Abstract
Mate choice can be sensitive to social cues from neighboring individuals, e.g., animals can copy mate choice decisions. Males that are at risk of being copied by others may respond to this with reduced preference expression (“audience effects”). We review the various pathways by which sperm competition risk affects (1) male mate copying behavior and (2) audience effects. For example, a recent study suggests that males gather complex social information on rivals' sexual competitiveness (sexual activity and attractiveness to females) and respond with reduced expression of mating preferences only “when it matters,” i.e., when a sexually competitive rival is present.
Key words: audience effect, communication networks, mate choice copying, non-independent mate choice, sexual selection
Sexual selection, e.g., through mate choice, is an important evolutionary driver and individuals may integrate a wide range of information in their assessment of mate quality.1 Mate choice is not only based on external characteristics (morphological, behavioral or other) of potential mating partners, but also the social environment in which mating occurs plays an essential role (non-independent mate choice2–4). Especially in group-living animals5 communication events—such as communicatory interactions between sending (courting) males and receiving (choosing) females—typically do not occur in privacy, but in a public domain.6–11 Living in a social (or communication) network11–14 enables animals to eavesdrop on other individuals' mating decisions and utilize the extracted information.8,10,15–17 The most intensely studied type of social eavesdropping is mate choice copying,2,3,18–21 but also males that are at risk of being copied by others may respond to this with reduced preference expression (“audience effects”14,22–24). In livebearing fishes (family Poeciliidae), where females often mate multiply,25,26 sperm competition risk (SCR) is a decisive factor affecting males' fitness (reproductive success), but its role for non-independent mate choice was largely unknown. We demonstrate that SCR plays a significant role for (1) male mate copying behavior (with males copying less when SCR is high27) and (2) audience effects. For instance, SCR leads males to deceive rivals about their actual mating preferences.28 Moreover, a recent study29 uncovered a surprisingly complex case of social information use: males eavesdrop on rivals' sexual competitiveness (sexual activity and attractiveness to females) and respond with reduced preference expression only “when it matters,” i.e., when being observed by a sexually competitive rival.
Sexual selection theory often assumes that mate choice decisions are based on genetically inherited internal factors (i.e., innate search images) that enable individuals to select among several potential mates1 (Fig. 1A) and accordingly, an increasing body of literature provides direct (e.g., through parent-offspring comparisons)30,31 or indirect evidence (e.g., from the investigation of common garden reared individuals)32–34 for a genetic basis of mating preferences. Still, several studies exemplified that mate choice in nature is a complex process that also involves the acquisition of information from the social environment2,3,21,35 (Fig. 1B). This does not come as a surprise as animal behavior in general typically is a product of both innate (genetic) and environmental factors.36,37
Figure 1.
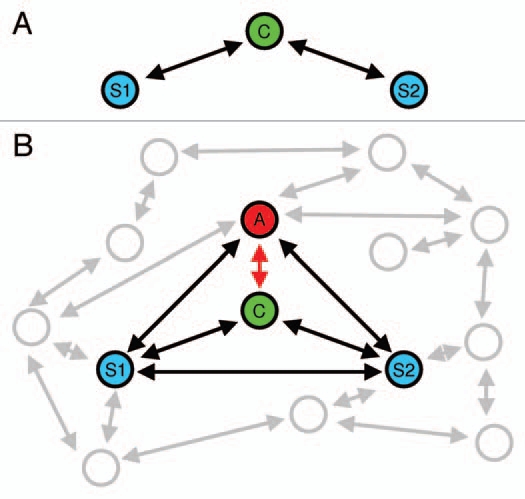
(A) Schematic view of an idealized mate choice situation in a classical binary choice test. Mate choice is thought of as a process of mate quality assessment involving the choosing individual (C) and two potential mating partners (stimuli, S1 and S2). (B) Mate choice in a communication network involving multiple senders and receivers of information. For simplicity, most studies to date have focused on interactions between four individuals. In this example, a by-standing individual (the audience, A) may affect the focal individual's mate choice, but also interactions between S1 and S2, and between A and both stimuli are acknowledged.
Much research has been conducted on female mate choice, and various theories have been forwarded to explain the adaptive significance of female mating preferences.1,38–40 However, it is well established that also males express mating preferences,41–45 especially if mating or sperm production is costly46–49 and if females differ in their resource value,50,51 such as numbers of oocytes in the female ovary.52 In modern bony fishes (Teleostei), for example, female fecundity is typically a correlate of body size, and males prefer to mate with larger, more fecund females (e.g., in two-spotted gobies, Gobiusculus flavescens,53 guppies, Poecilia reticulata,54 Atlantic mollies, P. mexicana,44,45,55 and haplochromine cichlids, Astatotilapia flaviijosephi).56
Male teleosts assess females' quality not only on the basis of body size, but a range of other phenotypic traits may also be relevant.50,57,58 However, in stark contrast to female mate choice, sperm competition risk (SCR) and intensity (SCI) can affect male mate choice.59–62 Obviously, a male's reproductive fitness is at stake when sperm from two or more males compete for fertilization of a clutch,63,64 or—in internally fertilizing species—within a single female's genital tract.26,65,66 In this review, we provide a brief overview of recent findings in the field of socially influenced (non-independent) male mate choice. We emphasize the role played by SCR for various aspects of non-independent male mate choice.
Especially in internal fertilizing species, such as livebearing fishes (family Poecilidae), where females mate multiply,67 and females can store sperm for several consecutive broods,26,68,69 sperm competition is intense. Poeciliid broods are typically sired by several males (e.g., P. reticulata;26,70 sailfin molly, P. latipinna;71,72 green swordtails, Xiphophourus hellerii73), which may be due, in part, to benefits for females of mating multiply,25 but also to coercive male mating behavior, i.e., forced copulations.74–77 Obviously, females' behavior (i.e., their past and anticipated future sexual interactions) and especially the behavior of surrounding males can have a profound effect on SCR and thus, are likely to affect males' fitness (reproductive success). It seems straightforward to predict that the SCR a male is facing while choosing a mate is likely to affect his mate choice,23,61,62,78 and males are predicted to evolve counter-strategies to reduce SCR.79
Social Eavesdropping: Males Copy Other Males' Choices
Social eavesdroppers are by-standing individuals that may extract information about the quality of the observed individuals by using information of signaling interactions3,16 (Fig. 2A). In the context of mate choice the most intensely studied form of social eavesdropping is mate choice copying, which has been defined as a process during which a female's probability of choosing a given male increases if other females have previously chosen that male18 (Fig. 2B). Most studies on mate choice copying have focused on females,2,21,35 but also males copy other males' choices (P. latipinna;80,81 pipefish, Syngnathus typhle;82 three-spined stickleback, Gasterosteus aculeatus83).
Figure 2.
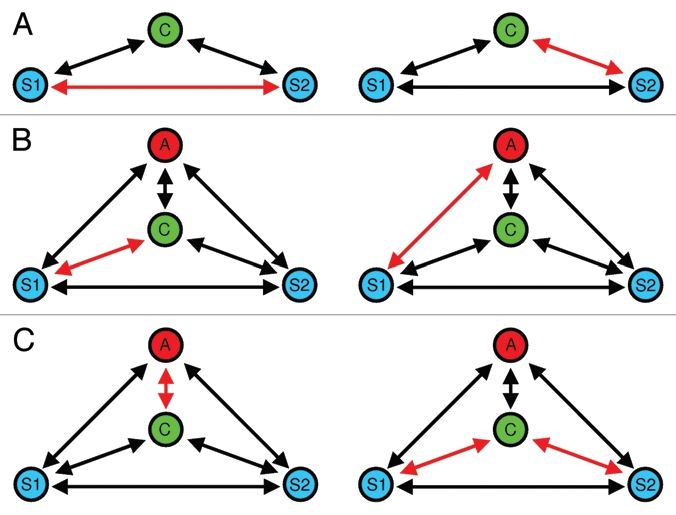
Different forms of social eavesdropping during mate choice in communication networks. (A) Eavesdropping on interactions between two potential mating partners: the choosing individual (C) extracts information about mate quality by observing aggressive92,93 or acoustic interactions17 between two potential mating partners (S1 and S2). (B) A special form of social eavesdropping is mate choice copying, where a by-standing individual (A) extracts information about mate quality from observing sexual interactions. In the present example, A is more likely to approach S1 after having observed (C) sexually interact with S1. (C) Audience-induced changes in mate choice. The mere presence of a by-stander (the audience, (A) affects the mate choice behavior of the choosing individual (C), which may be interpreted as a strategy to reduce sperm competition risk, as A might copy C's mate choice at a later point in time.
The adaptive significance of male mate choice copying in poeciliids could be linked to reduced costs for searching a receptive female.80 Poeciliid females are receptive only as virgins or for few days post partum68,84,85 and accordingly, only a small proportion of females in a population are receptive at a time.39,69 As poeciliid males need to approach females to test their receptivity by nipping at the female's genital opening,86 copying other males' mate choice may allow saving considerable energetic and opportunity costs (sensu80).
Nevertheless, male mate choice copying in internally fertilizing species—like livebearing fishes—remains a conundrum, as males incur increased SCR when choosing another male's previous mate. In a recent study, we therefore asked whether male Atlantic mollies (P. mexicana) would copy less under increased SCR.27 We created two copying situations with different levels of SCR: a fraction of the focal males were allowed to copy from visual interactions between a stimulus female and a model male (representing low SCR) while another fraction of males could observe direct (sexual) interactions (leading to increased SCR). As predicted, males from the latter group copied less, demonstrating that P. mexicana males indeed respond to perceived SCR when copying each other's mate choice.27
Audience Effects: Risk of Being Copied Affects Mate Choice
Audience effects are defined as behavioral changes induced by the presence of other (by-standing) individuals that may or may not extract information from the observed communication events23,24 (Fig. 2C). Beside audience induced changes in signaling and courtship behavior,87,88 the presence of a conspecific audience also has the potential to affect males' mate choice decisions.23,28,29,62,89–91 Poecilia mexicana males, e.g., cease expressing mating preferences and reduce their sexual activity when another male is present,89 which may be a response to avoid unintended interception of information about their mating preferences.23,62 On top of that, it appears that P. mexicana males deceive rivals about their mating preferences by directing their first sexual interaction (directly after they were presented with the audience male) towards the female they had rejected beforehand,28,91 (Fig. 3). This behavior was interpreted as an attempt employed by males to lead the rival away from their preferred mate, thereby exploiting mate choice copying through dishonest signaling.23 Ultimately, these behaviors may thus help to reduce the level of SCR.
Figure 3.
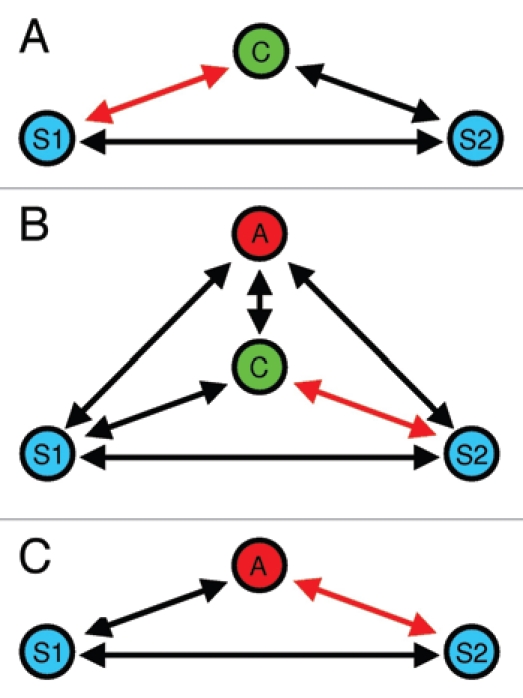
Deceptive signaling during male mate choice.28 In (A) the choosing male (C) exhibits a mating preference for S1. (B) When an audience male (A) enters the mate choice arena, C initially interacts with S2, (C) which will be copied by (A).
As SCR is clearly linked to the present motivational state of the by-stander, we asked whether P. mexicana males are able to asses and remember rivals' sexual motivation and attractiveness to females, and if they might integrate this information strategically into their mating behavior.29 Male-dyads were allowed to familiarize with one another in two adjacent compartments of a tank that was separated by a transparent Plexiglas divider. One of them was kept together with a female and thus, was perceived by the other male as sexually active, while the other male was alone and thus, perceived as sexually inactive. In subsequent mate choice tests with different stimulus females, the males from each dyad served as focal and audience males, and vice versa. Focal males ceased to show mating preferences only when they had perceived their rivals as sexually active (Fig. 4A). In addition, focal males that were observed by a sexually active rival showed a stronger behavioral response when rivals were larger and thus, more attractive to females29 (Fig. 4B). This suggests that male fish are indeed able to remember and strategically exploit information about rivals when performing mate choice; in essence, males respond to an audience only “when it matters.”
Figure 4.
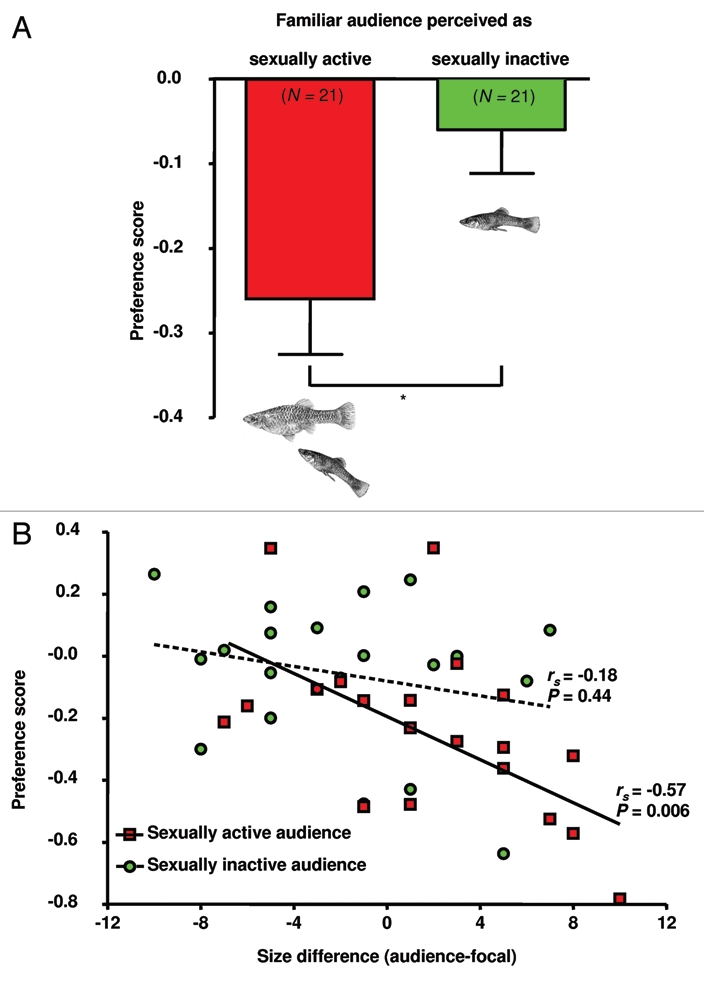
Changes in P. mexicana male mating preferences induced by the presence of a familiar audience male.29 Males of a dyad (one sexually active and one sexually inactive male) were tested for their preferences while successively serving both as focal and audience males. (A) A comparison of preference scores (fraction of time near preferred female during 2nd-1st test parts, means ± SEM) reveals that male preferences were more affected when the rival was perceived as sexually active (paired t-test; *p < 0.05). (B) Correlation between body size differences and changes in male mating preferences (preference scores). Body size differences in the tested male dyads (N = 21) were determined as the audience male's standard length (SL)—focal male's SL. rs- and p-values are from Spearman rank order tests. The strength of male preferences decreased with increasing rival body size when the audience was perceived as sexually active, but not in the case of sexually inactive audience males.
Altogether, the aforementioned studies exemplify the various effects of social eavesdropping on male mate choice. Males make use of socially acquired information (mate choice copying), but also males that are at risk of being copied respond to this (audience effects), which may be a strategy to reduce SCR.
Acknowledgements
We thank Holger Geupel for help with animal care. This work is supported by the German Research Foundation (DFG; PL 470/3-1).
References
- 1.Andersson M. Sexual Selection. Princeton: Princeton University Press; 1994. [Google Scholar]
- 2.Westneat DF, Walters A, McCarthy TM, Hatch MI, Hein WK. Alternative mechanisms of non-independent mate choice. Anim Behav. 2000;59:467–476. doi: 10.1006/anbe.1999.1341. [DOI] [PubMed] [Google Scholar]
- 3.Bonnie KE, Earley RL. Expanding the scope for social information use. Anim Behav. 2007;74:171–181. [Google Scholar]
- 4.Earley RL. Social eavesdropping and the evolution of conditional cooperation and cheating strategies. Phil Trans R Soc B. 2010;365:2675–2686. doi: 10.1098/rstb.2010.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause J, Ruxton GD. Living in Groups. Oxford: Oxford University Press; 2002. [Google Scholar]
- 6.Valone TJ, Templeton JJ. Public information for the assessment of quality: a widespread social phenomenon. Phil Trans R Soc Lond B. 2002;357:1549–1557. doi: 10.1098/rstb.2002.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danchin E, Giraldeau LA, Valone TJ, Wagner RH. Public information: from noisy neighbors to cultural evolution. Science. 2004;305:487–491. doi: 10.1126/science.1098254. [DOI] [PubMed] [Google Scholar]
- 8.Dabelsteen T. Public, private or anonymous? Facilitating and countering eavesdropping. In: McGregor PK, editor. Animal Communication Networks. Cambridge: Cambridge University Press; 2005. pp. 38–62. [Google Scholar]
- 9.Grosenick L, Clement TS, Fernald RD. Fish can infer social rank by observation alone. Nature. 2007;445:429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- 10.Valone TJ. From eavesdropping on performance to copying the behavior of others: a review of public information use. Behav Ecol Sociobiol. 2007;62:1–14. [Google Scholar]
- 11.Druen M, Dugatkin LA. Communication networks. In: Evans J, Pilastro A, Schlupp I, editors. Ecology and Evolution of Livebearing Fishes (Poeciliidae) Chicago: University Press; 201. [Google Scholar]
- 12.McGregor PK, Peake T. Communication networks: social environments for receiving and signaling behaviour. Acta Ethol. 2000;2:71–81. [Google Scholar]
- 13.Earley RL, Dugatkin LA. Fighting, mating and networking: pillars of poeciliid sociality. In: McGregor PK, editor. Animal communication networks. Cambridge: Cambridge University Press; 2005. pp. 84–113. [Google Scholar]
- 14.Matos R, Schlupp I. Performing in front of an audience: signallers and the social environment. In: McGregor PK, editor. Animal Communication Networks. Cambridge: Cambridge University Press; 2005. pp. 63–83. [Google Scholar]
- 15.Naguib M, Amrhein V, Kunc HP. Effects of territorial intrusions on eavesdropping neighbors: communication networks in nightingales. Behav Ecol. 2004;15:1011–1015. [Google Scholar]
- 16.Peake TM. Eavesdropping in communication networks. In: McGregor PK, editor. Animal Communication Networks. Cambridge: Cambridge University Press; 2005. pp. 13–37. [Google Scholar]
- 17.Fitzsimmons LP, Foote JR, Ratcliffe LM, Mennill DJ. Eavesdropping and communication networks revealed through playback and an acoustic location system. Behav Ecol. 2008;19:824–829. [Google Scholar]
- 18.Pruett-Jones S. Independent versus non-independent mate-choice: do females copy each other? Am Nat. 1992;140:1000–1009. doi: 10.1086/285452. [DOI] [PubMed] [Google Scholar]
- 19.Dugatkin LA. Sexual selection and imitation: females copy the mate choice of others. Am Nat. 1992;139:1384–1389. [Google Scholar]
- 20.Nordell SE, Valone TJ. Mate choice copying as public information. Ecol Letters. 1998;1:74–76. [Google Scholar]
- 21.Witte K. Learning and mate choice. In: Brown C, Laland KN, Krause J, editors. Fish cognition and behavior. Oxford: Blackwell Publishing; 2006. pp. 70–95. [Google Scholar]
- 22.Marler P, Dufty A, Pickert R. Vocal communication in the domestic chicken II. Is a sender sensitive to the presence and nature of a receiver? Anim Behav. 1986;34:194–198. [Google Scholar]
- 23.Plath M, Schlupp I. Misleading mollies—the effect of an audience on the expression of mating preferences. Comm Integr Biol. 2008;1:199–203. doi: 10.4161/cib.1.2.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuberbühler K. Audience effects. Curr Biol. 2008;11:189–190. doi: 10.1016/j.cub.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 25.Evans JP, Magurran AE. Multiple benefits of multiple mating in guppies. Proc Nat Ac Sci USA. 2000;97:10074–10076. doi: 10.1073/pnas.180207297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becher SA, Magurran AE. Multiple mating and reproductive skew in Trinidadian guppies. Proc R Soc Lond B. 2004;271:1009–1014. doi: 10.1098/rspb.2004.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bierbach D, Kronmarck C, Hennige-Schulz C, Stadler S, Plath M. Sperm competition risk affects male mate choice copying. Behav Ecol Sociobiol. 2011 [Google Scholar]
- 28.Plath M, Richter S, Tiedemann R, Schlupp I. Male fish deceive competitors about mating preferences. Curr Biol. 2008;18:1138–1141. doi: 10.1016/j.cub.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 29.Bierbach D, Girndt A, Hamfler S, Klein M, Mücksch F, Penshorn M, et al. Male fish use prior knowledge about rivals to adjust their mate choice. Biol Lett. 2011;7:349–351. doi: 10.1098/rsbl.2010.0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakker TMC. The study of intersexual selection using quantitative genetics. Behaviour. 1999;136:1237–1265. [Google Scholar]
- 31.Pierotti MER, Knight ME, Immler S, Barson NJ, Turner GF, Seehausen O. Individual variation in male mating preferences for female coloration in a polymorphic cichlid fish. Behav Ecol. 2008;19:483–488. [Google Scholar]
- 32.Rodd FH, Hughes KA, Grether GF, Baril CT. A possible non-sexual origin of mate preference: are male guppies mimicking fruit? Proc R Soc Lond B. 2001;269:475–481. doi: 10.1098/rspb.2001.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Endler JA, Houde AE. Geographic variation in female preferences for male traits in Poecilia reticulata. Evolution. 1995;49:456–468. doi: 10.1111/j.1558-5646.1995.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 34.Brooks R, Endler JA. Female guppies agree to differ: phenotypic and genetic variation in mate-choice behaviour and the consequences for sexual selection. Evolution. 2001;55:1644–1655. doi: 10.1111/j.0014-3820.2001.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 35.Dugatkin LA. Copying and mate choice. In: Heyes CM, Galef BG Jr, editors. Social learning in animals: the roots of culture. New York: Academic Press; 1996. pp. 85–105. [Google Scholar]
- 36.Jennions MD, Petrie M. Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev. 1997;72:283–327. doi: 10.1017/s0006323196005014. [DOI] [PubMed] [Google Scholar]
- 37.Cotton S, Small J, Pomiankowski A. Sexual selection and condition-dependent mate preferences. Curr Biol. 2006;16:755–765. doi: 10.1016/j.cub.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Ryan MJ. Sexual selection and mate choice. In: Krebs JR, Davies NB, editors. Behavioural ecology, an evolutionary approach. Fourth edition. Oxford: Blackwell; 1997. pp. 179–202. [Google Scholar]
- 39.Houde AE. Sex, Color and Mate Choice in Guppies. Princeton, New Jersey: Princeton University Press; 1997. [Google Scholar]
- 40.Clutton-Brock TH, McAuliffe K. Female mate choice in mammals. Quat Rev Biol. 2009;84:1–27. doi: 10.1086/596461. [DOI] [PubMed] [Google Scholar]
- 41.Bonduriansky R. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev. 2001;76:305–339. doi: 10.1017/s1464793101005693. [DOI] [PubMed] [Google Scholar]
- 42.Sæthers SA, Fiske P, Kalas JA. Male mate choice, sexual conflict and strategic allocation of copulations in a lekking bird. Proc R Soc Lond B. 2001;268:2097–2102. doi: 10.1098/rspb.2001.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong BBM, Jennions MD, Keogh JS. Sequential male mate choice in a fish, the Pacific blue-eye Pseudomugil signifer. Behav Ecol Sociobiol. 2004;56:253–256. [Google Scholar]
- 44.Plath M, Seggel U, Burmeister H, Heubel KU, Schlupp I. Choosy males from the underground: male mate choice in surface- and cave dwelling Atlantic mollies, Poecilia mexicana (Poeciliidae, Teleostei) Naturwissenschaften. 2006;93:103–109. doi: 10.1007/s00114-005-0072-z. [DOI] [PubMed] [Google Scholar]
- 45.Plath M, Kromuszczynski K, Tiedemann R. Audience effect alters male but not female mating preferences. Behav Ecol Sociobiol. 2009;63:381–390. [Google Scholar]
- 46.Milinski M, Bakker TCM. Costs influence sequential mate choice in sticklebacks, Gasterosteus aculeatus. Proc R Soc Lond B. 1992;250:229–233. [Google Scholar]
- 48.Bro-Jørgensen J. Reversed sexual conflict in a promiscuous antelope. Curr Biol. 2007;17:2157–2161. doi: 10.1016/j.cub.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 49.Kokko H, Jennions MD. Sexual conflict: The battle of the sexes reversed. Curr Biol. 2008;18:121–123. doi: 10.1016/j.cub.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 50.Amundsen T, Forsgren E. Male mate choice selects for female coloration in a fish. Proc Natl Acad Sci USA. 2001;98:13155–13160. doi: 10.1073/pnas.211439298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berglund A, Rosenqvist G. Male pipefish prefer dominant over attractive females. Behavioral Ecology. 2001;12:402–406. [Google Scholar]
- 52.Parker GA. Mate quality and mating decisions. In: Bateson P, editor. Mate Choice. Cambridge: Cambridge University Press; 1983. pp. 141–166. [Google Scholar]
- 53.Pélabon C, Borg ÅA, Bjelvenmark J, Forsgren E, Barber I, Amundsen T. Do male two-spotted gobies prefer large fecund females? Behav Ecol. 2003;14:787–792. [Google Scholar]
- 54.Herdman EJ, Kelley CD, Godin JGJ. Male mate choice in the guppy (Poecilia reticulata): Do males prefer larger females as mates? Ethology. 2004;110:97–111. [Google Scholar]
- 55.Riesch R, Plath M, Schlupp I. Toxic hydrogen sulfide and dark caves: life history adaptations in a livebearing fish (Poecilia mexicana, Poeciliidae) Ecology. 2010;91:1494–1505. doi: 10.1890/09-1008.1. [DOI] [PubMed] [Google Scholar]
- 56.Werner NY, Lotem A. Choosy males in a hamplochromine cichlid: first experimental evidence for male mate choice in a lekking species. Ethology. 2003;112:657–663. [Google Scholar]
- 57.Kolm N. Female courtship in the Banggai cardinalfish: Honest signals of egg maturity and reproductive output? Phil Trans R Soc Lond B. 2004;361:319–334. [Google Scholar]
- 58.Pierotti MER, Martín-Fernández JA, Seehausen O. Mapping individual variation in male mating preference space: Multiple choice in a color polymorphic cichlid fish. Evolution. 2009;63:2372–2388. doi: 10.1111/j.1558-5646.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 59.Wedell N, Gage MJG, Parker GA. Sperm competition, male prudence and sperm-limited females. Trends Ecol Evol. 2002;17:313–320. [Google Scholar]
- 60.Aspbury AS. Sperm competition effects on sperm production and expenditure in sailfin mollies, Poecilia latipinna. Behav Ecol. 2007;18:776–780. [Google Scholar]
- 61.Wong BBM, McCarthy M. Prudent male mate choice under perceived sperm competition risk in the eastern mosquito fish. Behav Ecol. 2009;20:278–282. [Google Scholar]
- 62.Ziege M, Mahlow K, Hennige-Schulze C, Kronmarck C, Tiedemann R, Streit B, et al. Audience effects in the Atlantic molly (Poecilia mexicana)—prudent male mate choice in response to perceived sperm competition risk? Front Zool. 2009;6:17. doi: 10.1186/1742-9994-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Largiadér CR, Fries V, Bakker TCM. Genetic analysis of sneaking and egg-thievery in a natural population of the three-spined stickleback (Gasterosteus aculeatus L.) Heredity. 2001;86:459–468. doi: 10.1046/j.1365-2540.2001.00850.x. [DOI] [PubMed] [Google Scholar]
- 64.Eizaguirre C, Yeates SE, Lenz TL, Kalbe M, Milinski M. MHC-based mate choice combines good genes and maintenance of MHC polymorphism. Mol Ecol. 2009;18:3316–3329. doi: 10.1111/j.1365-294X.2009.04243.x. [DOI] [PubMed] [Google Scholar]
- 65.Parker GA. Sperm competition and its evolutionary consequences in insects. Biol Rev. 1970;45:525–567. [Google Scholar]
- 66.Constanz GD. Sperm competition in poeciliid fishes. In: Smith RL, editor. Sperm competition and the evolution of animal mating systems. Orlando: Academic Press; 1984. pp. 465–485. [Google Scholar]
- 67.Haskins CP, Haskins EF, McLaughlin JJA, Hewitt RE. Polymorphism and Population Structure in Lebistes reticulatus, an Ecological Study. Austin: University of Texas Press; 1961. [Google Scholar]
- 68.Rosenthal HL. Observations of reproduction of the poeciliid Lebistes reticulatus (Peters) Biological Bulletin. 1952;102:30–38. [Google Scholar]
- 69.Magurran AE. Evolutionary Ecology: The Trinidadian Guppy. Oxford: Oxford University Press; 2005. [Google Scholar]
- 70.Neff BD, Pitcher TE, Ramnarine IW. Inter-population variation in multiple paternity and reproductive skew in the guppy. Mol Ecol. 2008;17:2975–2984. doi: 10.1111/j.1365-294X.2008.03816.x. [DOI] [PubMed] [Google Scholar]
- 71.Travis J, Trexler JC, Mulvey M. Multiple paternity and its correlates in female Poecilia latipinna (Poeciliidae) Copeia. 1990:3. [Google Scholar]
- 72.Trexler JC, Travis J, Dinep A. Variation among populations of the sailfin molly in the rate of concurrent multiple paternity and its implications for mating-system evolution. Behav Ecol Sociobiol. 1997;40:297–305. [Google Scholar]
- 73.Tatarenkov A, Healey CIM, Grether GF, Avise JC. Pronounced reproductive skew in a natural population of green swordtails, Xiphophorus helleri. Mol Ecol. 2008;17:4522–4534. doi: 10.1111/j.1365-294X.2008.03936.x. [DOI] [PubMed] [Google Scholar]
- 74.Evans JP, Pilastro A, Ramnarine IW. Sperm transfer through forced matings and its evolutionary implications in natural guppy (Poecilia reticulata) populations. Biol J Linn Soc. 2003;78:605–612. [Google Scholar]
- 75.Plath M, Makowicz AM, Schlupp I, Tobler M. Sexual harassment in live-bearing fishes: comparing courting and non-courting species. Behav Ecol. 2007;18:680–688. [Google Scholar]
- 76.Plath M. Male mating behavior and costs of sexual harassment for females in cavernicolous and extremophile populations of Atlantic mollies (Poecilia mexicana) Behaviour. 2008;145:73–89. [Google Scholar]
- 77.Köhler A, Hildenbrand P, Schleucher E, Riesch R, Arias-Rodriguez L, Streit B, et al. Effects of male sexual harassment on female time budgets, feeding behavior and metabolic rates in a tropical livebearing fish (Poecilia mexicana) Behav Ecol Sociobiol. 2011 [Google Scholar]
- 78.Dosen LD, Montgomerie R. Mate preferences by male guppies (Poecilia reticulata) in relation to the risk of sperm competition. Behav Ecol Sociobiol. 2004;55:266–271. [Google Scholar]
- 79.Bretman A, Fricke C, Chapman T. Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. Proc R Soc B Biol Sci. 2009;276:1705–1711. doi: 10.1098/rspb.2008.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schlupp I, Ryan MJ. Male sailfin mollies (Poecilia latipinna) copy the mate choice of other males. Behav Ecol. 1997;8:104–107. [Google Scholar]
- 81.Witte K, Ryan MJ. Mate-choice copying in the sailfin molly (Poecilia latipinna) in the wild. Anim Behav. 2002;63:943–949. [Google Scholar]
- 82.Widemo MS. Male but not female pipefish copy mate choice. Behav Ecol. 2006;17:255–259. [Google Scholar]
- 83.Frommen JG, Rahn AK, Schroth SH, Waltschyk N, Bakker TCM. Mate-choice copying when both sexes face high costs of reproduction. Evol Ecol. 2008;23:435–446. [Google Scholar]
- 84.Liley NR. Ethological isolating mechanisms in four sympatric species of Poeciliid fishes. Behav Suppl. 1966;13:1–197. [Google Scholar]
- 86.Parzefall J. Zur vergleichenden Ethologie verschiedener Mollienesia-Arten einschließlich einer Höhlenform von Mollienesia sphenops. Behaviour. 1969;33:1–37. [PubMed] [Google Scholar]
- 87.Fisher H, Rosenthal GG. Male swordtails court with an audience in mind. Biol Lett. 2007;3:5–7. doi: 10.1098/rsbl.2006.0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doutrelant C, McGregor PK, Oliveira RF. The effect of an audience on intrasexual communication in male Siamese fighting fish, Betta splendens. Behav Ecol. 2001;12:283–286. [Google Scholar]
- 89.Plath M, Blum D, Schlupp I, Tiedemann R. Audience effect alters mating preferences in Atlantic molly (Poecilia mexicana) males. Anim Behav. 2008;75:21–29. [Google Scholar]
- 90.Plath M, Blum D, Tiedemann R, Schlupp I. A visual audience effect in a cavefish. Behaviour. 2008;145:931–947. [Google Scholar]
- 91.Plath M, Richter S, Schlupp I, Tiedemann R. Misleading mollies: surface- but not cave-dwelling Poecilia mexicana males deceive competitors about mating preferences. Acta Ethol. 2010;13:49–56. [Google Scholar]
- 92.Doutrelant C, McGregor PK. Eavesdropping and mate choice in female fighting fish. Behaviour. 2000;137:1655–1669. [Google Scholar]
- 93.Ophir AG, Galef BG. Female Japanese quail that “eavesdrop” on fighting males prefer losers to winners. Anim Behav. 2003;66:399–407. [Google Scholar]


