Abstract
Eyeblink conditioning abnormalities have been reported in schizophrenia, but the extent to which these anomalies are evident across a range of delay intervals (i.e., ISI intervals) is unknown. In addition, the effects of interstimulus interval (ISI) shifts on learning are unknown, though such manipulations can be informative about the plasticity of cerebellar timing functions. Therefore, the primary purpose of the present study was to investigate the interactions between interstimulus interval (ISI) manipulations and learning in schizophrenia. A standard delay eyeblink conditioning procedure with four different interstimulus intervals (ISIs; 250, 350, 550, 850 ms) was employed. Each eyeblink conditioning experiment was immediately followed by another with a different ISI, thus permitting the characterization of conditioned response (CR) learning at one ISI and the extent to which CRs could be generated at a different latency following an ISI shift. Collapsing across all conditions, the schizophrenia group (n=55) had significantly fewer conditioned responses and longer onset latencies than age-matched controls (n=55). Surprisingly, shifting to a new ISI had negligible effects on conditioned response rates in both groups. These findings contribute to evidence of robust eyeblink conditioning abnormalities in schizophrenia and suggest impaired cerebellar function, but underscore the need for more research to clarify the source of these abnormalities and their relationship to clinical manifestations of schizophrenia.
Keywords: eyeblink conditioning, schizophrenia, cerebellum, interstimulus interval, associative learning
Introduction
The constellation of symptoms experienced by individuals with schizophrenia comprise a number of superficially unrelated domains, including cognitive deficits (e.g. impaired memory, executive function, visuospatial function), positive symptoms (hallucinations, delusions), and negative symptoms (e.g. avolition, blunted affect). Recent efforts to clarify relationships between this diverse array of symptoms have focused on identifying underlying “core” deficits that can unify these sometimes disparate symptom presentations (i.e. Silver et al., 2003; Andreasen et al., 1998). One influential theory proposes a putative core deficit in the temporal coordination of information processing in the brain, sometimes referred to as cognitive dysmetria (Andreasen et al.,1998;Andreasen and Pierson, 2008), which may lead to disturbances of consciousness as well as poor coordination of perceptual, affective, cognitive, and motor processes. In this model, dysfunction in the cortico-cerebellar-thalamic-cortical circuit (CCTCC) plays a cardinal role in the generation of schizophrenia symptoms. The model views cognitive symptoms as essential to the generation of the hallmark characteristics of schizophrenia—the positive and negative symptoms. Hence, cognitive function may be disrupted when the CCTCC is dysfunctional, which leads to cognitive dysmetria and, perhaps, the more obvious symptoms of schizophrenia. The centrality of cognition in this model is important because, regardless of symptom manifestation, cognition appears to be a relatively constant deficit in schizophrenia. Importantly, the core deficit that leads to cognitive impairment in this model is disrupted coordination or temporal synchrony of neural signals.
There is evidence of abnormalities in the cerebellar node of the CCTCC in schizophrenia, as indicated by structural, functional, neuropathological, and neurochemical studies, although to date a direct relationship between these abnormalities to clinical symptoms of schizophrenia has not been demonstrated (for review, see Picard et al., 2007). Nevertheless, a number of studies do provide indirect evidence linking disruptions to cerebellar circuitry to symptoms of schizophrenia. For example, the few previous studies that directly assessed structural cerebellar deficits in schizophrenia have yielded noteworthy abnormalities, some of which are correlated with greater clinical symptomatology (Ichimiya et al., 2001; Ho et al., 2004) and cognitive dysfunction (Nopoulos et al., 1999), and are reliable indicators of poor long-term outcome (Wassink, Andreasen, Nopoulos & Faum, 1999). In addition, Okugawa et al. (2006), found evidence of disrupted neural connectivity in the superior cerebellar peduncle in schizophrenia. The peduncles provide the anatomical pathway for cerebellar connectivity with the CCTCC. More disruption was associated with lower cognitive cluster scores on the PANSS, which provides further support for the cognitive dysmetria theory of schizophrenia (Andreasen, 1999).
Cerebellar abnormalities in schizophrenia are of particular interest because the functions of the cerebellum are increasingly believed to extend beyond its traditionally ascribed motor coordination role. Evidence of the cerebellum's role in both motor and perceptual timing (i.e. Ivry et al., 1988, 1989) and accumulating evidence of its role in cognition and affect (Katz & Steinmetz, 2002; Leiner, Leiner, & Dow, 1986; Schutter & Van Honk, 2005; Timmann et al., 2007, 2010) all point to an important role in non-motor psychological processes. Moreover, patients with cerebellar lesions sometimes exhibit symptoms that are remarkably similar to characteristic behaviors that define schizophrenia, including disturbances in executive function, perceptual anomalies, impaired attention, affective flattening, and contextually inappropriate behavior (Schmahmann & Sherman, 1998; Schmahmann, 2004).
As in any circuit, abnormalities in one node can impair function in the others, and in the circuit overall. The neuroanatomical connections of the cerebellum emphasize its potential importance within the CCTCC and in schizophrenia. For example, feedback and feedforward loops connect the cerebellum with areas of the brain implicated in the disorder, including the thalamus, basal ganglia, and prefrontal cortex (Middleton & Strick, 2001,1994; Ramnani et al., 2006; Schmahmann & Pandya, 1995). Taken together, these findings and the connectivity of the cerebellum with brain areas affected in schizophrenia implicate cerebellar dysfunction in the disorder. However, despite the evidence supporting the CCTCC model of schizophrenia, the specific functional abnormalities of the cerebellum in schizophrenia are incompletely understood.
This lack of knowledge was an impetus for this study of cerebellar-dependent eye-blink conditioning (EBC) in schizophrenia using an approach designed to further explicate the way in which previously observed structural cerebellar abnormalities (i.e., Nopoulos et al., 1999; Wassink et al., 1999; Ichimiya et al., 2001; Ho et al., 2004; Okugawa et al., 2006) may manifest behaviorally. The simplest EBC learning tasks (i.e. the single cue delay form of EBC) depend critically on the cerebellum, and even this most elementary task engages sensorimotor, affective, and cognitive components of learning (Stanton, 2000). Therefore, EBC may be informative about both the functional integrity of the cerebellum as well as fundamental aspects of cognition, i.e. associative learning. EBC has several advantages for testing cerebellar function in schizophrenia. First, the neural circuitry associated with EBC is distinct and well characterized. While structures besides the cerebellum modulate CR acquisition and performance in the delay version of EBC (e.g., the amygdala, septum, and hippocampus; Christian & Thompson, 2003), only the cerebellum is critical for performance in short interval, delay classical EBC (Steinmetz, 2004; Lavond et al., 1993; Kim & Thompson, 1997; Christian & Thompson, 2003). Furthermore, the magnitude of conditioning is related to the morphology and volume of the cerebellum in humans (Woodruff-Pak et al., 2000) and, thus, could be altered by structural anomalies observed in schizophrenia (Edwards et al., 2008).
Even though EBC is an associative learning task in which learning is expressed by predictive timing of conditioned responses, acquisition of the conditioned eyeblink response and timing of that response are also somewhat separable features of eyeblink conditioning. Poor acquisition may indicate one type of deficit in cerebellar processing (e.g., related to Lobule H-VI; Yeo et al., 1985; Lavond & Steinmetz, 1989), whereas poor timing of the response may indicate another (e.g., related to anterior lobe; Perett, Ruiz, & Mauk, 1993). The ability of the cerebellum to adaptively time CRs has most often been measured using the timing of either the CR onset or its peak latency. However, a more specific and sensitive test of cerebellar timing involves manipulation of the interstimulus interval (ISI) relationship between the conditioned stimulus and the unconditioned stimulus, which can be measured by shifting from a previously learned ISI to a new one. Such an approach may provide a means of dissociating CR acquisition from timing and may therefore provide a more direct measure of the integrity of the timing functions of the cerebellum. The primary purpose of the present study was to investigate the interactions between interstimulus interval (ISI) manipulations and learning in schizophrenia. More specifically, we were interested in the process of shifting from one ISI to another and what this shift process reveals about the plasticity of cerebellar timing functions. Another goal was to compare performance across delay intervals, which is afforded by the various ISI conditions. This second goal was important because there is evidence that optimal ISIs for acquisition of conditioned responding may differ according to population characteristics. For example, age-related acquisition deficits diminish at longer delay intervals (Woodruff-Pak et al., 1999).
Consistent with the theoretical and empirical evidence of a role for the cerebellum in non-motor psychological functions, abnormalities in cerebellar-mediated EBC have been reported in psychiatric disorders with abnormalities in these domains, including bipolar disorder (Bolbecker et al., 2009a) and schizophrenia (Spain, 1966; Sears et al., 2000; Hofer et al., 2001; Marenco et al., 2003; Brown et al., 2005; Bolbecker et al., 2009b). Most auditory delay EBC studies have found impaired conditioning in schizophrenia (Hofer et al., 2001; Brown et al., 2005; Edwards et al., 2008; Bolbecker et al., 2009b), although one study reported no differences in conditioning (Marenco et al., 2003), and another found facilitated learning (Sears et al., 2000). Earlier CR peak and onset latencies have also been rather consistently reported (Sears et al., 2000; Brown et al., 2005; Bolbecker et al., 2009b); however, Marenco et al. (2003) reported later CR peak and onset latencies. In the current study, ISI shift experiments were conducted using a total of 4 separate ISIs in a group of schizophrenia patients and age-matched healthy controls. The ISIs chosen for these experiments span a range which appears to optimize human learning in this procedure in humans (Woodruff-Pak & Finkbiner, 1995; McAllister, 1953, Solomon et al., 1991) with the additional benefit of allowing characterization of learning in schizophrenia and healthy controls at 4 unique ISIs.
Methods
Participants
Participants were 55 individuals (22 women) with DSM-IV schizophrenia spectrum disorders (47 schizophrenia, 8 schizoaffective, 1 schizotypal personality disorder) and 55 age-matched non-psychiatric healthy controls (29 women). Controls were matched to a corresponding schizophrenia participant if their ages were within 2 years of each other. Diagnostic status was determined using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) sections for mood disorders, psychotic disorders, and substance abuse disorders, and chart review. The study procedures were approved by the University's Human Subjects Institutional Review Board and written informed consent was obtained from all participants.
As expected, given the age-matching procedure, the mean age of schizophrenia participants (41.1 yrs, SD=11.1) did not differ from controls (40.9 yrs, SD=11.3), t(108)= -0.07, p=0.95. The schizophrenia group did not differ from the healthy control group on gender (X2(1)=1.79 p=.18). Inclusion criteria were completion of grade school level education, normal or corrected to normal hearing and vision, no history of cardiovascular or neurological disease, and no history of head injury that resulted in loss of consciousness. Participants who met criteria for substance dependency within three months prior to testing were not considered for the study.
Thirteen participants in the schizophrenia group were not on psychotropic medication. Among the schizophrenia participants who were taking such medications, 15 were on anticholinergic drugs, 16 on antidepressants, 16 on typical antipsychotic drugs, and 36 on atypical antipsychotic drugs.
Eye-blink Conditioning Procedure
Each participant completed one of two separate paradigms, each of which was composed of two single-cue tone delay eye-blink conditioning tasks: one having a short ISI (250 or 350 ms) and another with a long ISI (550 or 850 ms). Groups of participants were first recruited for the 350-850 ISI shift experiment, then for the 250-550 ISI shift experiment. The order of presentation was randomly assigned so that the short ISI was presented first, followed by the long ISI (short-to-long shift) or vise versa (long-to-short shift). The conditioned stimulus (CS) was a 1000 Hz (80 dB SPL) tone, which, on paired trials, co-terminated with a 50 ms air puff (50 ms, 10 psi at source) the unconditioned stimulus (US). The CS was one of four durations, depending on the condition: 300, 400, 600, or 900 ms. Participants were initially presented with 8 US alone trials (ITI=15 s). Acquisition trials for the initial ISI EBC task immediately followed. After completion of the initial EBC task and prior to the second EBC task, there was a brief pause in the experiment (approximately 5 minutes) to minimize fatigue. During the break, participants remained seated in the recording chamber, but were able to interact with examiners and were offered a drink of water. Each of the two EBC tasks consisted of 5 blocks of conditioning trials (mean ITI=15 s; range=10 to 20 s), with each trial block consisting of 18 CS-US paired trials and 2 CS-alone trials. Only paired trials were included in subsequent analyses. Participants were not informed of the shift in ISIs across EBC tasks. To maintain the participants' attention throughout the experiment, neutral photographs selected from the International Affective Picture System (Lang & Greenwald, 1988) were presented (2 s duration) between each trial and participants rated the pleasantness of the images by pressing a response pad button. In addition, participants were observed via a closed circuit monitor to ensure that their eyes remained open. The experiment was briefly suspended if signs of fatigue were observed so that the examiner could interact with the participant.
Procedure
Bipolar eletromyographic (EMG) electrodes (4mm Ag/Ag-Cl) were placed directly below the left eye and centered below the pupil, 1 cm from the eyelid and 1 cm apart. A ground electrode was placed on the forehead. The left eye was presented with a US air puff delivered via copper tubing (fused to the rim of lens-less glasses) connected to a regulator delivering air via plastic tubing (120″). The CS tone was delivered via ear inserts (E-A-RLINK – Aearo Company Auditory Systems). EMG recordings were made continuously with a SynAmps bio-amplifier (2.5 KHz A/D rate; high pass filter=1 Hz; low pass filter=500 Hz; gain=1000) and the Acquire data acquisition program ([4.1], NeuroScan, El Paso, TX) throughout the experiment and stored offline.
Data Analysis
Continuous data files for each subject were divided into 1650 ms epochs starting 500 ms prior to CS onset and ending 250 ms post-US. After a 10 Hz (6 dB/octave) high pass filter was applied, the data were rectified and smoothed using a 41-point Gaussian weighted moving average. Data were entered into DataMunch, a Matlab program written for eye-blink conditioning data analysis (unpublished data by King DAT and Tracy J, available upon request from Hetrick WP). Alpha responses, which are reflexive, non-associative orienting EMG responses to the tone CS, were assessed between 25 and 100 ms after CS onset. On a subject-by-subject and trial-by-trial basis, responses were recorded as blinks if the amplitude exceeded five standard deviations above the baseline (baseline window for each trial=125 ms prior to CS onset). For each of the 4 ISIs, eyeblinks that occurred during a 150 ms window extending from 150 ms prior to US onset until the US onset occurred were recorded as conditioned responses. While the possibility exists that this time window would miss CRs occurring earlier in the CR period during longer ISI conditions, use of longer CR windows runs the risk of capturing spontaneous blinks, which would then be included in the CR count. This would be less consequential if only one ISI were being considered, but in the present experimental design in which longer and shorter ISIs were being explicitly compared, measuring the entire window from CS onset through US onset could distort results. In contrast, the method we prefer and implemented standardized the CR window across ISIs, which should capture the most “adaptive” CRs, i.e., those that occur closest to the US onset and result in eyelid closure that is most likely to overlap with it as well as facilitate comparison across ISIs.1
The onset latency was calculated as the point in time where the conditioned response exceeded 0.5 standard deviations from the baseline EMG. The peak latency is the time point for the maximal value for that conditioned response. Trials in which spontaneous blinks occurred within a window from 75 ms prior to CS presentation to 25 ms following CS onset were designated bad trials and excluded from further analysis. There were no significant differences between groups on number of bad trials (p > .05).
In order to measure EBC performance at each of the various ISIs in the absence of possible interference by pre-exposure to a different ISI, a comparison of each group's performance on the initial ISI presentation was made using repeated measures ANOVAs with diagnosis (healthy control or schizophrenia) and ISI (250, 350, 550, or 850 ms) as between subjects variables separately for each primary CR and UR dependent variable (percent CRs, CR onset latency, UR amplitude, and UR peak latency).
In subsequent analyses, each EBC task was treated as a separate experiment so that the learning effects and their possible relationship with diagnosis could be explicitly examined. Repeated measures ANOVAs for all primary CR and UR dependent variables were conducted with a 2 (diagnosis: healthy control, schizophrenia) × 5 (block) design. In addition, the effects of order of presentation were examined for each ISI. To control for differences in acquisition rates that were due to ISI duration itself rather than due to shifting to a new ISI per se, performance at each ISI when it was presented first was then compared to performance at that same ISI when it was presented second in the sequence (i.e. after an ISI shift) on each dependent variable. To do this, the effects of order of presentation and possible interactions between order and diagnosis were examined in 2 (Order: short-to-long, long-to-short) × 2 (Diagnosis) × Block (5) repeated measures ANOVAs for each ISI. An additional measure of shift cost was obtained using a repeated measures 2 (diagnosis) × 2 (shift cost) ANOVA, where shift cost was the average of the last 2 blocks of the first ISI task and the first 2 blocks of the second ISI task. Shift cost was explored separately for percentage of conditioned responses and conditioned response onset latency for each ISI shift experiment, with diagnosis as a between subjects factor.
Because time (i.e. ISIs) was manipulated in these experiments, the onset latency was standardized across ISIs by calculating it as the time of CR onset relative to the US in order to get a clear measure of how onset latency was affected by different ISIs and orders of presentation. Therefore, larger latency values represent earlier occurrences of CRs whereas smaller values are indicative of later CR onsets. Earlier CRs are usually considered less adaptive than later ones, which are more coincident with the air-puff US and more likely to result in avoidance of this mildly aversive US. However, in the current ISI shift design, this definition may be more modifiable depending on the particular order of presentation and the length of the ISI and must be interpreted accordingly. For example, in a long-to-short shift, very late CRs could signal an inability to modify timing according to the new context.
Medication effects were assessed by comparing schizophrenia participants who were medicated (N=42) with those who were unmedicated (N=13) in repeated measures ANOVAs with block as a within subjects factor for all dependent variables. In addition, all dependent variables were analyzed using the following categorizations of the schizophrenia groups: typical antipsychotic (typical alone or typical+atypical; N=14), atypical antipsychotic only (N=30) and unmedicated (N=13). Finally, the unmedicated group was compared to age-matched controls (N=13 in each group) for all analyses. (No medication effects were detected, as described in the Results section.)
Results of the major dependent variables are reported with their corresponding effect sizes in the form of partial eta2 (ηP2): small effect sizes are less than .06; moderate effect sizes range from .06 to .14; large effect sizes are greater than .14 (Cohen, 1973). When violations of sphericity occurred, the Greenhouse-Geisser correction was used to report results.
Results
A total of 55 age-matched participants were included in each diagnostic group. In the 250-550 paradigm, there were 8 participants per group in the short-to-long condition. An additional 9 per group participated in the long-to-short condition. There were 21 participants per diagnostic group in the short-to-long condition of the 350-850 task and 17 per group in the long-to-short condition. An analysis of the initial presentation of each ISI for each dependent variable is presented in order to get an unbiased measure of the effects of ISI duration on conditioned and unconditioned response variables. Specifically, main effects of ISI and interactions of ISI duration with diagnosis were evaluated. In separate analyses, explicit comparisons of between-groups differences and differential effects of learning across blocks of the experiment were made, as well as measures of the effects of shifting to a given ISI after pre-exposure to ISI of a different duration.
Percentage of Conditioned Responses
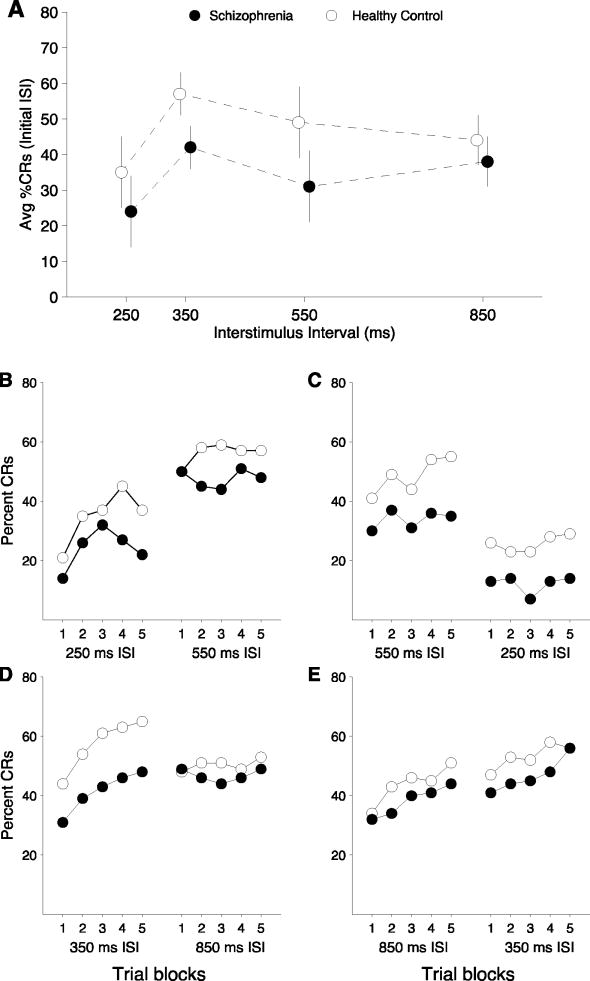
Table 1 contains a summary of the percentage of conditioned responses in the schizophrenia group and in controls in each paradigm, order, and duration. Analyses were conducted on the first presentation of each ISI because one aim of the present experiments was to examine the effects of changing ISI on CR acquisition rates to determine whether the optimal ISI range differed between groups. Figure 1a illustrates the average percent CRs at each ISI at its initial presentation for the schizophrenia and control groups. Figures 1b-e show the performance of both groups over the 10 blocks of each EBC task. Examination of Figure 1 indicates that the schizophrenia group has a lower percentage of conditioned responses at every ISI.
Table 1. Means and standard deviations of %CRs for each diagnostic group in each ISI task paradigm, order, and duration.
| Paradigm, Order, and ISI | Percent CRs by Diagnosis | Statistical values | ||||
|---|---|---|---|---|---|---|
| 250-550 Paradigm | Healthy Control % CRs | Schizophrenia % CRs | F-value | p-value | ||
| Short-to-Long Shift (N=8) | Mean | SD | Mean | SD | ||
| 250 ms ISI | 35 | 26 | 19 | 14 | F(1) = 1.00 | p = ns (0.33) |
| 550 ms ISI | 56 | 36 | 48 | 29 | F(1) = 0.38 | p = ns (0.55) |
| Long-to-Short Shift (N=9) | ||||||
| 550 ms ISI | 49 | 29 | 31 | 17 | F(1) = 4.39 | *p = 0.05 |
| 250 ms ISI | 26 | 25 | 12 | 16 | F(1) = 7.93 | *p = 0.01 |
| 350-850 Paradigm | Healthy Control % CRs | Schizophrenia % CRs | F-value | p-value | ||
| Short-to-Long Shift (N=21) | Mean | SD | Mean | SD | ||
| 350 ms ISI | 57 | 30 | 42 | 31 | F(1) = 4.11 | *p = .049 |
| 850 ms ISI | 50 | 28 | 47 | 30 | F(1) = 0.20 | p = ns (0.66) |
| Long-to-Short Shift (N=17) | ||||||
| 850 ms ISI | 44 | 27 | 38 | 28 | F(1) = 0.60 | p = ns (0.44) |
| 350 ms ISI | 53 | 26 | 47 | 33 | F(1) = 0.61 | p = ns (0.44) |
Indicates significant differences between diagnostic groups
Figure 1.
Percent CRs for the control (open circles) and schizophrenia (filled circles) groups at each ISI. Data points for the schizophrenia group are displayed with a slight rightward offset in order to allow visualization of standard error bars. The top panel shows the percent CRs for each group at each ISI at when it is presented first in the sequence, and illustrates a main effect of group on EBC. The bottom panel plots the percent CRs for each group across the 5 acquisition blocks when that ISI was presented first in the sequence and when it was presented second, i.e. when a “shift” was required.
Effects of ISI on Percent CRs
Analysis of performance on the initial presentation of each ISI revealed a main effect of block (F(9)=8.87, p < .001, ηP2 = .08), indicating that learning occurred as the experiments progressed. There was a main effect of ISI, F(3) = 3.92, p < .01, (ηP2 = .10) and a main effect of diagnosis, F(1)=8.64, p < .01 (ηP2 = .08), indicating that schizophrenia participants had lower rates of conditioned responses across ISIs. CR acquisition in both the healthy control and schizophrenia groups was highest in the 350 ms ISI task (57% and 42%, respectively) and the lowest in the 250 ms ISI task (35% and 19%, respectively). Consistent with this observation, post-hoc comparisons showed significant differences only between these tasks, with participants demonstrating higher conditioned response rates at the 350 ms ISI compared to the 250 ms ISI (p < .01).
ISI shift effects on percent CRs
Figure 1b-e graphically depicts conditioned responding for each group in each of the ISI shift combinations. No significant main effects of order were found at any ISI, nor were any interactions with diagnosis observed.
250-550 paradigm
In general, the schizophrenia group produced fewer CRs than the healthy control group in the 250-550 ms ISI regardless of order of presentation, as can be observed in Figure 1a-c. This reduction in CRs in the schizophrenia group was significant when the 250 ms ISI was presented after the 550 ISI, F(1)=7.93, p=.01, ηP2 = .33). When the 250 ISI was presented first, the groups were not statistically different. The schizophrenia group also generated significantly fewer CRs compared to controls in the long-to-short condition of the 550 ms ISI, F(1)=4.39, p=0.05, (ηP2 =0.22) (see Figure 1c). There were no main effects or interactions on the short-to-long version of the 550 ms ISI.
Analysis of shift cost revealed a significant main effect in both the 250-550 ISI shift and the 550-250 ISI shift conditions (both p < .01), due to significant increases in %CRs in the former and decreases in the latter, but there were no interactions with diagnosis in either condition.
350-850 paradigm
A significant effect of diagnosis was found in the short-to-long task of the 350 ms ISI, F(1)=4.11, p < .05 (ηP2 =.09), with a significantly lower percentage of conditioned responses in the schizophrenia group compared to the healthy control group. However, there were no significant differences between groups on the 350 ms ISI in the long-to-short task. A main effect of block was apparent in both the short-to-long task, F(2.53)=11.75, p < .001 (ηP2 = .23) and the long-to-short task, F(4)=3.59, p < .05 (ηP2 = .33). There was no block by diagnosis interaction in either 350 ms ISI task. In the 850 ms ISI, no significant differences between diagnostic groups emerged, nor were any interactions with diagnosis observed. However, when the 850 ISI was presented first, there was a main effect of block, F(6.17)=3.79, p < .001 (ηP2 = .11), suggesting that learning occurred as the acquisition period progressed. This learning effect was not apparent in when the 850 ISI was presented second in the sequence. Instead there was a relatively consistent level of responding in this condition compared with a more classical learning curve across the acquisition blocks when the 850 ms ISI was presented first (see Figure 1e). This resulted a in block by order interaction, F(3.53)=3.97, p < .01, (ηP2 =.05). By block 5, similar levels of conditioned responding occurred on the 850 ms ISI task regardless of order of presentation (Figure 1d-e).
Analysis of ISI shift cost in the 350-850 ISI shift condition produced a significant interaction between diagnosis and shift cost, F(1) = 3.95, p = .05, (ηP2 =.09). Follow-up tests indicated that the healthy control group had a significant drop in CRs upon shifting to the longer ISI (p = .01), whereas the schizophrenia group maintained their low levels of responding at the end of the 350 ms ISI task into the initial blocks of the 850 ISI (see Figure 1d).
Conditioned Response Onset Latency2
Effects of ISI on CR onset Latency
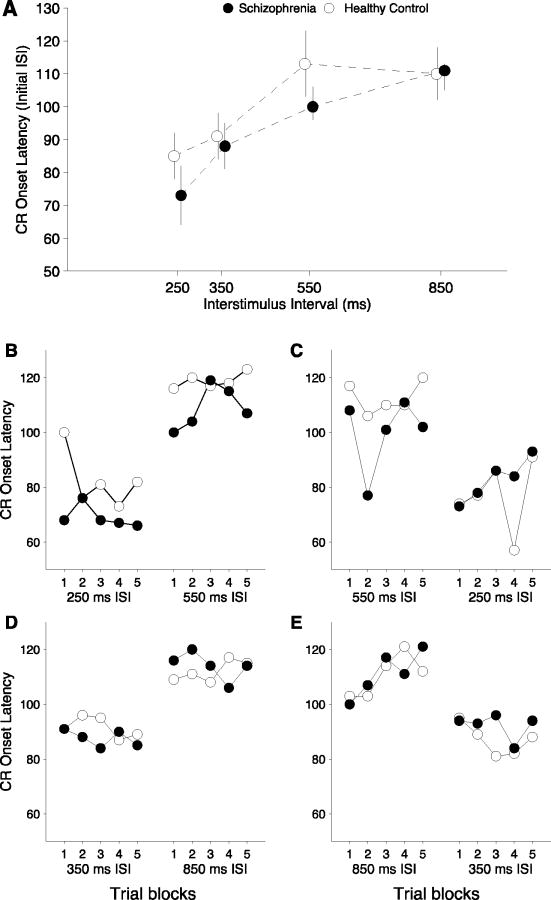
As can be observed from Figure 2a, when CR onset times were expressed in terms of ms prior to US onsets, the mean CR onset times ranged over only 50 ms (from 70 ms to 120 ms before US onset) across the four ISIs. The temporal relationship between the onset of conditioned response eyeblinks changed with ISI duration. Specifically, CRs occurred closer to US onset as ISI duration decreased, resulting in a main effect of ISI, F(3)=17.59, p < .001 (ηP2 =.34). Post-hoc tests indicated that this difference was significant in all pairwise comparisons, excepting the 550 and 850 ms ISI, which were not statistically distinguishable (p > .05). In addition, there was a block by ISI interaction, F(12)=1.97, p < .05 (ηP2 =.07), which was primarily due to CRs occurring progressively earlier, or more temporally distant, from the US onset, in the 850 ISI (see section below). Mean CR onset in the schizophrenia group (M=92.9, SD=19.1) was closer to US onset relative to controls (M=99.8, SD=21.9) overall, resulting in a main effect of diagnosis, F(1)=4.30, p = .05 (ηP2 =.04), although there were no significant differences between groups in follow-up pairwise comparisons.
Figure 2.
CR onset latency for the control (open circles) and schizophrenia (filled circles) groups expressed in terms of ms prior to US onsets at each ISI. Data points for the schizophrenia group are displayed with a slight rightward offset in order to allow visualization of standard error bars. Mean CR onset times ranged over only 50 ms (from 70 ms to 120 ms before US onset) across the four ISIs. The top panel shows the onset latency for each group at each ISI at when it is presented first in the sequence, and illustrates a main effect of group in which the schizophrenia group had slower CR onset latencies. The bottom panel plots the onset latency for each group across the 5 acquisition blocks when that ISI was presented first in the sequence and when it was presented second, i.e. when a “shift” was required.
ISI Shift effects on CR onset Latency
Overall, the effect of shifting from one ISI to another had negligible effects on performance as judged by the order of presentation (first or second in the sequence) on each ISI. When both orders of presentation were considered in the 550 ms ISI, the schizophrenia group had significantly later CR onsets, F(1)=4.13, p=.05 (ηP2 =.06). In the 850 ms ISI, when both orders of presentation were considered together there was a significant interaction between block and order, F(3.55)=3.22, p < .05 (ηP2 =.04). This effect was primarily due to CRs occurring progressively earlier in time relative to US onset in the long-to-short condition, F(3.22)=4.57, p < .01 (ηP2 =.13).
For onset latency, shift cost was significant in each of the 4 ISI shift experiments (p < .01), but there were no differential effects of shift cost across diagnostic groups.
UR Amplitude and Latency
Effects of ISI on URs
Analysis of the first presentation of each ISI showed that the schizophrenia group and healthy controls had similar UR amplitudes across ISIs, F(1)=.117, p=ns. Likewise, there were no differences between groups at any individual ISI. There was a main effect of block, F(2.41)=37.95, p < .001 (ηP2 = .27), signifying that UR amplitude decreased over the course of the experiment. When both orders of presentation were considered together, no main effects of diagnosis were observed, F(1)=0.08, p=ns. To ensure that increased conditioned responding in the control group did not diminish UR amplitude (due to eyelid closure at the UR onset), which could mask lower UR amplitudes in the schizophrenia group, the same analyses were conducted using percent CRs as a covariate. Results were not altered using this approach. The control group had significantly shorter UR latencies when the first presentation of each ISI was analyzed, F(1)=4.89, p < .05, (ηP2 = .05). Follow-up pairwise comparisons indicated this was due to differences in the 350 ms ISI (p < .01). However, when separate correlational analyses were conducted for each diagnostic group, UR latency did not significantly correlate with percent CRs and CR latency at the 350 ISI, nor at any other ISIs, suggesting that slower UR latencies in the schizophrenia group did not contribute to differences between groups at the 350 ms ISI or any other ISI task. When both orders of presentation were considered together, there were no differences between groups on UR latency at any ISI.
Medication analyses
No differences between medicated and unmedicated schizophrenia participants were observed on any eyeblink conditioning dependent variables, nor were any significant effects found when schizophrenia participants who were taking typical antipsychotics were compared with those on atypical antipsychotics only or who were unmedicated. When unmedicated schizophrenia participants were compared to their age-matched controls, the overall pattern of group differences was similar and, although it did not reach statistical significance, F(1)=1.35, p = ns, the differences in the percentage of conditioned responses was similar to what was observed using the entire sample (schizophrenia=33%; control=44%).
Discussion
The present study revealed several important findings. First, eyeblink conditioning is impaired in schizophrenia compared to healthy controls, particularly at the 350 ms ISI. Second, although the schizophrenia group consistently exhibited fewer conditioned responses across all ISIs compared to healthy controls, the pattern of performance across ISIs was similar (see Figure 1a) insofar as the highest levels of conditioning for both groups occurred at the 350 ms ISI, resulting in significant group differences. In addition, both groups had the lowest levels of conditioning at the shortest ISI (250 ms), with learning significantly more difficult at this ISI compared to the 350 ms ISI in both groups. Conditioning levels at the two longest ISIs (550 & 850) fell between the 250 ms ISI and the optimal 350 ms ISI, but conditioning did not differ between groups at these longer ISIs. Third, there was a shift cost to healthy controls in the 350-850 ISI shift paradigm, but this was not evident in schizophrenia group due to consistently low levels of CR acquisition, which were evident regardless of order of presentation. Fourth, CR onset latency was directly affected by ISI length, with shorter ISIs producing CRs that occurred closer to US onset. Finally, onset latencies in the schizophrenia group occurred significantly closer in time to UR onset overall.
Previous studies examining the effect of ISI duration on conditioned responding in humans in the auditory EBC delay paradigm have produced mixed results. For example, another study by our group in healthy college-age participants found that participants had higher CR rates at the 350 ms ISI compared to 850 ms ISI (Steinmetz et al., 2010). Although examination of Figure 1a suggests a similar pattern of results in the present study of middle-aged adults, CRs at the 350 and 850 ISIs were not statistically different from each other in either group. In a similar study, Woodruff-Pak and Finkbiner (1995) reported no differences in healthy college-age participants between a 400 ms and 750 ms delay conditioning paradigm. However, another study reported that healthy participants conditioned significantly better in a 400 ms delay paradigm than in a 650 ms delay or 900 ms delay task (Solomon et al., 1991). An additional early study examined optimal ISIs in humans and found the highest levels of conditioning at the 250 ms ISI, with decreased performance at 450 ms ISI and lower still at 750 ms ISI (McAllister, 1953). This finding is in marked contrast with the present study, where the 250 ms ISI showed a clear decrement in performance at the 250 ms ISI compared to longer ISIs. Nevertheless, although superficially contradictory, taken together these studies are broadly consistent with the present results in their overall pattern. In general, it appears that an ISI of approximately 350 ms is more ideal than longer ISIs.
In the 350-850 paradigm, when the 350 ms ISI was presented first, the schizophrenia group had significantly fewer CRs than controls, a finding that is consistent with our previous reports when this ISI was used in an auditory single-cue delay paradigm (Brown et al., 2005; Bolbecker et al., 2009b). However, there were no interactions with ISI order. This lack of order effects differs from our earlier ISI shift results using these ISIs in a healthy, college-age sample in which participants conditioned significantly better in the 350-850 paradigm relative to the 850-350 paradigm (Steinmetz et al., 2010). Significant shift costs were associated with the 250-550 ISI paradigm for both groups, with substantial declines in percent CRs in the 550 to 250 shift and gains in CRs in the 250 to 550 shift. However, taken together with the lack of shift effects in the 350-850 paradigm, the significant shift cost in the 250-550 paradigm was likely attributable to the very low conditioned response rates at the 250 ISI rather than shift cost per se (see Figure 1).
The onset of CRs occurred later, i.e. closer in time to the US onset, in shorter ISIs relative to longer ones, which is consistent with earlier findings from this laboratory in healthy adults (Steinmetz et al., 2010), using ISIs of 350 and 850 ms. The present results also indicate that the onset of conditioned responses occurred significantly later in the schizophrenia group relative to healthy controls overall. The present finding that conditioned response onset occurs later in schizophrenia is somewhat surprising, as it is at odds with previous findings from our laboratory and others who have on the whole reported earlier CR peak and onset latencies in schizophrenia (Bolbecker et al., 2009b; Brown et al., 2005; Sears et al., 2000. The finding in this study of later onset latencies in schizophrenia is consistent with a report of Marenco et al., (2003) that found later CRs in schizophrenia. They found onsets that were approximately 30 ms later compared to controls; however, the schizophrenia group in the present study had latencies that were on average only 7 ms later.
Lesion studies and evidence from structural imaging studies indicate that earlier CRs reflect disturbances in anterior lobe function in the cerebellum. For example, lesions to the anterior lobe in rabbits caused previously acquired responses to occur at a fixed, short latency, suggesting that this region plays a role in the delaying the onset of conditioned responses until just prior to the US onset (Perrett et al., 1993; Garcia et al., 1999). Findings from these animal studies are supported by the human literature (Perrett et al., 1993; Garcia et al., 1999). Moreover, in healthy volunteers, increased anterior lobe volume is associated with later CR peak responses that occur closer to UR onset (Edwards et al., 2008). Later CRs can be considered more adaptive than earlier ones because it is more likely to avoid the aversive unconditioned stimulus, in this case an airpuff. However, it is also possible that earlier onset latencies simply extend the duration of CRs without affecting eyelid closure at the US onset. Interestingly, the effects of switching to a new ISI had very little effect on onset latency in either group. It was anticipated that in the current ISI shift design, conditioned response latency would show some plasticity that would have manifested as an interaction between block and order. For example, in a long-to-short shift, it could be expected that early blocks in the short ISI would show late CRs that would become progressively earlier. No order effects were observed for onset latency, and the schizophrenia group did not show any differential effects compared to controls in adjusting to new ISIs following pre-exposure to a different ISI.
In considering CR timing, it may be relevant to point out that in the experimental design employed in this study, 90% of conditioning trials were paired CS-US trials. This general design is commonly used in human EBC experiments, but nevertheless could limit detection of late occurring CRs due to their occlusion by URs. While additional CS alone trials would circumvent this potential problem, in order to avoid interfering with acquisition of conditioned responses, a much longer task duration would be necessary in order to obtain enough CS-alone trials for meaningful analysis. While in non-human animals such designs are common, this is less feasible in humans, and in psychiatric populations, due to issues such as compliance, eye irritation, and participant fatigue. Therefore, the measurements of onset latency in these experiments should be interpreted with this caveat in mind. However, it seems unlikely that this possibility affected the current results because CR onsets were more than 90 ms prior to US onset in both groups with relatively small standard deviations.
The present results indicate significant EBC abnormalities in schizophrenia with respect to both CR learning and timing. On the basis of an extensive animal and human literature, the present findings implicate both the cerebellar cortex and interpositus nucleus in the deficits observed in schizophrenia (Thompson and Steinmetz, 2009). Several potential statistical and interpretive confounds were examined to further substantiate these conclusions. First, the schizophrenia group exhibited significantly fewer conditioned blink responses at each ISI, with statistically significant differences in several follow-up tests; however, the absence of a group by block statistical interaction raises the possibility that the schizophrenia group may have had a preexisting deficit in the performance of blinks rather than a cerebellar-mediated eyeblink conditioning deficit per se. Several findings argue against this supposition. First, UR amplitude in the schizophrenia group was not significantly different from the healthy controls at any ISI, meaning that overall the neural circuitry for producing eyeblinks was likely intact in the schizophrenia group and pointing to deficits in learning rather than production of conditioned responses. In addition, the schizophrenia and control groups had similar UR latencies. While the UR amplitude and latency can be confounded by CRs, covarying for CR rate did not influence UR results. Overall, these UR results suggest that problems with motor responsiveness in the schizophrenia group were unlikely to be responsible for the observed conditioned response deficits. Moreover, an analysis of the number of bad trials, which is an index of spontaneous blink rate, indicated that there was no difference between groups. An additional relevant point is that schizophrenia patients reportedly display higher spontaneous blink rates compared to non-psychiatric controls (Freed, 1980), yet in the present study this would have resulted in an artificial increase in their conditioned response rates and made it more difficult to detect differences between groups. Therefore, the reported group differences in conditioning rates are unlikely to have been influenced by increased spontaneous blinking in the schizophrenia group. Finally, the majority of patients in this sample were on psychotropic medications, and the effect of these medications on eyeblink conditioning is not completely understood. However, in the present study, the 13 unmedicated participants in the schizophrenia group were not statistically different from the medicated participants. When only unmedicated schizophrenia participants were compared with their age-matched controls, the pattern of results was consistent with that obtained for the entire sample, although statistical significance was not reached. Previous studies from our laboratory analyzed a subset of patients who were not medicated during EBC testing and found that impaired acquisition and timing in the schizophrenia group was evident, which was consistent with results observed in a larger sample, but with even larger effect sizes (Bolbecker et al., 2009b). This is consistent with findings of poorer EBC in unmedicated patients with bipolar disorder (Bolbecker et al., 2009a). In addition, a recent study from our laboratory found that a group of schizotypal personality disorder participants performed at an intermediate level on percentage of conditioned responses, performing better than a schizophrenia group, but acquiring significantly fewer conditioned responses compared to a healthy control group (Forsyth et al., 2010). This latter result is important because none of the schizotypal personality disorder participants were on antipsychotic drugs and because schizotypal personality disorder is widely believed to be an intermediate phenotype of schizophrenia (Siever & Davis, 2004). Taken together, these findings argue against a pre-conditioning blink performance deficit in schizophrenia that could have accounted for the observed conditioning abnormalities and strengthen the interpretation that the observed findings suggest cerebellar dysfunction in the disorder.
The present results add to evidence that cerebellar deficits exist in schizophrenia and may be part of the underlying pathophysiology of the disorder. Theoretical and empirical findings suggest that cerebellar abnormalities may result in abnormal coordination of information in non-motor psychological domains over which the cerebellum exerts modulatory influence. For example, the striking invariance of cerebellar circuitry suggests that it performs similar operations on its neuronal inputs regardless of their source (Katz & Steinmetz, 2002; Schmahmann, 2004; Schutter & Van Honk, 2005). Findings of topographic projections organized in bidirectional information streams connecting the cerebellum with motor, prefrontal, and posterior parietal cortex (Schmahmann, 2004) have led to suggestions that the cerebellum integrates information from different functional domains (Katz & Steinmetz, 2002; Schmahmann, 2004; Schutter & Van Honk, 2005). This integration may provide the moment-by-moment context for behavioral output, binding together perceptual, cognitive, emotional, and motivational information from disparate brain regions (Schutter & Van Honk, 2005). Given such a model, the deficits in cerebellar-dependent associative learning reported here may be indicative of abnormal integration of cognitive, perceptual, and affective information. This cerebellar malfunction could be a key contributor to observed disruptions in the CCTCC (i.e. Wilson et al., 2009; Honey et al., 2005) and to clinical features of schizophrenia.
More research will be necessary, however, to fully understand the nature of cerebellar dysfunction in EBC in schizophrenia. Imaging studies would be especially helpful in illuminating cerebellar activity in schizophrenia compared with healthy people. Such research will be helpful for disentangling the extent to which schizophrenia is associated with cerebellar-mediated conditioned response acquisition versus pure production deficits. In addition, appropriately designed transcranial magnetic stimulation (TMS) may prove valuable in studies of the cerebellum and EBC in schizophrenia (Demirtas-Tatlidede et al., 2010). In other clinical populations with cerebellar deficits, rTMS over the lateral cerebellum improved timing and reduced timing variability on a cerebellar-dependent paced finger-tapping task (i.e Avanzino et al., 2009). If improved EBC could be demonstrated in schizophrenia using similar methods, and corresponding improvements in cognition were also observed, this would provide more confidence that the cerebellum is involved in psychological functions. Furthermore, this may indicate that cerebellar-based treatments may be a valid therapeutic target for schizophrenia (c.f., Bolbecker et al., 2009c).
Table 2. Means and standard deviations for UR peak latency and amplitude for each diagnostic group during the first presentation of each ISI task.
| First Presentation UR Peak Amplitude | Healthy Control | Schizophrenia | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| 250 ms ISI | 50.1 | 33.5 | 23.4 | 19.5 |
| 350 ms ISI | 42.4 | 37.3 | 39.4 | 38.0 |
| 550 ms ISI | 27.7 | 12.3 | 46.6 | 30.0 |
| 850 ms ISI | 40.8 | 19.7 | 53.2 | 34.1 |
| First Presentation UR Peak Latency | Healthy Control | Schizophrenia | ||
| Mean | SD | Mean | SD | |
| 250 ms ISI | 106 | 50 | 105 | 48 |
| 350 ms ISI | 102 | 50 | 114 | 65 |
| 550 ms ISI | 103 | 48 | 106 | 47 |
| 850 ms ISI | 109 | 39 | 106 | 42 |
Acknowledgments
We are grateful to Colleen Merrill, Ashley Steffen, and Sam Kaiser for their assistance in collecting finger-tapping data. We also thank the clinical research team at Larue D. Carter Memorial Hospital and the Indiana University Neuroscience Clinical Research Center for their support. This research was supported by a National Institute of Mental Health grant (R01 MH074983) and NARSAD Young Investigator Award to William P. Hetrick.
Footnotes
It was important to confirm that use of a 150 ms CR window across ISIs did not overlook important response patterns occurring at longer ISIs. Therefore, data were analyzed for each ISI using CR windows that extended from 100 ms post-CS onset through US onset. As expected, there was a main effect of window length due to increased CRs in the 350, 550, and 850 ISI conditions, but there were no interactions of window length with any other variables, including order. Therefore, although more CRs were detected using the longer window, the overall pattern of results was not altered in any way by extending the length of the CR window.
Peak latency was also examined, and yielded effects that were similar in pattern to those reported for onset latency. No main effect of diagnosis was found, nor were any interactions with diagnosis evident.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Anand BK, Malhotra CL, Singh B, Dua S. Cerebellar projections to limbic system. Journal of Neurophysiology. 1959;22:451–7. doi: 10.1152/jn.1959.22.4.451. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O'Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophrenia Bulletin. 1998;4:203–18. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biological Psychiatry. 2008;64:81–8. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolbecker AR, Mehta C, Johannesen JK, Edwards CR, O'Donnell BF, Shekhar A, Nurnberger JI, Steinmetz JE, Hetrick WP. Eyeblink conditioning anomalies in bipolar disorder suggest cerebellar dysfunction. Bipolar Disorders. 2009a;11:19–32. doi: 10.1111/j.1399-5618.2008.00642.x. [DOI] [PubMed] [Google Scholar]
- Bolbecker AR, Mehta CS, Edwards CR, Steinmetz JE, O'Donnell BF, Hetrick WP. Eye-blink conditioning deficits indicate temporal processing abnormalities in schizophrenia. Schizophrenia Research. 2009b;111:182–91. doi: 10.1016/j.schres.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolbecker AR, Hetrick WP, Johannesen JK, O'Donnell BF, Steinmetz JE, Shekhar AS. Secretin effects on cerebellar-dependent motor learning in schizophrenia. American Journal of Psychiatry. 2009c;166:460–6. doi: 10.1176/appi.ajp.2008.08040597. [DOI] [PubMed] [Google Scholar]
- Brown SM, Kieffaber PD, Carroll CA, Vohs JL, Tracy JA, Shekhar A, O'Donnell BF, Steinmetz JE, Hetrick WP. Eyeblink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain & Cognition. 2005;58:94–108. doi: 10.1016/j.bandc.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learning & Memory. 2003;10:427–55. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Edwards CR, Newman S, Bismark A, Skosnik PD, O'Donnell BF, Shekhar A, Steinmetz JE, Hetrick WP. Cerebellum volume and eyeblink conditioning in schizophrenia. Psychiatry Research. 2008;162:185–94. doi: 10.1016/j.pscychresns.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Freitas C, Cromer JR, Safar L, Ongur D, Stone WS, Seidman LJ, Schmahmann JD, Pascual-Leone A. Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophrenia Research. 2010;124:91–100. doi: 10.1016/j.schres.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed W. Eye-blink rates and platelet monoamine oxidase activity in chronic schizophrenic patients. Biological Psychiatry. 1980;15:329–332. [PubMed] [Google Scholar]
- Garcia KS, Steele PM, Mauk MD. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. Journal of Neuroscience. 1999;19:10940–7. doi: 10.1523/JNEUROSCI.19-24-10940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwig M, Hajjar K, Dimitrova A, Maschke M, Kolb FP, Frings M, Thilmann AF, Forsting M, Diener HC, Timmann D. Timing of conditioned eyeblink responses is impaired in cerebellar patients. Journal of Neuroscience. 2005;25:3919–31. doi: 10.1523/JNEUROSCI.0266-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naïve schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biological Psychiatry. 2004;55:1146–53. doi: 10.1016/j.biopsych.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Hofer E, Doby D, Anderer P, Dantendorfer K. Impaired conditional discrimination learning in schizophrenia. Schizophrenia Research. 2001;51:127–36. doi: 10.1016/s0920-9964(00)00118-3. [DOI] [PubMed] [Google Scholar]
- Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, Fletcher PC. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimiya T, Okubo Y, Suhara T, Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biological Psychiatry. 2001;49:20–7. doi: 10.1016/s0006-3223(00)01081-7. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW, Diener HC. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Experimental Brain Research. 1988;73:167–80. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing of functions of the cerebellum. Journal of Cognitive Neuroscience. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Katz DB, Steinmetz JE. Psychological functions of the cerebellum. Behavioral and Cognitive Neuroscience Reviews. 2002;1:229–241. doi: 10.1177/1534582302001003004. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends in Neurosciences. 1997;20:177–81. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK. The International Affective Picture System standardization procedure and initial group results for affective judgements: Technical Report 1A. The Center for Research in Psychophysiology, University of Florida; Gainseville, FL: 1988. [Google Scholar]
- Lavond DG. Mammalian brain substrates of aversive classical conditioning. Annual Review of Psychology. 1993;44:317–42. doi: 10.1146/annurev.ps.44.020193.001533. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Steinmetz JE. Acquisition of classical conditioning without cerebellar cortex. Behavioural Brain Research. 1989;33:113–64. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Does the cerebellum contribute to mental skills? Behavioral Neuroscience. 1986;100:443–54. doi: 10.1037//0735-7044.100.4.443. [DOI] [PubMed] [Google Scholar]
- Marenco S, Weinberger DR, Schreurs BG. Single-cue delay and trace classical conditioning in schizophrenia. Biological Psychiatry. 2003;53:390–402. doi: 10.1016/s0006-3223(02)01506-8. [DOI] [PubMed] [Google Scholar]
- McAllister WR. The effect on eyelid conditioning of shifting the CS-US interval. Journal of Experimental Psychology. 1953;45:423–8. doi: 10.1037/h0057118. [DOI] [PubMed] [Google Scholar]
- Nopoulos PC, Ceilley JW, Gailis EA, Andreasen NC. An MRI study of cerebellar vermis morphology in patients with schizophrenia: evidence in support of the cognitive dysmetria concept. Biological Psychiatry. 1999;46:703–11. doi: 10.1016/s0006-3223(99)00093-1. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Nobuhara K, Sugimoto T, Kinoshita T. Diffusion tensor imaging study of the middle cerebellar peduncles in patients with schizophrenia. Cerebellum. 2005;4:123–7. doi: 10.1080/14734220510007879. [DOI] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. Journal of Neuroscience. 1993;13:1708–18. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard H, Amado I, Mouchet-Mages S, Olie JP, Krebs MO. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bulletin. 2008;34:155–72. doi: 10.1093/schbul/sbm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:367–78. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Prefrontal cortex projections to the basilar pons in rhesus monkey: implications for the cerebellar contribution to higher function. Neuroscience Letters. 1995;199:175–8. doi: 10.1016/0304-3940(95)12056-a. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The cerebrocerebellar system: anatomic substrates of the cerebellar contribution to cognition and emotion. International Review of Psychiatry. 2001a;13:247–260. [Google Scholar]
- Schmahmann JD. The cerebellar cognitive affective syndrome: clinical correlations of the dysmetria of thought hypothesis. International Review of Psychiatry. 2001b;13:313–322. [Google Scholar]
- Schutter DJ, van Honk J. The cerebellum on the rise in human emotion. Cerebellum. 2005;4:290–4. doi: 10.1080/14734220500348584. [DOI] [PubMed] [Google Scholar]
- Sears LL, Andreasen NC, O'Leary DS. Cerebellar functional abnormalities in schizophrenia are suggested by classical eyeblink conditioning. Biological Psychiatry. 2000;48:204–9. doi: 10.1016/s0006-3223(00)00247-x. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. American Journal of Psychiatry. 2004;161:398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. American Journal of Psychiatry. 2003;160:1809–16. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Blanchard S, Levine E, Velazquez E, Groccia-Ellison M. Attenuation of age-related conditioning deficits in humans by extension of the interstimulus interval. Psychology and Aging. 1991;6:36–42. doi: 10.1037//0882-7974.6.1.36. [DOI] [PubMed] [Google Scholar]
- Spain B. Eyelid conditioning and arousal in schizophrenic and normal subjects. Journal of Abnormal Psychology. 1966;71:260–6. doi: 10.1037/h0023596. [DOI] [PubMed] [Google Scholar]
- Steinmetz AB, Skosnik PD, Edwards CR, Steinmetz JE, Hetrick WP. Evaluation of bidirectional interstimulus interval shift in auditory delay eyeblink conditioning in healthy humans. 2010 doi: 10.3758/s13420-011-0031-9. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Steinmetz JE. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience. 2009;162:732–55. doi: 10.1016/j.neuroscience.2009.01.041. [DOI] [PubMed] [Google Scholar]
- Timmann D, Drepper J, Frings M, Maschke M, Richter S, Gerwig M, Kolb FP. The human cerebellum contributes to motor, emotional and cognitive associative learning. Cortex. 2010;46:845–57. doi: 10.1016/j.cortex.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Timmann D, Daum I. Cerebellar contributions to cognitive functions: a progress report after two decades of research. Cerebellum. 2007;6:159–62. doi: 10.1080/14734220701496448. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Andreasen NC, Nopoulos P, Flaum M. Cerebellar morphology as a predictor of symptom and psychosocial outcome in schizophrenia. Biological Psychiatry. 1999;45:41–8. doi: 10.1016/s0006-3223(98)00175-9. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Goldenberg G, Downey-Lamb MM, Boyko OB, Lemieux SK. Cerebellar volume in humans related to magnitude of classical conditioning. Neuroreport. 2000;11:609–15. doi: 10.1097/00001756-200002280-00035. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Jaeger ME, Gorman C, Wesnes KA. Relationships among age, conditioned stimulus-unconditioned stimulus interval, and neuropsychological test performance. Neuropsychology. 1999;13:90–102. doi: 10.1037//0894-4105.13.1.90. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Finkbiner RG. Larger nondeclarative than declarative deficits in learning and memory in human aging. Psychology and Aging. 1995;10:416–26. doi: 10.1037//0882-7974.10.3.416. [DOI] [PubMed] [Google Scholar]