, even during degeneration (Fig. 3). Supporting this finding, Hoyland and her colleagues showed that in the degenerate human nucleus pulposus, unlike cytokeratins 8 and 18, brachyury expression remained constant.22
FIGURE 3.
Brachyury expression in cells of the nucleus pulposus. A) Low magnification saggital section of the E15.5 mouse embryo showing brachyury staining in the developing nucleus pulposus. Note that the annulus fibrosus and endplate cartilage is negative (10X). B) High magnification image of the developing nucleus pulposus that is shown by an arrow in panel A (20X). All cells show intense nuclear staining of brachyury. C) Western blot analysis of brachyury expression in nucleus pulposus tissue isolated from progressively degenerate human discs (Thompson Grade II–V). A robust expression of brachyury was seen in all the nucleus pulposus samples. Reproduced with permission.23
While there is considerable agreement on the expression of brachyury, it is acknowledged that Vujovic et al. were unable to show that this gene was expressed by the adult nucleus pulposus.79 Tempering the conclusion that this tissue was not derived from the notochord was the observation that other characteristic proteins such as the cytokeratins were also not detected in this tissue.21,22,77,78,80,81 In the absence of these related proteins, there is little support for the conclusion by Vujovic and colleagues that the notochord does not contribute to the cellular composition of the adult nucleus pulposus.
The ontological studies mentioned earlier provide a rational explanation for the observed variations in cell size within the nucleus pulposus. That variations exist is not surprising and may reflect one or more of the following possibilities. First, within any tissue, cell size differences are the norm, not the exception. Rather than indicating differences in lineage, they may signal variations in metabolic/secretive activity and cell cycle status. Indeed, it would be very unusual if differences in cell size were not apparent in this tissue. Noteworthy, the presence of two cell types with distinct morphologies in the late stages of development of the notochord has been reported: large vacuolated and smaller, spindle-shaped cells.82 Thus, the notochord contains cells of differing size that are similar to those seen in the nucleus pulposus itself. Also relevant to this discussion is that in the growth plate, chondrocyte volumes can vary by as much as eight- to ten-fold.83 Second, it would not be unreasonable to assume that within a single population, cells of differing size express different gene profiles.84 In the study by Chen et al., the two cell types in the nucleus pulposus evidenced substantive differences in expression levels of integrins, collagens, biglycan, and MMPs.52 Again, this is similar to the maturing chondrocytes where substantive differences in transcript expression are reported; there is even the expression of new transcript (collagen type X) by the large mature hypertrophic cells.85 Based on the limitations discussed above and the lack of a highly specific marker that is uniformly expressed within a specific population, it is difficult to accept the view that cells with different lineages (notochord and mesenchymal cells) are present within the nucleus pulposus. Moreover, without unequivocal lineage information, the argument that notochordal cells are lost and replaced by cells of a different lineage is far from convincing.
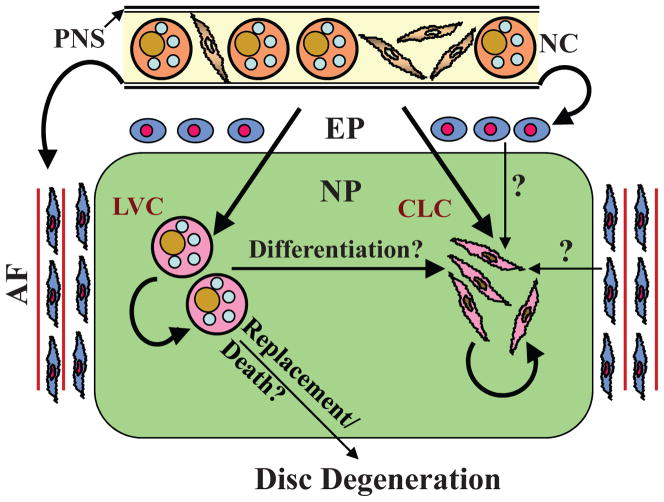
Findings from our own investigations as well as the state-of-the-art lineage mapping studies favor the hypothesis that in all of the animal species mentioned earlier, including human, nucleus pulposus tissue retains notochordal cells throughout life.23 Although it is still possible that development of disc disease is linked to loss of a subpopulation of notochordal cells, ie, “large cells,” either by death, or by de-differentiation to a modified phenotype,68 more definitive studies using molecular genetic approaches are required. We propose that all nucleus pulposus cells, including chondrocyte-like cells, are derived from notochordal precursors and that variations in morphology and size are representative of different stages of maturation and/or function (Fig. 4). Nevertheless, since notochordal cells may retain some stem cell characteristics, it would not be unreasonable to assume that they would continue to proliferate within the disc to maintain cell homeostasis and tissue function.86 Moreover, in the disease state, a change in the hypoxic and hyperosmotic environment, as well as expression of cytokines and growth factors, may serve to limit cell proliferation while provoking terminal differentiation along chondrocyte, fibroblast, and osteoblast lineages. Finally, while an overall decrease in cell number and a reduction in their functional activity may contribute to development of degenerative disc disease, the existing notion that degeneration is due to a selective loss of the notochordal fraction of cells is untenable.
FIGURE 4.
Schematic indicating current theories concerning the origin of cells of the nucleus pulposus (NP). Although it is well established that the notochord (NC) gives rise to large NP cells during embryogenesis, there is discord concerning the origin of small chondrocytic cells in the adult disc. As the NC contains both small spindle-shaped cells as well as large vacuolated cells, we hypothesize that both the large vacuolated cells (LVC) and chondrocyte-like cells (CLC) of the NP are derived from the notochord. These cells then undergo self renewal to maintain cellular homeostasis of the NP tissue. It is possible that LVC may differentiate into CLC. It is known that the perinotochordal sheath (PNS) gives rise to both endplate (EP) chondrocytes and annulus fibrosus (AF) cells. Some researchers are of the opinion that in the adult, endplate chondrocytes and inner annulus fibrosus cells give rise to CLC at the same time replacing LVC. There is debate that loss or replacement of LVC in the nucleus pulposus initiates disc degeneration. Reproduced with permission.23
V. REGULATION OF CELLULAR HOMEOSTASIS: REGENERATION OF THE DEGENERATE DISC
In this review, we have drawn attention to the critical role and fate of notochordal cells in the postnatal intervertebral disc. Where applicable, we have included a discussion of the relationship of notochordal cells to the development of the disease state. It should be noted that in concert with most connective tissues, cell turnover within this hypoxic tissue niche is slow: as indicated in Figure 4, some cells retain their proliferative potential. Moreover, we have shown that a small notochordal progenitor cell population is present within the adult disc and can differentiate along the mesengenic pathway to replace dying cells.86 Thus, tissue renewal in the intervertebral disc is dependent on the ability of notochordal progenitors to commit to the nucleus pulposus lineage and undergo terminal differentiation.
It is now recognized that the notch signaling pathway is central to both of these activities. In blood, skin, and gut epithelium, the notch pathway maintains stem cells in a proliferative, pluripotent, and undifferentiated state. Additionally, it directs cell fates. In the canonical notch pathway, there is interaction between two cells or two cell types; in the disc, these cells could be in the nucleus pulposus itself or possibly between the nucleus pulposus and the inner annulus fibrosus cells. Proteins on one cell (Jagged-1 and -2; and Delta-like-1, Delta-like-3, and Delta-like-4) serve as ligands to the single-pass notch transmembrane receptor. Following two sequential proteolytic cleavages, the notch intracellular domain (NICD) is released from the plasma membrane and translocates to the nucleus, where it interacts with a transcription factor of the CSL (RBPJκ) family and activates the transcription of target genes that include Hes and Hey. The transmembrane domain cleavage and release of NICD is mediated by the γ-secretase complex, which in mammals contains either presenilin-1 or presenilin-2 as the catalytic subunit. Removal of both presenilin-1 (encoded by Psen1) and presenilin-2 (encoded by Psen2) completely abolishes NICD production.
We have recently shown that the notch signaling pathway is active in the intervertebral disc and is responsive to hypoxia.87 In skeletal tissues, disruption of notch signaling markedly increases trabecular bone mass: with aging, the mice become osteopenic due to a sharp reduction in mesenchymal progenitor populations.88,89 Hypoxia also increases the expression of known notch target genes. Accordingly, in the nucleus, HIF-1α may directly interact with the notch ICD and direct cell fate. Based on what is known of cell replacement in other tissues, it is more than likely that this HIF-regulated pathway is a critical component of cell renewal and replacement.
From a disease viewpoint, an oxemic shift, possibly mediated by alterations in the vascular supply to the endplate cartilage, or even the annulus fibrosus, would be expected to lead to a failure in notochordal progenitor cell activation and a decrease in the number of differentiated cells. In turn, this would lead to decrements in function and enhancement of the effect of agents that are known to promote disc degeneration. From a therapeutic viewpoint, it should be possible to modulate the niche environment to enhance renewal and promote differentiation of precursors into functional cells of the nucleus or the annulus. Accordingly, rather than relying on surgical and other interventional strategies, which may themselves damage the disc or cause infection, it should be possible to promote tissue repair by manipulating oxemic conditions within the niche, or by using proteins of the notch signaling pathway to reactivate the endogenous notochordal precursors in the nucleus pulposus. Restoration of disc cell function and prevention of degeneration remains the ultimate goal of current intervertebral disc research.
Acknowledgments
This work was supported by grants from the National Institutes of Health: R01-AR050087 and R01-AR055655. The authors wish to thanks Dr. Richard Dryden for the diagrams shown in Figure 2.
References
- 1.Agrawal A, Guttapalli A, Narayan S, Albert TJ, Shapiro IM, Risbud MV. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293:C621–31. doi: 10.1152/ajpcell.00538.2006. [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–63. [PubMed] [Google Scholar]
- 3.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–97. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Hunter CJ, Bianchi S, Cheng P, Muldrew K. Osmoregulatory function of large vacuoles found in notochordal cells of the intervertebral disc. Mol Cell Biomech. 2007;4:227–37. [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter CJ, Matyas JR, Duncan NA. The three-dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. J Anat. 2003;202:279–91. doi: 10.1046/j.1469-7580.2003.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajghate S, Hiyama A, Shah M, Sakai D, Anderson DG, Shapiro IM, Risbud MV. Osmolarity and intracellular calcium regulate aquaporin2 expression through TonEBP in nucleus pulposus cells of the intervertebral disc. J Bone Miner Res. 2009;24:992–1001. doi: 10.1359/JBMR.090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai TT, Danielson KG, Guttapalli A, Oguz E, Albert TJ, Shapiro IM, Risbud MV. TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J Biol Chem. 2006;281:25416–24. doi: 10.1074/jbc.M601969200. [DOI] [PubMed] [Google Scholar]
- 8.Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003;9:667–77. doi: 10.1089/107632703768247368. [DOI] [PubMed] [Google Scholar]
- 9.Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–30. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- 10.Hogan B, Beddington R, Costantini F, Lacey E. Manipulating the Mouse Embryo: A Laboratory Manual. 2. New York: Cold Spring Harbor Laboratory Press; 1994. Early mouse development; pp. 63–81. [Google Scholar]
- 11.Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–12. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich S, Schubert FR, Gruss P. Altered Pax gene expression in murine notochord mutants: the notochord is required to initiate and maintain ventral identity in the somite. Mech Dev. 1993;44:189–207. doi: 10.1016/0925-4773(93)90067-8. [DOI] [PubMed] [Google Scholar]
- 13.Pourquié O, Coltey M, Teillet MA, Ordahl C, Le Douarin NM. Control of dorsoventral patterning of somitic derivatives by notochord and floor plate. Proc Natl Acad Sci U S A. 1993;90:5242–6. doi: 10.1073/pnas.90.11.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halpern ME, Ho RK, Walker C, Kimmel CB. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell. 1993;75:99–111. [PubMed] [Google Scholar]
- 15.Danos MC, Yost HJ. Linkage of cardiac left-right asymmetry and dorsal-anterior development in Xenopus. Development. 1995;121:1467–74. doi: 10.1242/dev.121.5.1467. [DOI] [PubMed] [Google Scholar]
- 16.Bell GR. Anatomy of the lumbar spine: developmental to normal adult anatomy. In: Wiesel SW, Weinstein JN, Herkowitz HN, editors. The Lumbar Spine. Philadelphia: Saunders; 1996. pp. 43–52. [Google Scholar]
- 17.Horwitz T. The Human Notochord: A Study of Its Development and Regression, Variations, and Pathologic Derivative, Chordoma. Indianapolis: Horwitz; 1977. [Google Scholar]
- 18.Aszódi A, Chan D, Hunziker E, Bateman JF, Fässler R. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J Cell Biol. 1998;143:1399–1412. doi: 10.1083/jcb.143.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rufai A, Benjamin M, Ralphs JR. The development of fibrocartilage in the rat intervertebral disc. Anat Embryol(Berl) 1995;192:53–62. doi: 10.1007/BF00186991. [DOI] [PubMed] [Google Scholar]
- 20.Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237:3953–8. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilson A, Dreger M, Urban JP. Differential expression level of cytokeratin 8 in cells of the bovine nucleus pulposus complicates the search for specific intervertebral disc cell markers. Arthritis Res Ther. 2010;12:R24. doi: 10.1186/ar2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010;12:R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn. 2010;239:2141–8. doi: 10.1002/dvdy.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunliffe VT, Ingham PW. Switching on the notochord. Genes Dev. 1999;13:1643–6. doi: 10.1101/gad.13.13.1643. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann BG, Kispert A. The T genes in embryogenesis. Trends Genet. 1994;10:280–6. doi: 10.1016/0168-9525(90)90011-t. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–9. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- 27.Dobrovolskaïa-Zavadskaïa N. Sur la mortification sponta-nee de la queue chez la souris nouveau-nee et sur l’existence d’un caractere (facteur) hereditaire “non-viable”. Crit Rev Seance Soc Biol. 1927;97:114–6. [Google Scholar]
- 28.Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78:561–74. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- 29.Weinstein DC, Ruiz i Altaba A, Chen WS, Hoodless P, Prezioso VR, Jessell TM, Darnell JE., Jr The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78:575–88. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- 30.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 31.Teillet M, Watanabe Y, Jeffs P, Duprez D, Lapointe F, Le Douarin NM. Sonic hedgehog is required for survival of both myogenic and chondrogenic somitic lineages. Development. 1998;125:2019–30. doi: 10.1242/dev.125.11.2019. [DOI] [PubMed] [Google Scholar]
- 32.McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–52. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li SW, Prockop DJ, Helminen H, Fassler R, Lapvetelainen T, Kiraly K, Peltarri A, Arokoski J, Lui H, Arita M, Khillan JS. Transgenic mice with targeted inactivation of the Col2 alpha 1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 1995;9:2821–30. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]
- 34.Tribioli C, Lufkin T. The murine Bapx1 homeobox gene plays a critical role in embryonic development of the axial skeleton and spleen. Development. 1999;126:5699–711. doi: 10.1242/dev.126.24.5699. [DOI] [PubMed] [Google Scholar]
- 35.Lettice LA, Purdie LA, Carlson GJ, Kilanowski F, Dorin J, Hill RE. The mouse bagpipe gene controls development of axial skeleton, skull, and spleen. Proc Natl Acad Sci U S A. 1999;96:9695–700. doi: 10.1073/pnas.96.17.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallin J, Wilting J, Koseki H, Fritsch R, Christ B, Balling R. The role of Pax-1 in axial skeleton development. Development. 1994;120:1109–21. doi: 10.1242/dev.120.5.1109. [DOI] [PubMed] [Google Scholar]
- 37.Peters H, Wilm B, Sakai N, Imai K, Maas R, Balling R. Pax1 and Pax9 synergistically regulate vertebral column development. Development. 1999;126:5399–408. doi: 10.1242/dev.126.23.5399. [DOI] [PubMed] [Google Scholar]
- 38.Lefebvre V. Toward understanding the functions of the two highly related Sox5 and Sox6 genes. J Bone Miner Metab. 2002;20:121–30. doi: 10.1007/s007740200017. [DOI] [PubMed] [Google Scholar]
- 39.Smits P, Lefebvre V. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development. 2003;130:1135–48. doi: 10.1242/dev.00331. [DOI] [PubMed] [Google Scholar]
- 40.Wellik DM. Hox genes and vertebrate axial pattern. Curr Top Dev Biol. 2009;88:257–78. doi: 10.1016/S0070-2153(09)88009-5. [DOI] [PubMed] [Google Scholar]
- 41.Lohnes D, Mark M, Mendelsohn C, Dollé P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–48. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 42.Kessel M, Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- 43.Sakai Y, Meno C, Fujii H, Nishino J, Shiratori H, Saijoh Y, Rossant J, Hamada H. The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev. 2001;15:213–25. doi: 10.1101/gad.851501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Latimer AJ, Appel B. Notch signaling regulates midline cell specification and proliferation in zebrafish. Dev Biol. 2006;298:392–402. doi: 10.1016/j.ydbio.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 45.Latimer AJ, Dong X, Markov Y, Appel B. Delta-Notch signaling induces hypochord development in zebrafish. Development. 2002;129:2555–63. doi: 10.1242/dev.129.11.2555. [DOI] [PubMed] [Google Scholar]
- 46.Latimer AJ, Shin J, Appel B. her9 promotes floor plate development in zebrafish. Dev Dyn. 2005;232:1098–104. doi: 10.1002/dvdy.20264. [DOI] [PubMed] [Google Scholar]
- 47.Zecchin E, Conigliaro A, Tiso N, Argenton F, Bortolussi M. Expression analysis of jagged genes in zebrafish embryos. Dev Dyn. 2005;233:638–45. doi: 10.1002/dvdy.20366. [DOI] [PubMed] [Google Scholar]
- 48.Oates AC, Mueller C, Ho RK. Cooperative function of deltaC and her7 in anterior segment formation. Dev Biol. 2005;280:133–49. doi: 10.1016/j.ydbio.2005.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sprague J, Bayraktaroglu L, Clements D, Conlin T, Fashena D, Frazer K, Haendel M, Howe DG, Mani P, Ramachandran S, Schaper K, Segerdell E, Song P, Sprunger B, Taylor S, Van Slyke CE, Westerfield M. The Zebrafish Information Network: the zebrafish model organism database. Nucleic Acids Res. 2006;34:581–5. doi: 10.1093/nar/gkj086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto M, Morita R, Mizoguchi T, Matsuo H, Isoda M, Ishitani T, Chitnis AB, Matsumoto K, Crump JG, Hozumi K, Yonemura S, Kawakami K, Itoh M. Mib-Jag1-Notch signalling regulates patterning and structural roles of the notochord by controlling cell-fate decisions. Development. 2010;137:2527–37. doi: 10.1242/dev.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205:357–62. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Yan W, Setton LA. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J. 2006;15 (Suppl 3):S303–11. doi: 10.1007/s00586-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:129–37. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- 54.Henriksson H, Thornemo M, Karlsson C, Hägg O, Junevik K, Lindahl A, Brisby H. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine. 2009;34:2278–87. doi: 10.1097/BRS.0b013e3181a95ad2. [DOI] [PubMed] [Google Scholar]
- 55.Risbud MV, Schipani E, Shapiro IM. Hypoxic regulation of nucleus pulposus cell survival: from niche to notch. Am J Pathol. 2010;176:1577–83. doi: 10.2353/ajpath.2010.090734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–14. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 57.Butler WF. Comparative anatomy and development of the mammalian disc. In: Ghosh P, editor. The Biology of the Intervertebral Disc. Boca Raton: CRC Press; 1989. pp. 84–108. [Google Scholar]
- 58.Walmsley R. The development and growth of the intervertebral disc. Edinburgh Med J. 1953;60:341–63. [PMC free article] [PubMed] [Google Scholar]
- 59.Trout JJ, Buckwalter JA, Moore KC, Landas SK. Ultra-structure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982;14:359–69. doi: 10.1016/0040-8166(82)90033-7. [DOI] [PubMed] [Google Scholar]
- 60.Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine. 2003;28:982–90. doi: 10.1097/01.BRS.0000061986.03886.4F. [DOI] [PubMed] [Google Scholar]
- 61.Kim KW, Ha KY, Lee JS, Nam SW, Woo YK, Lim TH, An HS. Notochordal cells stimulate migration of cartilage end plate chondrocytes of the intervertebral disc in in vitro cell migration assays. Spine. 2009;9:323–9. doi: 10.1016/j.spinee.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Gan JC, Ducheyne P, Vresilovic EJ, Shapiro IM. Intervertebral disc tissue engineering II: cultures of nucleus pulposus cells. Clin Orthop. 2003:315–24. doi: 10.1097/01.blo.0000063797.98363.d3. [DOI] [PubMed] [Google Scholar]
- 63.Poiraudeau S, Monteiro I, Anract P, Blanchard O, Revel M, Corvol MT. Phenotypic characteristics of rabbit intervertebral disc cells. Comparison with cartilage cells from the same animals. Spine. 1999;24:837–44. doi: 10.1097/00007632-199905010-00002. [DOI] [PubMed] [Google Scholar]
- 64.Guilak F, Ting-Beall HP, Baer AE, Trickey WR, Erickson GR, Setton LA. Viscoelastic properties of intervertebral disc cells: identification of two biomechanically distinct cell populations. Spine. 1999;24:2475–83. doi: 10.1097/00007632-199912010-00009. [DOI] [PubMed] [Google Scholar]
- 65.Stevens JW, Kurriger GL, Carter AS, Maynard JA. CD44 expression in the developing and growing rat intervertebral disc. Dev Dyn. 2000;219:381–90. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1060>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 66.Trout JJ, Buckwalter JA, Moore KC. Ultrastructure of the human intervertebral disc. II. Cells of the nucleus pulposus. Anat Rec. 1982;204:307–14. doi: 10.1002/ar.1092040403. [DOI] [PubMed] [Google Scholar]
- 67.Pazzaglia UE, Salisbury JR, Byers PD. Development and involution of the notochord in the human spine. J R Soc Med. 1989;82:413–5. doi: 10.1177/014107688908200714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang F, Leung VY, Luk KD, Chan D, Cheung KM. Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J Pathol. 2009;218:113–21. doi: 10.1002/path.2519. [DOI] [PubMed] [Google Scholar]
- 69.Tian H, Jeong J, Harfe BD, Tabin CJ, McMahon AP. Mouse Disp1 is required in sonic hedgehog-expressing cells for paracrine activity of the cholesterol-modified ligand. Development. 2005;132:133–42. doi: 10.1242/dev.01563. [DOI] [PubMed] [Google Scholar]
- 70.Chesley P. Development of the short-tailed mutant in the house mouse. J Exp Zool. 1935;70:429–59. [Google Scholar]
- 71.Schulte-Merker S, Ho RK, Herrmann BG, Nüsslein-Volhard C. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development. 1992;116:1021–32. doi: 10.1242/dev.116.4.1021. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi H, Hotta K, Erives A, Di Gregorio A, Zeller RW, Levine M, Satoh N. Brachyury downstream notochord differentiation in the ascidian embryo. Genes Dev. 1999;13:1519–23. doi: 10.1101/gad.13.12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang XR, Ng D, Alcorta DA, Liebsch NJ, Sheridan E, Li S, Goldstein AM, Parry DM, Kelley MJT. Brachyury gene duplication confers major susceptibility to familial chordoma. Nat Genet. 2009;41:1176–8. doi: 10.1038/ng.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salisbury JR, Isaacson PG. Demonstration of cytokeratins and an epithelial membrane antigen in chordomas and human fetal notochord. Am J Surg Pathol. 1985;9:791–7. doi: 10.1097/00000478-198511000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Salisbury JR, Deverell MH, Cookson MJ, Whimster WF. Three-dimensional reconstruction of human embryonic notochords: clue to the pathogenesis of chordoma. J Pathol. 1993;171:59–62. doi: 10.1002/path.1711710112. [DOI] [PubMed] [Google Scholar]
- 76.Kim JH, Deasy BM, Seo HY, Studer RK, Vo NV, Georgescu HI, Sowa GA, Kang JD. Differentiation of intervertebral notochordal cells through live automated cell imaging system in vitro. Spine. 2009;34:2486–9. doi: 10.1097/BRS.0b013e3181b26ed1. [DOI] [PubMed] [Google Scholar]
- 77.Stosiek P, Kasper M, Karsten U. Expression of cytokeratin and vimentin in nucleus pulposus cells. Differentiation. 1988;39:78–81. doi: 10.1111/j.1432-0436.1988.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 78.Weiler C, Schaaf R, Nerlich A, Boss N. Immunohistochemical identification of notochordal cell phenotype in the ageing human lumbar intervertebral disc. Pathol Res Pract. 2007;203:233–4. doi: 10.1007/s00586-010-1392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vujovic S, Henderson S, Presneau N, Odell E, Jacques TS, Tirabosco R, Boshoff C, Flanagan AM. Brachyury, a crucial regulator of notochordal development, is a novel bio-marker for chordomas. J Pathol. 2006;209:157–65. doi: 10.1002/path.1969. [DOI] [PubMed] [Google Scholar]
- 80.Lee CR, Sakai D, Nakai T, Toyama K, Mochida J, Alini M, Grad S. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16:2174–85. doi: 10.1007/s00586-007-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sakai D, Nakai T, Mochida J, Alini M, Grad S. Differential phenotype of intervertebral disc cells: microarray and immunohistochemical analysis of canine nucleus pulposus and annulus fibrosus. Spine. 2009;34:1448–56. doi: 10.1097/BRS.0b013e3181a55705. [DOI] [PubMed] [Google Scholar]
- 82.Zavala G, Vázquez-Nin GH. Analysis of nuclear ribo-nucleoproteic structures during notochordal cell differentiation and maturation in chick embryos. Anat Rec. 2000;259:113–23. doi: 10.1002/(SICI)1097-0185(20000601)259:2<113::AID-AR1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 83.Hunziker EB, Schenk RK, Cruz-Orive LM. Quantitation of chondrocyte performance in growth-plate cartilage during longitudinal bone growth. J Bone Joint Surg Am. 1987;69:162–73. [PubMed] [Google Scholar]
- 84.Gebhardt R. Metabolic zonation of the liver: regulation and implications for liver function. Pharmacol Ther. 1992;53:275–354. doi: 10.1016/0163-7258(92)90055-5. [DOI] [PubMed] [Google Scholar]
- 85.Reginato AM, Shapiro IM, Lash JW, Jimenez SA. Type X collagen alterations in rachitic chick epiphyseal growth cartilage. J Biol Chem. 1988;263:9938–45. [PubMed] [Google Scholar]
- 86.Risbud MV, Guttapalli A, Tsai TT, Lee JY, Danielson KG, Vaccaro AR, Albert TJ, Gazit Z, Gazit D, Shapiro IM. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine. 2007;32:2537–44. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- 87.Hiyama A, Skubutyte R, Markova D, Anderson DG, Yadla S, Sakai D, Mochida J, Albert TJ, Shapiro IM, Risbud MV. Hypoxia activates notch signaling pathway in cells of the intervertebral disc: Implications in degenerative disc disease. Arthritis Rheum. 2011 Jan 18; doi: 10.1002/art.30246. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM, Chen Y, Wang L, Zheng H, Sutton RE, Boyce BF, Lee B. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14:299–305. doi: 10.1038/nm1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long F. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14:306–14. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]