Abstract
The sound of one's own name is one of the most salient auditory environmental stimuli. Several studies of human brain potentials have revealed some characteristic waveforms when we hear our own names. In a recent work, we investigated event-related potentials (ERPs) in a female chimpanzee and demonstrated that the ERP pattern generated when she heard her own name differed from that generated when she heard other sounds. However, her ERPs did not exhibit a prominent positive shift around 300 ms (P3) in response to her own name, as has been repeatedly shown in studies of human ERPs. The present study collected comparative data for adult humans using basically the same procedure as that used in our previous study of the chimpanzee. These results also revealed no prominent P3 to the human subjects' own names. The lack of increased P3 is therefore likely due to our experimental protocol, in which we presented the subject's own name relatively frequently. In contrast, our results revealed prominent negativity to the subject's own name at around 500 ms in the chimpanzee and around 200 ms in human subjects. This may indicate that initial orientation to the sound of one's own name is delayed in the chimpanzee.
Key words: auditory processing, ERP, chimpanzee, name, self
Chimpanzees, phylogenetically closest to humans, have been studied intensively from ecological, behavioral and cognitive perspectives to clarify the evolutionary basis of the human mind.1 Dozens of laboratory and field studies have conducted direct comparisons of chimpanzees and humans, often using behavioral responses as indices. Human studies have increasingly been using non-invasive techniques to investigate the neural basis of the human mind, but this has rarely been the case for studies of chimpanzees. Recently, we were the first to measure event-related potential (ERP), a scalp surface potential that reflects neural activities in the brain, in a fully conscious adult female chimpanzee.23 This success was made possible by the very close relationship between the subject chimpanzee and the human experimenters along with step-by-step training with positive reinforcement.
Our recent paper presented ERP results related to the subject chimpanzee's hearing her own name.3 Chimpanzees are known to recognize themselves in a mirror, so they are capable of some kind of self-recognition.4 Chimpanzee self-recognition has mainly been assessed using mirrors as test devices, and other aspects of self-recognition or self-awareness have rarely been investigated. Studies of human subjects have demonstrated several different aspects of self-recognition, one of which is event-related potential in response to hearing one's own name.5,6 Caretakers and researchers of chimpanzees at zoos and institutes are aware that chimpanzees are capable of recognizing their names; it is a routine procedure to use an individual's name when inviting a specific chimpanzee to come.7,8 However, no studies have investigated chimpanzees' responses when they hear their own names. This study focused on clarifying the neural basis of vocal processing when a chimpanzee heard several types of auditory stimuli including her own name.
We measured ERPs for each of the following auditory stimuli: the vocal sound of the subject's own name (SON), the vocal sound of a familiar name of another group member (FN), the vocal sound of an unfamiliar name (UN) and a nonvocal sound (NV). A negative shift was observed particularly in response to SON at approximately 500 ms latency following stimulus onset. This negative shift was similar to Nc in human infants, which is considered to reflect some attentional states9 and indicates that this chimpanzee processes her name differently from other sounds.
Our results were unexpected in one regard: we did not observe a larger positive component at around 300 ms following stimulus onset. This positive deflection, known as P3 in human ERP studies, has appeared in response to the subject's own name in several studies of human adult subjects.5,6 Many possibilities could explain our findings. One hypothesis is that the neural processing of one's own name differs between human adults and chimpanzee adults. Another possibility is that our experimental protocol would not elicit the P3 component even in human adult subjects. Our protocol was modified from that used in earlier human studies, based on the limited time period during which our subject chimpanzee could sit quietly.
After our previous chimpanzee experiment,3 we conducted additional testing using a similar methodology on human adults as subjects. These subjects were eight Japanese males (aged 22–32 years). Our stimuli were the sounds of family names spoken by a familiar individual, because Japanese normally use family names to call one another. Testing involving humans and testing involving the chimpanzee differed in the following ways: the requirement that human subjects look at a particular spot during stimulus-sound presentation (but no other requirements were imposed), the place of the experiment, the number of stimulus presentations and the device used to record the EEG. Otherwise, the methodology was identical to that used previously (reviewed in ref. 3).
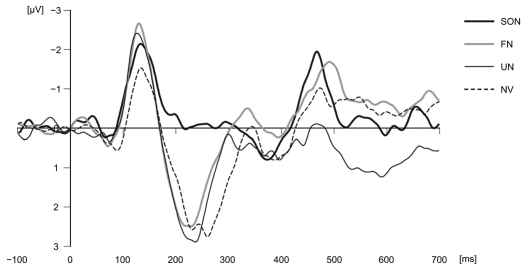
The results revealed some positive and negative shifts in ERP (Fig. 1). In particular, we observed significant differences in potentials in response to subjects' own names compared with responses to other types of sounds at the vertex (Cz) in a certain latency range (negative deflection around 200–300 ms following stimulus onset: Tukey's HSD, SON vs. FN, p = 0.09; SON vs. UN, p = 0.02; SON vs. NV, p = 0.02). Previous studies have reported this negative deflection in response to a human subject's own name around 200 ms, and this component can be described as the initiation of an attention-switching mechanism.10 However, the peak amplitude of potentials at Cz in response to subjects' own names within the P3 range (300–450 ms) did not differ significantly from those in response to other types of sounds (in our previous study with a chimpanzee subject, we set the P3 range at 200–450 ms, but for human subjects we excluded 200–300 ms from the P3 range because this range included a P2 peak). In addition, the peak amplitude of potentials at Cz in response to subjects' own names within the range of 450–600 ms following stimulus onset did not differ from those for other types of sounds (except for SON vs. UN), in contrast to chimpanzee test results.
Figure 1.
Average waveforms in response to each stimulus at Cz in human adults. A 64-channel Geodesic EEG System (Electrical Geodesics, Inc., Eugene, OR) was used to record the EEG. The left mastoid was used as reference.
The absence of increased P3 in response to subjects' own names in our additional testing contradicts previous reports about ERP in human adults. Methodological differences can explain this variation. Various studies have indicated that the generation of P3 is influenced by the novelty of the stimulus, so habituation to the stimulus may preclude the P3 component.11 Our testing used the four types of sounds, including the subjects' own names, equally (25% each), so this relatively frequent presentation of the stimulus might have resulted in the decreased P3.
In sum, we evaluated ERP in response to one's own name by collecting comparative data from a chimpanzee and human adults. We observed a greater negative deflection in response to one's own name relative to other types of sounds in both chimpanzee and human subjects. This negativity can be interpreted as the initiation of orientation to the sound of one's own name. However, the latency of this negativity differed between the chimpanzee and the humans: 500 ms in the chimpanzee and 200–300 ms in the humans. These results may indicate that the initiation of orientation to the sound of one's own name is delayed in chimpanzees. Next, we focused on the lack of increased P3 in the chimpanzee. Data from human subjects did not reveal increased P3 in response to their own names. Our results therefore did not indicate species-based differences with respect to the generation of P3; it was not elicited in our protocol as well as it was in some earlier studies of human subjects.12 Further study will be needed to determine which factors affect variation in negative shifts and P3 to one's own name. Because the generation of P3 is an important topic in the study of human ERP, it will also be worthwhile to investigate the presence or absence of P3 in chimpanzees by designing a protocol that applies to both humans and chimpanzees and that enables P3 generation in human subjects. The study of chimpanzee ERP is a new way to investigate the neural basis of human cognition from comparative and evolutionary perspectives.
Acknowledgements
We thank Kosuke Itoh for his comments. This study was supported by the Center for Evolutionary Cognitive Science at the University of Tokyo and Grants-in-Aid for Scientific Research (grant nos. 20680015 to S.H., 18200018 to K.H., 19300091 and 20002001 to M.T.) from J.S.P.S.
References
- 1.Lonsdorf EV, Ross SR, Matsuzawa T. The Mind of the Chimpanzee: Ecological and Experimental Perspectives. Chicago: The University of Chicago Press; 2010. [Google Scholar]
- 2.Ueno A, Hirata S, Fuwa K, Sugama K, Kusunoki K, Matsuda G, et al. Auditory ERPs to stimulus deviance in an awake chimpanzee (Pan troglodytes): towards hominid cognitive neurosciences. PLoS ONE. 2008;3:1442. doi: 10.1371/journal.pone.0001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueno A, Hirata S, Fuwa K, Sugama K, Kusunoki K, Matsuda G, et al. Brain activity in an awake chimpanzee in response to the sound of her own name. Biol Lett. 2010;6:311–313. doi: 10.1098/rsbl.2009.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallup GG., Jr Chimpanzees: self-recognition. Science. 1970;167:86–87. doi: 10.1126/science.167.3914.86. [DOI] [PubMed] [Google Scholar]
- 5.Berlad I, Pratt H. P300 in response to the subject's own name. Electroencephalogr Clin Neurophysiol. 1995;96:472–474. doi: 10.1016/0168-5597(95)00116-a. [DOI] [PubMed] [Google Scholar]
- 6.Perrin F, Maquet P, Peigneux P, Rubya P, Degueldre C, Balteau E, et al. Neural mechanisms involved in the detection of our first name: a combined ERP and PET study. Neuropsychologia. 2005;43:12–19. doi: 10.1016/j.neuropsychologia.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Sousa C, Okamoto S, Matsuzawa T. Behavioral development in a matching-to-sample task and token use by an infant chimpanzee reared by his mother. Anim Cogn. 2003;6:259–267. doi: 10.1007/s10071-003-0186-7. [DOI] [PubMed] [Google Scholar]
- 8.de Waal FBM, Pokorny JJ. Faces and behinds: chimpanzee sex perception. Adv Sci Lett. 2008;1:99–103. [Google Scholar]
- 9.Reynolds GD, Richards JE. Familiarization, attention and recognition memory in infancy: an event-related potential and cortical source localization study. Dev Psychol. 2005;41:598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holeckova I, Fischer C, Giard MH, Delpuech C, Morlet D. Brain response to a subject's own name uttered by a familiar voice. Brain Res. 2006;1082:142–152. doi: 10.1016/j.brainres.2006.01.089. [DOI] [PubMed] [Google Scholar]
- 11.Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr Clin Neurophysiol. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- 12.Müller HM, Kutas M. What's in a name? Electrophysiological differences between spoken nouns, proper names and one's own name. NeuroReport. 1996;8:221–225. doi: 10.1097/00001756-199612200-00045. [DOI] [PubMed] [Google Scholar]