Abstract
The corticospinal tract (CST) is the principal motor control pathway for skilled movements. It has a protracted postnatal development, creating a protracted period of vulnerability to perinatal brain and spinal cord injury. Research has shown that the motor signs in cerebral palsy (CP) reflect the loss of CST connections as well as development of abnormal motor systems connections, especially between the developing CST and spinal motor circuits. In this paper, we discuss a feline model of CP that we have developed. The animals develop a pattern of abnormal CST connections that are remarkably similar to those seen in hemiplegic CP and visuomotor impairments. Using this model we devised neural activity-based therapeutic approaches to repair the abnormal CST connections and restore normal skilled movement control. Our studies stress that more active CST connections are better able to maintain strong synaptic connections with spinal motor circuits. We propose that perinatal trauma initiates a vicious cycle in which CST axons that are spared after an injury are at a disadvantage for maintaining spinal connections, leading to further reductions in connections and motor signs. If this is so, targeted activation of the spared CST might interrupt this process and lead to functional improvement.
The corticospinal tract (CST) is the principal motor control pathway for skilled movements.1 It has a protracted development, matching closely development of skilled movements.2 Along with delayed development, there is a prolonged period of vulnerability when damage to the CST or its cortical origins can have long-term consequences for motor function. Perinatal damage to the developing CST can result in cerebral palsy (CP). Research in humans with CP shows that the motor signs reflect the loss of CST connections and development of miswired motor-system connections.3–6 Important among these miswired connections are those between the developing CST and spinal circuits. A person with hemiplegic CP has weakened or ineffective connections between the affected cortex and contralateral spinal cord along with stronger, effective, bilateral connections between the less affected side and the spinal cord.2
Here, we focus on our research using a feline model that produces motor circuit changes that are remarkably similar to those seen in CP. We focus on activity-dependent synaptic competition, a mechanism key to (1) normal CST development, (2) understanding changes in CST organization in hemiplegic CP, and (3) repairing the damaged CST and restoring skilled motor functions. Our work provides a scientific basis for exploring novel biological-based strategies for CST repair and recovery of skilled movement control.
CST CONNECTION DEVELOPMENT REFLECTS THE INTERPLAY BETWEEN ACTIVITY AND INTRINSIC GUIDANCE MECHANISMS
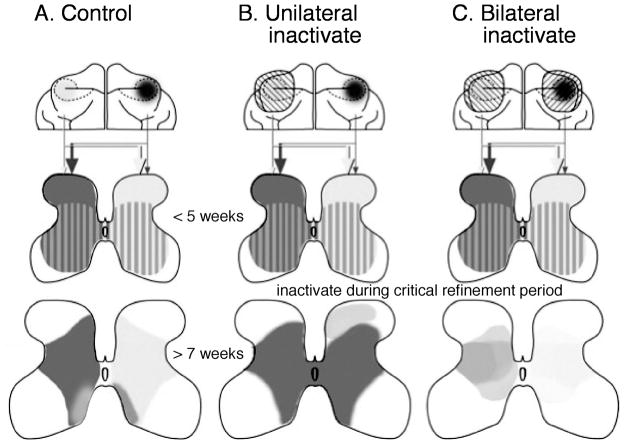
Establishing CST connections with spinal neurons requires axon guidance and activity. Diverse guidance cues under important genetic regulation steer CST axon growth to the spinal cord and help establish a coarse pattern of spinal terminations.2,7–12 The coarse pattern of CST connectivity is refined later during postnatal development. In cats, and probably humans (see below), there are bilateral CST terminations early in development that are subsequently eliminated.2,13,14 Because motor impairments in hemiplegic CP reflect miswiring of CST circuits during development – bilateral CST projections – we probed the mechanisms of CST circuit formation to obtain fundamental insights into the neuronal underpinnings of this developmental motor disorder. We studied development of the laterality of CST terminations in cats, which involves elimination of most ipsilateral terminations. At ages younger than 5 weeks, the cat CST has dense contralateral spinal terminations that terminate throughout the gray matter. At this age, ipsilateral terminations are robust but not as dense as the contralateral terminations (Fig. 1a, middle row, striped pattern2). Between postnatal weeks 5 and 7, most of the ipsilateral terminations are eliminated to establish the predominantly contralateral termination pattern seen in mature cats (Fig. 1a, bottom row). We determined if activity-dependent synaptic competition is key to refinement of the initially coarse pattern of CST connections with spinal neurons. We blocked CST activity unilaterally by infusion of an inhibitory neurotransmitter (GABAA [γ-aminobutyric acid A receptor]) agonist muscimol directly into the motor cortex (M1), the principal origin of the CST in cats (Fig. 1b, top row, left cortex with cross hatching). We found that the silenced axons failed to establish dense, strong, spinal terminations (Fig. 1b, bottom row, light gray2,15). By contrast, the CST that originated in the opposite, active, motor cortex developed dense bilateral connections (Fig. 1b, bottom row, dark gray). This indicates an important role for activity. This pattern of CST connections resembles that of hemiplegic CP. Bilateral M1 activity blockade produced a more balanced laterality pattern of CST terminations, supporting an important role for activity-dependent synaptic competition, not just activity alone16 (Fig. 1c).
Figure 1.
Semi-schematic representation of maturation of corticospinal tract (CST) terminations in cats (a) and the role of activity-dependent processes (b, c). (a) Projections from motor cortex (top row; light and dark shading) to the spinal cord gray matter are bilateral before 5 weeks of age and become refined to a predominantly contralateral projection after 7 weeks of age. (b) Unilateral inactivation of motor cortex (left cortex) during the critical refinement period (between 5 and 7wk) results in an aberrant dorsal projection (light shading on right side of spinal cord in bottom row) and aberrant bilateral projection from the non-inactivated side (dark shading). (c) Bilateral motor cortex inactivation during the critical refinement period results in a more balanced termination pattern of the corticospinal tract (bottom row).
Because the contralateral CST is denser and stronger than the ipsilateral tract, the contralateral connections stabilize at the expense of the weaker ipsilateral terminations. The larger contralateral CST projection has a ‘competitive edge’ over the smaller ipsilateral projection, thereby enabling it to strengthen its connections, at the expense of the ipsilateral tract. Importantly, we also found that blocking CST activity impedes development of spinal cholinergic interneurons, suggesting a trophic role for the CST in spinal circuit development.17 With this trophic influence diminished after brain damage, spinal circuits will not develop normally, even though the trauma does not involve the spinal cord.
MOTOR CORTEX MOTOR MAP DEVELOPMENT DEPENDS ON AN ACTIVE CST
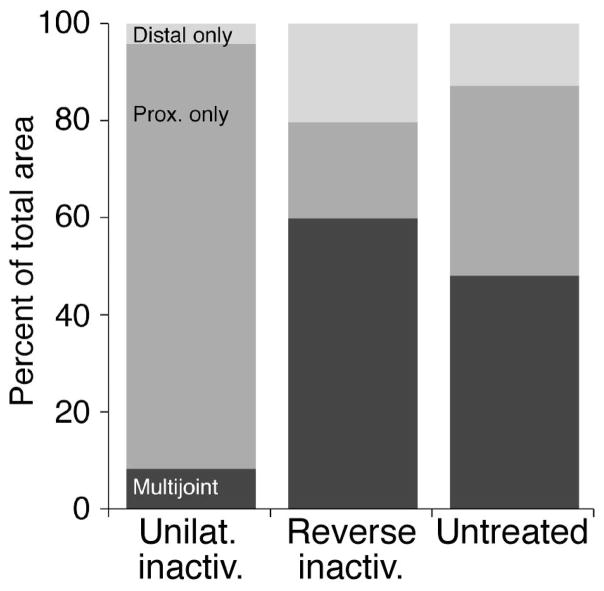
We determined the spatial representation of forelimb muscles and joints, the motor map, in motor cortex (M1). Surprisingly, the map begins to develop after the mature laterality pattern of CST termination is achieved, at 7 weeks of age, and continues until about 11 to 14 weeks (Chakrabarty and Martin18), not earlier, when CST connections first form. We found that between these ages the map develops its key mature characteristics: (1) most of M1 represents a motor response; and (2) proximal and distal forelimb responses become represented in a balanced way. Unilateral M1 inactivation permanently impedes motor-map development. There is a substantial reduction in the distal representation and a disproportionate increase in the proximal joint representation (Fig. 2, compare Unilat. inactiv., Reverse inact., and Untreated). Normally, many sites co-represent multiple forelimb joints, which reflects a role of M1 in interjoint coordination. After inactivation, such multijoint sites are rare.
Figure 2.
Activity-dependent maturation of the M1 motor representation. Proximal sites are represented predominantly after unilateral inactivation whereas proximal and distal sites are represented after alternate inactivation. Data from the M1 that received unilateral inactivation (between weeks 5 and 7 only; a), M1 that received the initial inactivation between weeks 5 and 7, but examined after inactivation of the other M1 from weeks 7 to 11 (reverse inactivation; b), and the untreated motor cortex (c), are shown. (Modified from Chakrabarty et al.24).
HUMAN CST DEVELOPMENT PARALLELS THAT OF THE CAT IN HEALTH AND DISEASE
Our finding of refinement of the CST and its activity dependence in cats parallels closely normal development in humans and aberrant CST development in hemiplegic CP. Using transcranial magnetic stimulation (TMS), humans have a bilateral CST until about 6 to 12 months of age, when the predominantly contralateral projection is established.6 Children and adults with hemiplegic CP maintain ipsilateral CST projections from the non-affected side; the contralateral projection from the affected side is weak.4,6 In a longitudinal study,6 babies at risk for CP were followed. Babies with unilateral infarction had normal TMS effects at term and 3 months. But by 6 months, the infarcted side showed reduced contralateral and ipsilateral TMS-evoked responses. The non-infarcted side showed a normal contralateral response. Surprisingly, evoked ipsilateral responses at 6 and 12 months were large, as in neonates, suggesting that the ipsilateral CST spinal projection was maintained. This supports the synaptic competition hypothesis, derived from our cat experiments.2 With elimination of strong contralateral projections from the affected side, the ipsilateral CST from the unaffected side maintained its connections and was able to elicit motor responses.
UNILATERAL M1 INACTIVATION IS A MODEL FOR CST CIRCUIT ABNORMALITIES IN HEMIPLEGIC CP
The aberrant CST projection after unilateral inactivation pattern (see Fig. 1 for summary of published findings) is similar to what is seen in hemiplegic CP.3–6 Animals subjected to unilateral M1 inactivation also develop reaching and visually guided adaptive locomotion impairments, while walking on a horizontal ladder.19,20 Both of these tasks require the animal to use complex sensory information to generate motor control signals for accurately guiding the limb to the target. For reaching, the target is a piece of food to be grasped. For adaptive locomotion, the target is the ladder rung; it must place its paws squarely on the ladder rungs to prevent slipping. Healthy young cats are excellent performers on both tasks, accurately grasping the food or placing its paws on the ladder rungs. After unilateral inactivation, reaching and stepping on the affected (contralateral) side become impaired. The reaching and stepping become hypermetric, possibly owing to impairments in integrating limb proprioceptive and visual information with motor control signals. Note that whereas disorders of movement extent (hypo- and hypermetria) can be associated with cerebellar lesion, these animals have no cerebellar damage. Nevertheless, internal signals between the corticospinal and cerebellar control systems might be dysfunctional. Grasping becomes ineffective, with the loss of coordination between digit flexion and forearm supination. It follows from our cat model that, in individuals with unilateral (hemiplegic) CP, the impaired contralateral CST is at a disadvantage early in development, compared with the unimpaired ipsilateral CST, in establishing and maintaining synaptic connections in the spinal cord.
BIOLOGICAL STRATEGIES TO HARNESS ACTIVITY-DEPENDENT PLASTICITY FOR CST CIRCUIT REPAIR
We reasoned that if we could repair the aberrant CST circuitry in the cat model, to restore a normal spinal termination pattern (Fig. 1, bottom row; b to a), this would restore a more normal M1 motor map and improve limb control. We adopted two complementary biological strategies for repairing connections and restoring function.
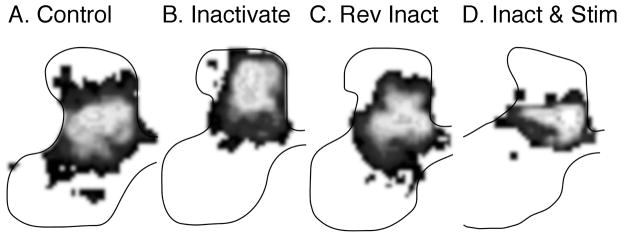
First, we promoted the capacity of the inactivated CST to compete more effectively for spinal terminations. This was accomplished by electrical stimulation of the previously silenced CST21,22 (2h/d). Figure 3a shows the normal distribution of CST terminations to the contralateral spinal cord, as a grayscale with the regions of highest CST axon density shaded white. After unilateral inactivation (Fig. 3b), the CST shifts dorsally to an aberrant location. Electrical stimulation after inactivation shifted the axon terminations back to within the normal zone (Fig. 3d). Thus, activation of the previously silent (and aberrant dorsal) CST resulted in re-establishment of connections within the normal spinal gray-matter territory. This also partly restored normal adaptive locomotor performance; the extent of hypermetria was substantially reduced (2.12cm [SD 0.11] with stimulation versus 2.72cm [SD 0.15] without stimulation).22 The partial nature of motor recovery might be because the tract was stimulated for only 2h/d, rather than longer.
Figure 3.
Effects of activity manipulations on corticospinal tract (CST) development and repair. Each panel shows the local density of CST axon terminations as grayscale in which white is most dense and black least dense. Areas of the background without label are show as white. Data are from cats at approximately 11 to 14 weeks of age. (a) Control. (b) Unilateral inactivation. (c) Reverse inactivation. (d) Inactivation followed by stimulation. Data are re-graphed in the grayscale from the original publications.22,23
Second, we reduced the competition that the previously silenced CST faces in the spinal cord23 by inactivation of the previously active/unaffected side (i.e. Fig. 1b; bottom row, dark gray), termed reverse inactivation (Fig. 3c). The CST axon terminations are restored to their normal location. This results in normal motor control returns.23 Without treatment, step distance is 2.98cm (SD 0.11); after treatment step distance is 1.91cm (0.11), which is not significantly different from control values. The M1 motor map, which was examined after reverse inactivation, was also restored, achieving balanced distal–proximal limb representation and increased multijoint responses.24
BEHAVIORAL MANIPULATIONS CAN RESTORE CST CONNECTIVITY AND FUNCTION
Whereas our work stresses biological approaches to manipulate activity-dependent synaptic competition, we have also studied if behavioral manipulations alone lead to CST repair and motor recovery. We constrained the unimpaired limb and trained the impaired limb (constraint-induced movement therapy). Indeed, this therapy restored the normal distribution of CST spinal connections; virtually identical to what we have observed with CST stimulation and reverse inactivation (data not shown). Behavior improved as well. Before training, step distance was 2.6cm (SD 0.27), and after 4 weeks of training 1.7cm (SD 0.14). Thus, training resulted in a 35.6% decrease in step distance, giving a step distance similar to controls. By contrast, 4 weeks of constraint of the unimpaired limb alone – without training of the affected limb – resulted in no reduction of step distance (0.46%; data not shown). The similarity with the other approaches strongly suggests that CST synaptic competition is at work in re-establishing normal connections after the activity-based treatments.
DIVERSE ACTIVITY MANIPULATIONS CAN BE HARNESSED TO MAKE CST CONNECTIONS WITH SPINAL MOTOR CIRCUITS MORE OR LESS COMPETITIVE
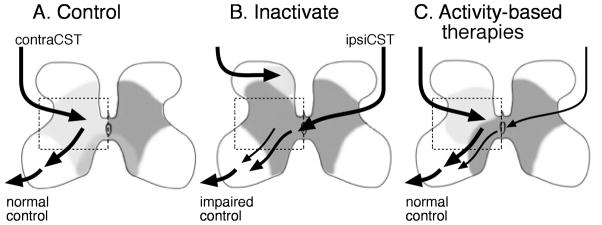
Our animal studies are summarized in Figure 4. There are three key points: (1) inactivation produces an aberrant CST spinal termination pattern that resembles hemiplegic CP (Fig. 4b); (2) activity-based therapies restore these connections (Fig. 4c); (3) activity-based therapies restore improved/normal limb control and this is associated with the normal CST connections pattern. The period of effective activity treatments in cats immediately follows the period of CST refinement. In humans, this occurs by about 6 months and 1 year of age, suggesting that this very early age is the optimal time to intervene in the child at risk for developing CP. Having a more biological-based therapy is, therefore, important at this age. We can non-invasively promote activity-dependent competitiveness among developing CST connections using TMS and anodal transcranial direct current stimulation. Competitiveness can be reduced through cathodal transcranial direct current stimulation or particular repetitive TMS (rTMS) pulse sequences that promote intracortical inhibition.25 When the CST is most plastic is when the effects of therapeutic intervention will probably be most effective. At this age, babies are too young to follow a therapist’s instructions, which limits behavioral approaches alone. Moreover, children with CP who have impairments in cognition or attention also cannot participate as effectively in therapy compared with those less impaired. A combination of biological and behavioral therapies might be most appropriate for most people with CP. Our behavioral findings using constraint and training are also consistent with an activity-dependent competition mechanism. Constraint of the unimpaired limb might be akin to inactivation of the controlling, or unimpaired, M1; this would lead to creating a competitive disadvantage for its CST. Training of the impaired limb might create a competitive advantage to the controlling CST, akin to stimulation.
Figure 4.
Model for connectional and functional changes in healthy controls (a), after unilateral inactivation between weeks 5 and 7 (b), and after harnessing activity-dependent processes (c, either reverse inactivation, corticospinal tract [CST] stimulation, or constraint-induced movement therapy). Left side shows contralateral spinal projections (light gray). Line thickness and location denotes density and strength of connections as well as location. After unilateral inactivation, CST terminations are in an aberrant dorsal territory (b; light gray patch). The impaired movements are controlled by a combination aberrant contralateral and ipsilateral connections (black arrows from ipsilateral tract).19 Reverse inactivation, CST electrical stimulation, or combined restraint of the unimpaired limb and training of the impaired limb each help to restore CST connections to more intermediate and ventral regions (c; gray field) and less aberrant ipsilateral connections.
Little is known, however, about the safety of stimulation procedures in neonates.26 Safety of behavioral approaches also must be assessed, especially for constraint, with the same rigor as electrical stimulation. Behavior is a very powerful intervention in the developing brain, as shown by the extraordinary efficacy of patching the impaired eye for amblyopia.6,27,28 Obviously, these concerns will have to be addressed before methods for harnessing activity-dependent developmental plasticity move to the clinic.
The importance of activity-dependent competition goes beyond potential new therapeutic strategies. Our findings, together with those in humans,6 suggest that perinatal trauma initiates a vicious cycle. Spared CST connections are at a competitive disadvantage, which leads to further reduction in connections, and less competitiveness. If so, targeted activation of the spared CST, or de-activation of the unimpaired side, might interrupt this process and lead to functional improvement.
Acknowledgments
We thank Xiuli Wu for histochemistry and histology. This work was supported by National Institutes of Health grants NS36835 (to JHM) and NIH K01NS62116 (to KMF).
LIST OF ABBREVIATIONS
- CST
Corticospinal tract
- TMS
Transcranial magnetic stimulation
References
- 1.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 2.Martin J, Friel K, Salimi I, Chakrabarty S. Corticospinal development. In: Squire L, editor. Encyclopedia of Neuroscience. Oxford: Academic Press; 2009. pp. 203–14. [Google Scholar]
- 3.Farmer SF, Harrison LM, Ingram DA, Stephens JA. Plasticity of central motor pathways in children with hemiplegic cerebral palsy. Neurology. 1991;41:1505–10. doi: 10.1212/wnl.41.9.1505. [DOI] [PubMed] [Google Scholar]
- 4.Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–47. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- 5.Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–54. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- 6.Eyre JA, Smith M, Dabydeen L, et al. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann Neurol. 2007;62:493–503. doi: 10.1002/ana.21108. [DOI] [PubMed] [Google Scholar]
- 7.Kullander K, Croll SD, Zimmer M, et al. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 2001;15:877–88. doi: 10.1101/gad.868901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dottori M, Hartley L, Galea M, et al. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc Natl Acad Sci U S A. 1998;95:13248–53. doi: 10.1073/pnas.95.22.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Leary DD, Wilkinson DG. Eph receptors and ephrins in neural development. Curr Opin Neurobiol. 1999;9:65–73. doi: 10.1016/s0959-4388(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 10.Beg AA, Sommer JE, Martin JH, Scheiffele P. α2-chimaerin is an essential EphA4 effector in the assembly of neuronal locomotor circuits. Neuron. 2007;55:768–78. doi: 10.1016/j.neuron.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 11.Iwasato T, Katoh H, Nishimaru H, et al. Rac-GAP α-chimerin regulates motor- circuit formation as a key mediator of ephrinB3/EphA4 forward signaling. Cell. 2007;130:742–53. doi: 10.1016/j.cell.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Asante C, Chu A, Fisher M, et al. Cortical control of adaptive locomotion in wild- type mice and mutant mice lacking the Ephrin-Eph effector protein α2-chimaerin. J Neurophysiology. 2010;104:3189–202. doi: 10.1152/jn.00671.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theriault E, Tatton WG. Postnatal redistribution of pericruciate motor cortical projections within the kitten spinal cord. Dev Brain Res. 1989;45:219–37. doi: 10.1016/0165-3806(89)90041-2. [DOI] [PubMed] [Google Scholar]
- 14.Alisky JM, Swink TD, Tolbert DL. The postnatal spatial and temporal development of corticospinal projections in cats. Exp Brain Res. 1992;88:265–76. doi: 10.1007/BF02259101. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarty S, Martin JH. Postnatal development of a segmental switch enables corticospinal tract transmission to spinal forelimb motor circuits. J Neurosci. 2010;30:2277–88. doi: 10.1523/JNEUROSCI.5286-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin JH, Lee S. Activity-dependent competition between developing corticospinal terminations. NeuroReport. 1999;10:2277–82. doi: 10.1097/00001756-199908020-00010. [DOI] [PubMed] [Google Scholar]
- 17.Chakrabarty S, Shulman B, Martin JH. Activity-dependent codevelopment of the corticospinal system and target interneurons in the cervical spinal cord. J Neurosci. 2009;29:8816–27. doi: 10.1523/JNEUROSCI.0735-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakrabarty S, Martin JH. Postnatal development of the motor representation in primary motor cortex. J Neurophysiol. 2000;84:2582–94. doi: 10.1152/jn.2000.84.5.2582. [DOI] [PubMed] [Google Scholar]
- 19.Martin JH, Hacking A, Donarummo L. Impairments in prehension produced by early postnatal sensorimotor cortex activity blockade. J Neurophysiol. 2000;83:895–906. doi: 10.1152/jn.2000.83.2.895. [DOI] [PubMed] [Google Scholar]
- 20.Friel KM, Drew T, Martin JH. Differential activity-dependent development of corticospinal control of movement and final limb position during visually-guided locomotion. J Neurophysiol. 2007;97:3396–406. doi: 10.1152/jn.00750.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salimi I, Martin JH. Rescuing transient corticospinal terminations and promoting growth with corticospinal stimulation in kittens. J Neurosci. 2004;24:4952–61. doi: 10.1523/JNEUROSCI.0004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salimi I, Friel K, Martin JH. Pyramidal tract stimulation restores normal corticospinal tract connections and visuomotor skill after early postnatal motor cortex activity blockade. J Neurosci. 2008;28:7426–34. doi: 10.1523/JNEUROSCI.1078-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friel K, Martin JH. Bilateral activity-dependent interactions in the developing corticospinal system. J Neurosci. 2007;27:11083–90. doi: 10.1523/JNEUROSCI.2814-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakrabarty S, Friel K, Martin JH. Activity-dependent plasticity improves M1 motor representation and corticospinal tract connectivity. J Neurophysiol. 2009;110:1283–93. doi: 10.1152/jn.91026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2:241–5. doi: 10.1016/j.brs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Kirton A, Deveber G, Gunraj C, Chen R. Cortical excitability and interhemispheric inhibition after subcortical pediatric stroke: plastic organization and effects of rTMS. Clin Neurophysiol. 2010;121:1922–9. doi: 10.1016/j.clinph.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell DE, MacKinnon S. The present and potential impact of research on animal models for clinical treatment of stimulus deprivation amblyopia. Clin Exp Optom. 2002;85:5–18. doi: 10.1111/j.1444-0938.2002.tb03067.x. [DOI] [PubMed] [Google Scholar]
- 28.Sale A, Maya Vetencourt JF, Medini P, et al. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–81. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]