Abstract
Transition-state theory has led to the design of Immucillin-H (Imm-H), a picomolar inhibitor of purine nucleoside phosphorylase (PNP). In humans, PNP is the only route for degradation of deoxyguanosine, and genetic deficiency of this enzyme leads to profound T cell-mediated immunosuppression. This study reports the biological effects and mechanism of action of Imm-H on malignant T cell lines and on normal activated human peripheral T cells. Imm-H inhibits the growth of malignant T cell leukemia lines with the induction of apoptosis. Imm-H also inhibits activated normal human T cells after antigenic stimulation in vitro. However, Imm-H did not inhibit malignant B cells, colon cancer cell lines, or normal human nonstimulated T cells, demonstrating the selective activity of Imm-H. The effects on leukemia cells were mediated by the cellular phosphorylation of deoxyguanosine and the accumulation of dGTP, an inhibitor of ribonucleotide diphosphate reductase. Cells were protected from the toxic effects of Imm-H when deoxyguanosine was absent or when deoxycytidine was present. Guanosine incorporation into nucleic acids was selectively blocked by Imm-H with no effect on guanine, adenine, adenosine, or deoxycytidine incorporation. Imm-H may have clinical potential for treatment of human T cell leukemia and lymphoma and for other diseases characterized by abnormal activation of T lymphocytes. The design of Imm-H from an enzymatic transition-state analysis exemplifies a powerful approach for developing high-affinity enzyme inhibitors with pharmacologic activity.
Aberrant T lymphocyte activity is implicated in the development of graft-versus-host disease, the pathogenesis of diverse autoimmune diseases, the growth of T cell malignancies, and the evolution of organ allograft rejection. Suppression of abnormal T cell responses has relied on pharmacologic agents that interfere with essential cell surface receptors or metabolic pathways of reactive lymphocytes. However, these agents are usually not selective for T cells, and thus their use results in generalized host immunosuppression and other serious side effects. Purine nucleoside phosphorylase (PNP, EC 2.4.2.1) is an enzyme involved in the recycling of nucleosides and deoxynucleosides in cellular remodeling. Although PNP is present in all mammalian cells, T cells are especially sensitive to deficiencies of this enzyme. A rare genetic deficiency of PNP results in a gradual specific loss of T cell function after birth and is associated with significant cellular immunodeficiency (1). DNA synthesis in other lymphoid and nonlymphoid cells of affected individuals is usually normal, suggesting that inhibition of PNP may provide an effective mechanism for selectively targeting T cells.
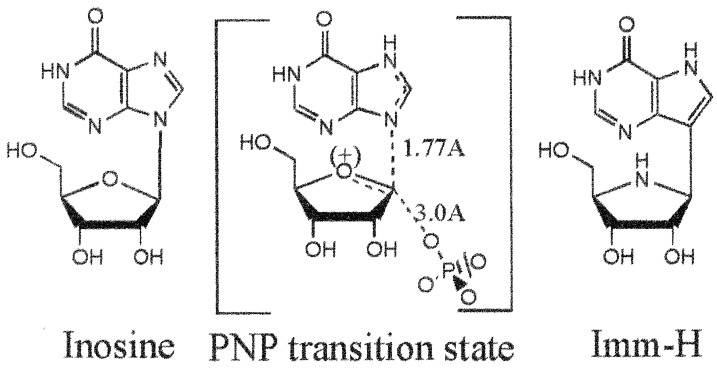
Inhibitors of PNP were first developed by using a structure-based inhibitor design focused on iterative group alignment established from the PNP crystal structures (2, 3). These inhibitors achieved only nanomolar dissociation constants, which limited their effectiveness because greater than 95% continuous inhibition of PNP is required for significant reduction in T cell function (4). Another approach for designing enzyme inhibitors is based on the identification of the transition-state structure stabilized by the target enzyme. Transition-state analogs preferentially bind their cognate enzyme with high affinity (5–7). The transition-state structure for the arsenolysis of inosine by PNP was used to guide the design of an inhibitor designated Immucillin-H (Imm-H) (Fig. 1) (6, 8, 9). This is, to our knowledge, the first report characterizing the biochemical and biologic effects of Imm-H, a picomolar inhibitor of PNP.
Figure 1.
Imm-H mimics the transition state of PNP. Atomic substitutions in Imm-H provide ribooxocarbenium ion character and elevated N7 pKa similar to the transition state. These substitutions produce a competitive inhibitor that binds >105 times tighter than substrate.
Imm-H selectively inhibited the in vitro growth of malignant T cell lines in the presence of deoxyguanosine (dGuo) without affecting non-T cell tumor lines. Activated human peripheral blood T lymphocytes were also sensitive to inhibition by Imm-H. Toxicity in leukemia cells was related to an accumulation of dGTP and the subsequent failure to synthesize cellular DNA. Under normal physiologic conditions, dGuo undergoes phosphorolysis by PNP. However, when PNP is inhibited, deoxycytidine kinase (dCK, EC 2.7.1.74) shunts unmetabolized dGuo into dGTP, which accumulates and blocks DNA synthesis. A correlation between the degree of T cell inhibition and the level of dCK activity was observed. These powerful biological effects of Imm-H suggest that this agent may have utility in the treatment of certain human diseases characterized by abnormal T cell growth or activation.
Materials and Methods
Reagents.
Imm-H [(1S)-1-(9-deazahypozanthin-9-yl)-1,4-dideoxy-1,4-imino-d-ribitol] was synthesized from d-gulonolactone and chemically protected 9-deazahypoxanthine (10, 11). Incorporation of 14C at the 2 position of the deazahypoxanthine ring was accomplished by including 14C-formamidine at the appropriate step in the chemical synthesis. Purity and structure were established by NMR, and radiochemical purity was checked by HPLC. Nucleosides and deoxynucleosides were purchased from Sigma.
Malignant Cell Lines.
The human T cell leukemia cell lines MOLT-4 and CCRF-CEM were obtained from the American Type Culture Collection (Rockville, MD). The human colon cancer line, GEO, was provided by J. Kantor (National Cancer Institute, Bethesda, MD), and the human B cell line BL2 was provided by M. Scharff (Albert Einstein College of Medicine, Bronx, NY). The human Jurkat T cell line was kindly provided by B. Bloom (Harvard School of Public Health, Boston, MA). Cell lines were cultured in RPMI medium 1640 with 2 mM l-glutamine, 10% heat-inactivated FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin (Life Technologies, Gaithersburg, MD). Other tumor cell lines were provided by Bristol-Myers Squibb and were cultured in RPMI medium 1640 supplemented with 10% FBS.
Human Peripheral T Cells.
Collection of blood from normal volunteers was performed after obtaining informed consent under a protocol approved by the Committee on Clinical Investigations at the Albert Einstein College of Medicine. Blood was obtained from volunteers, and peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation by using Ficoll/Hypaque (Amersham Pharmacia, Pharmacia Biotech, Piscataway, NJ). T cells were isolated from PBMC by negative selection by using the Pan T-cell Isolation Kit (Miltenyi Biotec, Auburn, CA). Magnetic bead sorting was accomplished by using an AutoMacs instrument (Miltenyi Biotec) according to the manufacturer's instructions. Isolated T cells were characterized as CD3+, CD45+, CD14−, and CD16−/CD56− (99%) by FACScan analysis (Becton Dickinson) by using fluorescent-labeled monoclonal antibodies (Becton Dickinson). Viability was assessed by using trypan blue exclusion in cells cultured in DMEM supplemented with 10% FBS/100 units/ml penicillin/100 μg/ml streptomycin/2 mM glutamine (Life Technologies) in a humidified 5% atmosphere at 5% CO2 37°C.
Cell Proliferation Assays.
Cell proliferation was measured by a colorimetric assay based on formazan production from tetrazolium salts or standard [3H]thymidine incorporation. Cells were grown in 96-well plates at 1 × 106 cells/ml, 200 μl/well, and cultured for 72 h at different concentrations of Imm-H (10 pM–10 μM), with or without 20 μM dGuo, and with or without 20 μM deoxycytidine. Concentration of dGuo used in assays was chosen on the basis of measurements of serum dGuo in patients with PNP deficiency (2–19 μM) (12) and from previously described methods (13, 14). This concentration guided the dGuo concentration. Selected samples were stimulated with a mouse anti-human CD3 mAb (0.5 μg/ml) (Ancell, Bayport, MN) and recombinant human IL-2 (rhIL-2, 20 units/ml). Other samples were incubated for 6 days and stimulated with rhIL-2 (200 units/ml) and T cell-depleted mononuclear cells (5 × 105 cells/ml) pretreated with 50 μg/ml of mitomycin. All experiments were done in triplicate.
For the colorimetric assay, tetrazolium salt WST-1 was added according to the manufacturer's instructions after 72 h of incubation (Boehringer Mannheim). Absorbance of formazan product was measured at 440 nm, and the fraction of viable cells was calculated as (A at 440 nm sample/A at 440 nm control). Alternatively, proliferation was measured by [3H]thymidine incorporation where 1 μCi was added to each well, and cells were incubated for another 18 h. Inhibition of DNA synthesis, as detected by thymidine incorporation, is an early event that precedes cell lysis. Formazan formation results from mitochondrial electron transfer, a reaction that persists until mitochondrial lysis. Cells were harvested onto glass fiber filters and analyzed by scintillation counting by using a 1414 Winspectral (Wallac, Gaithersburg, MD). Cell proliferation was measured as (cpm sample/cpm control). Controls for [3H]thymidine incorporation into cells were samples unaffected by Imm-H (0, 10−11, and 10−10 M).
For human T cell assays, the results represent an average of 10 experiments by using four individual donors. [3H]Thymidine incorporation in stimulated samples was nine times greater than incorporation in unstimulated cells. Incorporation of [3H]thymidine into unstimulated cells was greater than five times above controls containing no cells.
Apoptosis Assay.
CCRF-CEM, MOLT-4, and BL2 cells were cultured in RPMI as described above and plated at 1 × 106 cells/ml with 20 μM dGuo, 10 μM Imm-H, or 20 μM deoxycytidine, as indicated in Figs. 2–4. At 36 h, apoptosis was detected by annexin staining by using the TACS Annexin V-FITC kit according the manufacturer's protocol (Trevigen, Rockville, MD). Samples were analyzed by FACScan by using cellquest software (Becton Dickinson Immunocytometry Systems).
Figure 2.
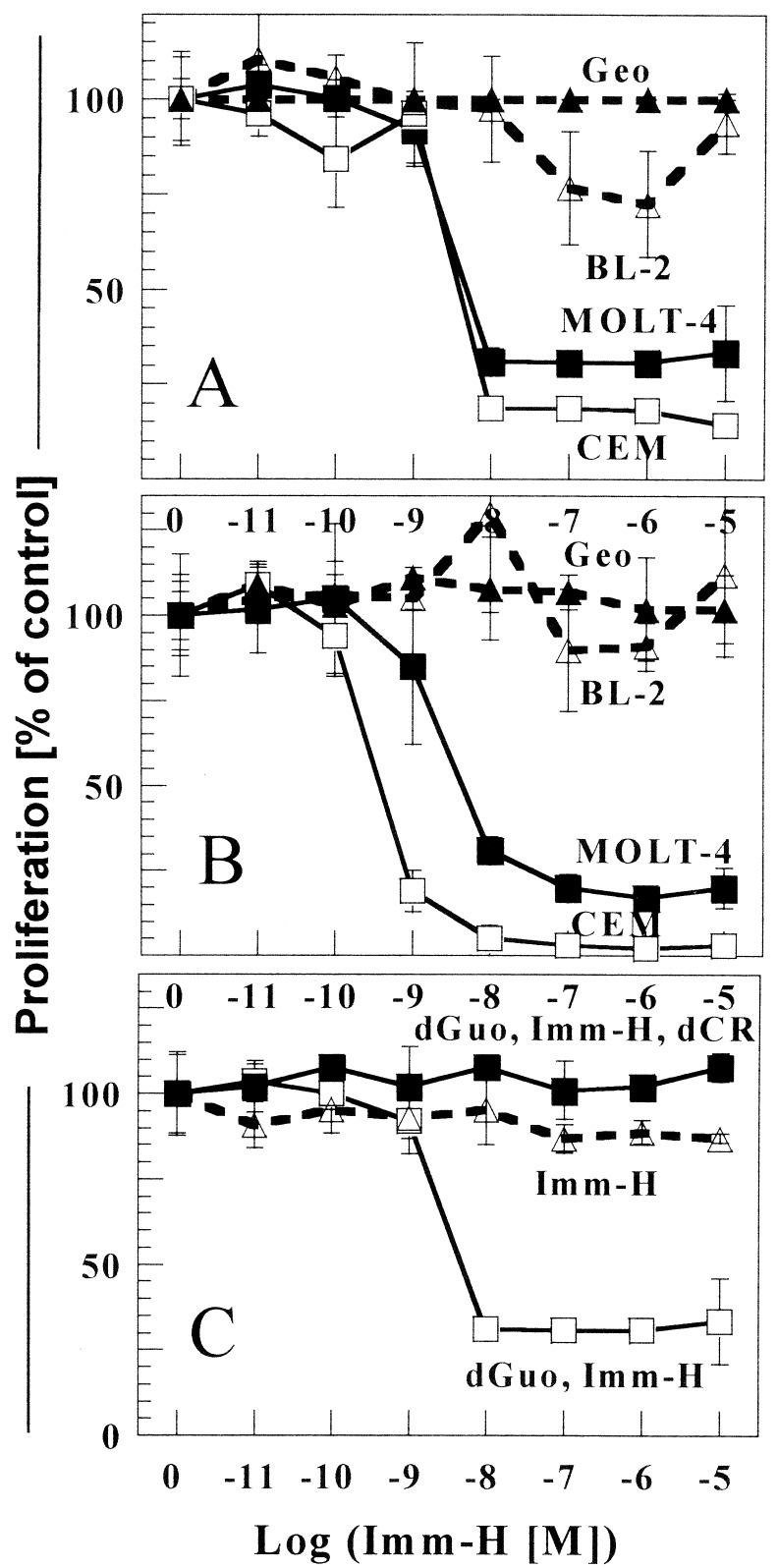
Inhibition of PNP by Imm-H selectively prevents proliferation of malignant T cells. Human T cell leukemia cell lines CCRF-CEM (CEM) and MOLT-4, the human B cell leukemia line BL2, and the colon carcinoma line GEO were incubated for 3 days in the presence of 20 μ M dGuo and Imm-H at the indicated concentrations. Proliferation was determined by WST-1 activity (A) or by [5−3 [H]thymidine incorporation (B). Cell proliferation was expressed as the percent of control cell growth (see Materials and Methods). In C, CCRF-CEM cells cultured in the absence of dGuo or with added dCyd were protected from the inhibitory effects of Imm-H. Lines labeled “Imm-H”, “dGuo, Imm-H”, and “dGuo, Imm-H, dCyd” represented cells exposed to 10 μM Imm-H, 20 μM dGuo, and Imm-H, dGuo with Imm-H, and 20 μM dCyd, respectively.
Figure 4.
Activated human peripheral T cells are inhibited by Imm-H. (A) Human T cells were negatively selected from four individual donors and incubated for 3 days with the indicated concentrations of Imm-H. Proliferation was measured by [3H]thymidine incorporation (see Materials and Methods). Cells were unstimulated (□) or stimulated (■) with 20 units/ml IL-2 and 0.2 μg/ml anti-CD3 mAb. Results shown are the percentage of control proliferation averaged for the four donors and represent 10 separate experiments. (B) T cells from one donor were subjected to increased stimulation by culturing for 6 days with 200 units/ml rhIL-2 and autologous mitomycin-pretreated mononuclear cells. Under these conditions, proliferation was inhibited (>95%) at increasing concentrations of Imm-H. Unstimulated T cells from 6 days of culturing were not inhibited (data not shown).
dGuo Accumulation.
The CCRF-CEM-cultured T cells were incubated for 24 h in complete medium as described above, containing 10 μM dGuo with or without 10 μM Imm-H. Cells (5 × 106) were pelleted, washed once with ice-cold PBS, and lysed with 0.5 M perchloric acid. The supernatant was adjusted to pH 6–8 with 2 M ammonium phosphate and analyzed for dGTP and GTP on a Partsil10 SAX 4.6 × 250 mm column (Millipore) with a gradient of ammonium phosphate (5 mM, pH 2.8, to 750 mM, pH 3.7, 50 min). The quantities of these guanine nucleotides were determined from standards of dGTP and GTP analyzed under similar experimental conditions.
Base and Nucleoside Incorporation into CCRF-CEM Cells.
Cultured CCRF-CEM cells (106) were preincubated with or without 10 μM Imm-H at 37°C for 30 min followed by addition of 1 μM (1 μCi) [8-3H]guanine, [8-3H]guanosine, [5-3H]deoxycytidine, [2-3H]adenine, or [2,8-3H]adenosine. At the indicated times, cells were washed twice with PBS followed by treatment with 1 ml 5% trichloroacetic acid (TCA). The pellets were washed with 5% TCA, solubilized in formic acid and radioactivity measured in a 1414 Winspectral (Wallac).
Imm-H Metabolism.
CCRF-CEM cells were incubated under conditions similar to methods described above for cell proliferation assays. Briefly, 1 × 106 cells/ml were incubated for 72 h without dGuo followed by 18-h incubation with 0.5 μCi [2-14C]Imm-H (50 μCi/μmol). Cells were harvested and analyzed for 14C incorporation into nucleic acids as described above.
dCK Assay.
T cell lines were cultured in RPMI medium 1640 as described above. After 72 h of incubation, 107 cells with >95% viability (by trypan blue exclusion) were washed once with PBS and resuspended in 200 μl of 50 mM KPO4, pH 7.6, 1 mM DTT, and 10% vol/vol glycerol. Cells were sonicated for 20 seconds (30% duty cycle), and the lysate was centrifuged at 14,000 × g for 10 min. The supernatant was analyzed for total protein content with the dye-binding assay of Bradford (Bio-Rad) by using BSA as a standard. Extracts were assayed for dCK following previously described methods (15). Briefly, extracts from 2 × 105 and 1 × 106 cells were added to reaction mixtures containing 5 μM [3H]deoxycytidine, 2 mM ATP, 25 mM MgCl2, 0.5 mM cytidine, 0.5 mM deoxyuridine, and 50 mM potassium phosphate, pH 7.6. The mixture was incubated at 37°C, and aliquots were loaded on a 1-ml column of DEAE A-25. The column was washed with 5 volumes of 100 mM ammonium bicarbonate, pH 8.0, to elute deoxynucleosides. dCMP was eluted with five volumes of 1 M ammonium bicarbonate, pH 8.0, and fractions were analyzed for 3H content in a Wallac 1414 liquid scintillation counter by using Liquiscint (National Diagnostics).
5′-Nucleotidase Assay.
Initially, 1 × 107 cells were cultured in RPMI medium 1640 for 3 days and washed with PBS followed by 0.29 mM sucrose and 10 mM Tris⋅HCl, pH 7.4. The cells were resuspended in sucrose buffer at a concentration of 1 × 108 cells/ml and sonicated twice for 10 seconds at 30% duty cycle. Samples were centrifuged at 100,000 × g for 60 min at 4°C, and supernatant equivalent to 2 × 105 and 1 × 106 cells was added to reaction mixtures containing 100 mM Tris⋅HCl, pH 7.4, 10 mM MgCl2, 3 mM ATP, 500 mM NaCl, 0.4 mM inosine monophosphate (IMP), and 1.25 μCi/μmol [8-14C]IMP (16). The nucleotide/nucleoside ratio was determined by using DEAE A-25 column analysis as described above for the dCK assay.
Results
Imm-H Inhibits Growth and Induces Apoptosis of Malignant Human T Cell Lines.
The effects of Imm-H on T cell leukemia cell lines CCRF-CEM and MOLT-4 were evaluated by treating cell cultures with increasing concentrations of Imm-H with and without dGuo for 3 days. The addition of dGuo mimics the extracellular accumulation of this metabolite in human PNP deficiency. Imm-H selectively inhibited proliferation of both the CCRF-CEM and MOLT-4 cell lines with an IC50 of 5 × 10−9 M (Fig. 2A). Thymidine incorporation was also blocked by Imm-H with an IC50 of 4 × 10−10 M (Fig. 2B). These effects were selective for the malignant T cell lines, as proliferation of the human B cell leukemia lines BL2, SKW 4.2 (data not shown), and the human colon carcinoma cell line GEO were not inhibited. Inhibition of PNP has been reported to be toxic to all T cell lines; however, no effect of Imm-H was observed against the Jurkat T cell leukemia line (data not shown) (17, 18). CCRF-CEM cells could be rescued from Imm-H sensitivity by excluding dGuo from the culture medium or by adding 20 μM deoxycytidine (dCyd) in the presence of dGuo (Fig. 2C). dCyd rescues cells by competing for dGuo phosphorylation and by dCMP product inhibition of dCK (18). MOLT-4 cells demonstrated a similar pattern (data not shown); toxicity was obviated in the absence of dGuo and was overcome by the addition of dCyd.
Imm-H (up to 50 μM) had no direct toxic effects when added to cultures of a variety of non-T cell lines derived from various tissues (Table 1). Although the IC50 for T cell lines was near 5 nM, these cell lines tolerated concentrations at least 104 times greater. To further define the cytotoxic effects of Imm-H on the CCRF-CEM and MOLT-4 lines, treated cells were analyzed for the induction of apoptosis. Annexin staining revealed significant apoptosis of CCRF-CEM (Fig. 3) and MOLT-4 (data not shown) cells treated with Imm-H and dGuo for 36 h. Apoptosis was not detected in the same cells when 20 μM dCyd was added or in the absence of dGuo. Apoptosis was not detected in BL-2 cells under similar culture conditions (Fig. 3).
Table 1.
Toxicity screening of Imm-H against cultured cell lines
| Cell line | Tissue origin* | IC50, μM |
|---|---|---|
| A2780 | Ovarian cancer | >150 |
| OVCAR-3 | Ovarian cancer | >150 |
| MCF-7 | Breast cancer | >150 |
| SKBR3 | Breast cancer | >150 |
| LNCAP | Prostate cancer | >150 |
| PC3 | Prostate cancer | >150 |
| HCT116 | Colon cancer | >150 |
| LS174t | Colon cancer | >150 |
| MIP | Colon cancer | >150 |
| CACO-2 | Colon cancer | >150 |
| A549 | Lung cancer | >150 |
| LX-1 | Lung cancer | >150 |
| HL60 | Leukemia | >150 |
| HS27 | Fibroblast | >150 |
| ABAE | Aortic endothelium† | 142 |
| M109 | Lung cancer‡ | 60 |
| MLF | Lung fibroblast‡ | 92 |
All cell lines are human unless otherwise specified.
Bovine cell line.
Murine cell line. Cells were cultured with Imm-H concentrations to 50 μM. No effect was scored as IC50 >150 μM.
Figure 3.
Imm-H induces apoptosis in human T cell lines but not in the BL2 B cell leukemia line. In A, flow cytometry analysis of the malignant CCRF-CEM T cell leukemia line gated for annexin+/pI− incubated for 36 h with 10 μM Imm-H and 20 μM dGuo. The percent of cells within each quadrant is indicated. The lower right quadrant corresponds to cells undergoing apoptosis. In B, the gated percent of apoptotic cells from flow cytometry for CCRF-CEM and BL2 cells incubated for 36 h with 10 μM Imm-H, 20 μM dGuo, and 20 μM dCyd, as indicated (Imm-H, dGuo, dCyd). Only CCRF-CEM T cell line undergoes apoptosis in the presence of dGuo and Imm-H (Imm-H, dGuo). Culture conditions that rescue CCRF-CEM from growth inhibition by dGuo and Imm-H do not induce apoptosis (Imm-H and Imm-H, dCyd). Similar results were obtained with the MOLT-4 leukemia cell line (data not shown). BL2 are resistant to apoptosis after exposure to Imm-H.
Imm-H Inhibits Activated Normal Peripheral Blood T Lymphocytes.
The effect of Imm-H on normal human T cells was determined by in vitro culture in the presence of dGuo and variable concentrations of Imm-H. Peripheral blood T cells were derived from normal donors by negative selection to obtain a pure population of CD3+ lymphocytes (>99%). Exposure to increasing concentrations of Imm-H had no effect on the viability of unstimulated T cells after 3 days of culture (Fig. 4A). However, activation of the T cells with an anti-CD3 monoclonal antibody and rhIL-2 (20 units/ml) for 3 days rendered the cells sensitive to Imm-H suppression (Fig. 4A). When cells were maximally stimulated by prolonged culture (6 days) with autologous monocytic cells and high-dose rhIL-2 (200 units/ml), treatment with Imm-H resulted in a greater inhibitory effect (95%) with an IC50 of 5 nM (Fig. 4B). Growth of unstimulated T cells was unchanged under similar culture conditions (data not shown).
Imm-H Causes the Accumulation of dGTP.
PNP normally catabolizes dGuo to 2-deoxyribose-1-phosphate and guanine. When PNP is inhibited, increased intracellular levels of dGTP result from phosphorylation by dCK. Accumulation of dGTP causes allosteric inhibition of ribonucleotide diphosphate reductase, preventing balanced production of deoxynucleotides essential for DNA synthesis. When dGTP levels were measured by HPLC in the Imm-H sensitive CCRF-CEM cell line exposed to dGuo alone, only GTP was detected. However, in cells exposed to dGuo and Imm-H, dGTP was elevated, and the level of GTP was reduced (Table 2).
Table 2.
Effect of Imm-H on dGTP levels in CCRF–CEM cells
| Growth condition* | GTP | dGTP |
|---|---|---|
| (nmol/5 × 106 cells†) | ||
| Control‡ | 1.86 | ND§ |
| dGuo | 2.17 | ND¶ |
| dGuo + Imm-H | 0.16 | 1.53 |
Cells were cultured for 24 hr in the presence of 10 μM dGuo with or without 10 μM Imm-H.
Nucleotide content was determined from GTP and dGTP standards analyzed by using HPLC chromatography. Experiments were performed in duplicate with errors estimated at ±10%.
Result from Bantia et al. (22).
¶ Not detectable (limit of detection = 0.2 and 0.08, respectively).
The site of Imm-H action was established by measuring the incorporation of precursors into RNA and DNA. CCRF-CEM cells were exposed briefly to Imm-H without dGuo followed by the addition of radiolabeled guanine, dCyd, adenine, adenosine, or guanosine. Under these conditions, ribonucleotide diphosphate reductase is not inhibited because dGTP does not accumulate. Imm-H suppressed only the incorporation of guanosine into nucleic acids (Fig. 5) with no effect on adenine and guanine phosphoribosyltransferases, dCK, adenosine kinase, or the enzymes of RNA and DNA synthesis, consistent with selective inhibition of PNP. Imm-H bears close structural similarity to inosine and may inhibit oligonucleotide synthesis through incorporation into DNA. This particular case was excluded by demonstrating the lack of incorporation of [2-14C]Imm-H into oligonucleotides (data not shown).
Figure 5.
Imm-H selectively blocks incorporation of guanosine into RNA and DNA of cultured CCRF-CEM cells. Cells were incubated for 30 min with 10 μ M Imm-H (□) or without Imm-H (■) followed by the addition of 3 H-base, nucleoside or deoxynucleoside. After the indicated times, incorporation into the trichloroacetic acid insoluble nucleic acids was determined. Guanosine incorporation was completely inhibited by incubation with Imm-H. In contrast adenine, adenosine, dCyd, and guanine incorporation remained unchanged.
Imm-H Toxicity Correlates with dCK Activity.
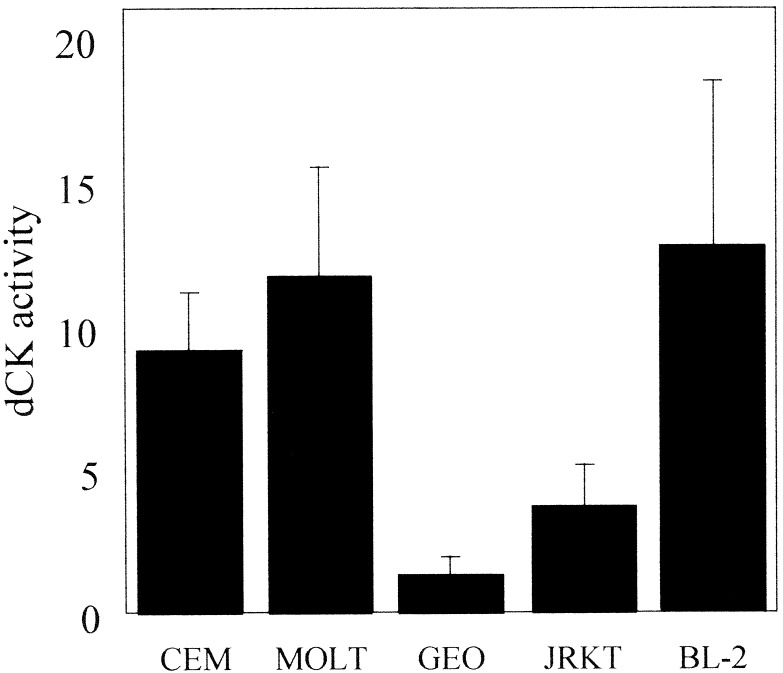
Imm-H toxicity requires the conversion of dGuo to dGTP, therefore the enzymes involved in dGuo metabolism were evaluated, namely dCK and cytoplasmic 5′-nucleotidase (5′-NT, EC 3.1.3.5). dCK activity was assayed in cell extracts by using an assay based on the conversion of [3H]dCyd to [3H]dCMP. The CCRF-CEM and MOLT-4 T cell leukemia lines are highly sensitive to inhibition by Imm-H and exhibited dCK activity at least 3-fold higher than resistant cell lines such as Jurkat and the colon carcinoma line GEO (Fig. 6). Of the normal human T cells, only the activated T cells were sensitive to suppression by Imm-H. Activated cells consistently showed an increase in dCK compared with unstimulated T cells that were not affected by Imm-H. 5′-NT activity did not significantly correlate with Imm-H sensitivity in any of the cell lines tested and did not vary by more than 50% (data not shown).
Figure 6.
Imm-H toxicity correlates with dCK activity. dCK activity was determined by discontinuous assay of cellular extracts (see Materials and Methods). T cell leukemia lines CCRF-CEM and MOLT-4 have more than three times the dCK activity (pmol/min/106 cells) than the resistant T cell cell line, Jurkat (Jrkt), or the colon carcinoma line GEO. The BL2 B cell leukemia line has elevated dCK activity but may avoid Imm-H toxicity through other mechanisms (see Discussion).
Discussion
Human genetic deficiency of PNP provides unequivocal evidence that loss of this enzymatic activity causes suppression of T cell function with minimal effects on B cells or other tissues (1). The challenge of inhibitor design for PNP arises from the abundance of the enzyme in human tissues. Individuals with genetic deficiencies of PNP that retain as little as 5% of normal catalytic activity do not exhibit manifestations of T cell deficiency and do not accumulate circulating dGuo (4, 19). Therefore, nearly complete inhibition of PNP (>95%) must be achieved to increase the dGuo concentration to the level required for T cell toxicity (4, 19). Paradoxically, inhibition of PNP also causes accumulation of PNP substrates, which further increases the requirement for the inhibitor. A similar competition has been seen with inhibitors directed against dihydrofolate reductase (20). This requirement for near-complete PNP inhibition has prevented the practical success of previous agents directed against this enzyme. One of these [peldesine (IC50, 36 nM)] has undergone clinical evaluation but in the first clinical trials did not produce sufficient inhibition of PNP to be an effective anti-T cell agent (4).
Imm-H binds ≈1,000 times more tightly to PNP than previous inhibitors and provides inhibition of catalytic activity when only one of the three identical catalytic sites is filled (6). For instance, the IC50 of Imm-H is 60–4,000 times lower than those for six other PNP inhibitors against cultured CCRF-CEM cells (4). We show here that Imm-H is a potent cytotoxic agent against T cells and that this cytotoxicity requires dGuo, which is linked to dGTP accumulation (21). Further, Imm-H is selective in suppressing the growth of the CCRF-CEM and MOLT-4 human T cell leukemia lines and activated normal peripheral blood T cells without affecting the BL-2 or SKW 4.2 B cell leukemia cell lines or the GEO colon carcinoma cell line. Imm-H induced apoptosis in sensitive T cells but not in B cells or in T cells protected by the addition of dCyd or in the absence of dGuo. The specificity of Imm-H action is demonstrated by complete PNP inhibition with unhindered incorporation of dCyd, adenine, adenosine, or guanine into the nucleic acids of CCRF-CEM cells.
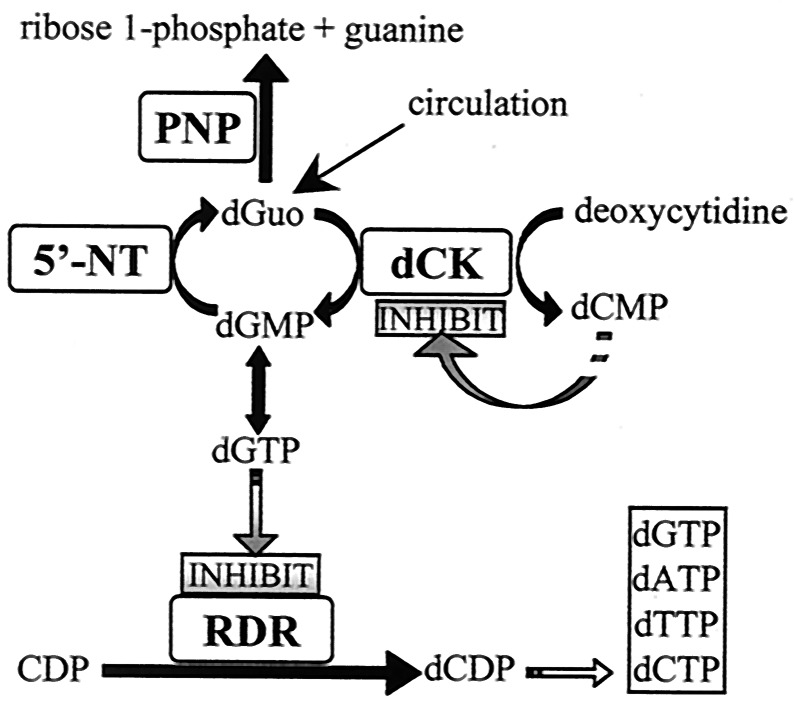
Accumulation of dGTP in T cells causes allosteric inhibition of ribonucleotide diphosphate reductase (Fig. 7), an activity essential for DNA production in the clonal expansion required in response to an antigenic challenge (22). Cells resistant to anti-PNP agents may not accumulate dGTP because of decreased dGuo uptake, low dCK activity, and/or increased dGMP phosphatase activity. The levels of dCK activity were low in the Imm-H-resistant GEO and Jurkat cell line but were elevated in the BL-2 B cell leukemia line. Thus, increased dCK activity or T cell lineage alone is insufficient to predict Imm-H susceptibility. Other mechanisms that limit the accumulation of dGTP may include reduced dGuo transport or high ecto-5′-NT activity. In fact, B lymphoblasts have been reported to contain higher levels of ecto-5′-NT than T lymphoblasts (23). In one case, a patient was found to have variable immunodeficiency related to partial deficiencies of both PNP and ecto-5′-NT activity (24), implicating a role of ecto-5′-NT in modulating the impact of PNP deficiency.
Figure 7.
Proposed mechanism of dGuo toxicity in PNP deficiency. DNA recycling and cellular uptake of plasma dGuo in the absence of PNP activity causes elevated intracellular levels. dGuo is phosphorylated by dCK in cells with high dCK activity, leading to high levels of dGTP that inhibit ribonucleotide diphosphate reductase.
An important finding was that Imm-H and dGuo had no toxic effect on normal unstimulated peripheral T cells but markedly suppressed the proliferation of activated T cells. Hence, Imm-H is likely to produce a selective inhibitory effect on T cells activated in autoimmune diseases, transplantation, and graft-versus-host disease (25). Imm-H may be especially beneficial in diseases caused by a select population of activated T cells, such as acute graft rejection, or for ex vivo depletion of donor T cells before organ transplantation (26).
In summary, Imm-H is a powerful and selective agent that suppresses the growth of T cell leukemia cell lines as well as activated normal human peripheral T lymphocytes. These effects depended on the presence of dGuo and could be reversed by the addition of dCyd, supporting the mechanism of T cell growth inhibition as caused by accumulation of dGTP, the subsequent block in DNA synthesis and induction of apoptosis. Sensitivity to Imm-H also correlated with increased dCK activity in the T cell leukemia cell lines and activated peripheral T cells. These results suggest that Imm-H may be useful for the treatment of human T cell leukemias and lymphomas and the treatment of diseases characterized by activated T cell responses, such as autoimmunity, organ transplantation, and graft-versus-host disease.
Acknowledgments
We thank Dr. M. Fukushima, Taiho Pharmaceutical Company, Limited, Saitama, Japan, for the nucleoside incorporation studies and Drs. I. David Goldman and Anne Davidson for critical review of this manuscript. This work was supported by research grants from the National Institutes of Health (GM41916) and the New Zealand Foundation for Research, Science, and Technology.
Abbreviations
- Immucillin-H
Imm-H
- PNP
purine nucleoside phosphorylase
- dGuo
deoxyguanosine
- dCK
deoxycytidine kinase
- rhIL-2
recombinant human IL-2
- dCyd
deoxycytidine
References
- 1.Stoop J W, Zegers B J, Hendrickx G F, van Heukelom L H, Staal G E, de Bree P K, Wadman S K, Ballieux R E. N Eng J Med. 1977;296:651–655. doi: 10.1056/NEJM197703242961203. [DOI] [PubMed] [Google Scholar]
- 2.Ealick S E, Rule S A, Carter D C, Greenhough T J, Babu Y S, Cook W J, Habash J, Helliwell J R, Stoeckler J D, Parks R E, Jr, et al. J Biol Chem. 1990;265:1812–1820. doi: 10.2210/pdb2pnp/pdb. [DOI] [PubMed] [Google Scholar]
- 3.Ealick S E, Babu Y S, Bugg C E, Erion M D, Guida W C, Montgomery J A, Secrist J A d. Proc Natl Acad Sci USA. 1991;88:11540–11544. doi: 10.1073/pnas.88.24.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris P E, Montgomery J A. Exp Opin Ther Patents. 1998;8:283–299. [Google Scholar]
- 5.Schramm V L. Annu Rev Biochem. 1998;67:693–720. doi: 10.1146/annurev.biochem.67.1.693. [DOI] [PubMed] [Google Scholar]
- 6.Miles R W, Tyler P C, Furneaux R H, Bagdassarian C K, Schramm V L. Biochemistry. 1998;37:8615–8621. doi: 10.1021/bi980658d. [DOI] [PubMed] [Google Scholar]
- 7.Wolfenden R. Acc Chem Res. 1972;5:10–18. [Google Scholar]
- 8.Kline P C, Schramm V L. Biochemistry. 1993;32:13212–13219. doi: 10.1021/bi00211a033. [DOI] [PubMed] [Google Scholar]
- 9.Kline P C, Schramm V L. Biochemistry. 1995;34:1153–1162. doi: 10.1021/bi00004a008. [DOI] [PubMed] [Google Scholar]
- 10.Horenstein B, Zabinski R F, Schramm V L. Tetrahedron Lett. 1993. 7213–7216. [Google Scholar]
- 11.Evans G B, Furneaux R H, Gainsford G J, Schramm V L, Tyler P C. Tetrahedron. 2000;56:3053–3062. [Google Scholar]
- 12.Hershfield M S, Mitchell B S. In: The Metabolic Basis of Inherited Disease. Schriber C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 1725–1768. [Google Scholar]
- 13.Bantia S, Montgomery J A, Johnson H G, Walsh G M. Immunopharmacology. 1996;35:53–63. doi: 10.1016/0162-3109(96)00123-3. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell B S, Mejias E, Daddona P E, Kelley W N. Proc Natl Acad Sci USA. 1978;75:5011–5014. doi: 10.1073/pnas.75.10.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ullman B, Gudas L J, Clift S M, Martin D W., Jr Proc Natl Acad Sci USA. 1979;76:1074–1078. doi: 10.1073/pnas.76.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawasaki H, Carrera C J, Piro D, Saven A, Kipps T J, Carson D A. Blood. 1993;81:597–601. [PubMed] [Google Scholar]
- 17.Cohen A, Barankiewicz J, Gelfand E W. Ann NY Acad Sci. 1985;451:26–33. doi: 10.1111/j.1749-6632.1985.tb27093.x. [DOI] [PubMed] [Google Scholar]
- 18.Osborne W R, Scott C R. Biochem J. 1983;214:711–718. doi: 10.1042/bj2140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staal G E, Stoop J W, Zegers B J, Siegenbeek van Heukelom L H, van der Vlist M J, Wadman S K, Martin D W. J Clin Invest. 1980;65:103–108. doi: 10.1172/JCI109639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White J C, Goldman I D. J Biol Chem. 1981;256:5722–5727. [PubMed] [Google Scholar]
- 21.Chottiner E G, Shewach D S, Datta N S, Ashcraft E, Gribbin D, Ginsburg D, Fox I H, Mitchell B S. Proc Natl Acad Sci USA. 1991;88:1531–1535. doi: 10.1073/pnas.88.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cory J G, Sato A, Brown N C. Adv Enz Regul. 1986;25:3–19. doi: 10.1016/0065-2571(86)90005-1. [DOI] [PubMed] [Google Scholar]
- 23.Edwards N L, Recker D, Manfredi J, Rembecki R, Fox I H. Am J Physiol. 1982;243:C270–C277. doi: 10.1152/ajpcell.1982.243.5.C270. [DOI] [PubMed] [Google Scholar]
- 24.Ostergaard P A, Deding A, Eriksen J, Mejer J. Acta Pathol Microbiol Scand Suppl C. 1980;88:299–302. doi: 10.1111/j.1699-0463.1980.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 25.Mason D, Powrie F. Curr Opin Immunol. 1998;10:649–655. doi: 10.1016/s0952-7915(98)80084-8. [DOI] [PubMed] [Google Scholar]
- 26.Davison G M, Novitzky N, Kline A, Thomas V, Abrahams L, Hale G, Waldmann H. Transplantation. 2000;69:1341–1347. doi: 10.1097/00007890-200004150-00022. [DOI] [PubMed] [Google Scholar]