Abstract
Membrane protein activity is affected by the properties of the lipid bilayer hosting them. These properties are established by both the lipid composition and the thermodynamic state of the bilayer. In the latter case, any parameter that can alter the state of the bilayer is indirectly able to affect the activity of membrane proteins. In a recent study, we have demonstrated that the activity of the KcsA ion channel is strongly related to the thermodynamic state of the lipid bilayer. In particular, when the lipid bilayer is in its main phase transition region, the conductivity of KcsA is increased and all its characteristic times change according to the characteristic times of the lipid bilayer. We propose here that the lipid bilayer can affect the distribution among many conformational substates of the open channel, affecting the corresponding channel conductivity.
Key words: Membrane proteins, phase transition, KcsA, lipid bilayer, single channel conductance, fluctuations
Biological membranes are one of the typical complex systems that are usually found in biology. Many crowded proteins and many different lipids are the main constituents of this system. The membrane plays the crucial roles of controlling the exchanges between the inner and the outer regions of the cells, the generation and propagation of neural signals and, by means of changes in shape, fundamental processes like cell division and endocytosis. A physical approach to this system should start from very simplified models trying to gain insights in the general physical principles ruling the behavior of biological membranes. Simplifying the system does not mean, however, that one necessarily looks for simple laws that could control the membrane behavior. We are favorably disposed to accept also complex behaviors that should eventually emerge.
Since its introduction in the 19th century, thermodynamics is considered the framework of choice to investigate complex systems, and biological membranes belong to that ensemble. The advent of new single molecule techniques has prompted the development of thermodynamic approaches to single molecule experiments. When these techniques are applied to single transmembrane proteins, the thermal bath in which proteins are mainly included is represented largely by the lipid bilayer. It is therefore foreseeable that a modification of the thermal bath could affect the functional behavior of the proteins.2 In recent years, the idea of an involvement of bilayer lateral heterogeneity in ruling aspecific interactions with transmembrane proteins that modulate protein activity is re-emerging from the Seventies.3 The lateral pressure profile of the lipid bilayer,4 the lateral compressibility and elastic moduli of the mono- and bi-layers5,6 are among the physical parameters that can potentially affect the conformational transitions, hence the activity, of transmembrane proteins.
At the same time, many experimental evidences support the idea that biological systems sit near an edge.7 Biological systems are attracted near borderline regions. From a thermodynamic point of view the edge is represented, for example, by phase transition points and critical points.
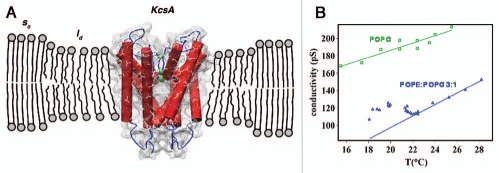
In a recent paper, we have studied the behavior of a single proteinaceous ion channel through the main phase transition region of the bilayer that hosts it.8 The phase transition of the lipid bilayer has been induced by changing the temperature in a Black Lipid Membrane set-up. We chose to work with KcsA in bilayers composed by the phospholipids POPE and POPG in different relative proportions. The main result of the investigation was that every functional aspect of the channel undergoes a trend inversion upon temperature changes when the bilayer goes through its main phase transition. In particular, single channel conductance decreases with temperature when the bilayer remains in the liquid disordered phase, but upon entering the phase transition region the single channel conductance increases counter-intuitively with decreasing temperature (Fig. 1). Many factors exclude the possibility of an exclusively specific lipid effect connected to a modification of the compositional environment for the channel in the coexistence region of solid ordered and liquid disordered phases. The most striking one is the variation of the characteristic dwell times for the channel in the transition region. The open dwell times increase by almost two orders of magnitude when the channel performs its activity while in the bilayer phase transition region, and they are far longer than the corresponding KcsA dwell times in a pure POPG bilayer at the same temperature. The relation between the thermodynamic state of the lipid bilayer and the channel behavior was confirmed by Differential Scanning Calorimetry (DSC) analysis which assured that the single channel behavior changed according to the variations in melting temperature with lipid composition. The relevant point of the observed behavior is that the physical state of the membrane can be modified by temperature but also by other parameters, such as chemicals which can partition in the lipid bilayer or even only interact with the lipid headgroups/water interface. Chemicals which interact with a lipid bilayer can affect its properties and, as a secondary effect, can modify the behavior of proteins in the membrane.9 As such, the effect of drugs can be little specific, especially at high concentrations. This drug action mechanism does not exclude the possibility of a specific interaction with membrane proteins.10 The problem is that usually it is not possible to distinguish clearly between those two types of actions. Moreover, local pH variations in biological systems can have a relevant effect on the phase state of lipid bilayers11 which are usually electrically charged. pH can consequently perform an indirect gating control of membrane protein functionality.
Figure 1.
(A) Scheme of a KcsA molecule in the lipid bilayer at the phase transition region. The channel is confined in the liquid disordered (ld) domains and mainly excluded from the solid ordered (so) ones.14 (B) By decreasing the temperature the single channel conductance initially decreases and, in the case of a POPE:POPG 3:1 bilayer, at a temperature of about 22°C a trend inversion is observed. The trend inversion occurs at the temperature when the phase transition of the bilayer is supposed to start. For a POPG bilayer, for which no phase transition is expected in the observed temperature range, a monotonic behavior is observed for the single channel conductance.
The second relevant aspect of this study is that the possible conformations transmembrane proteins can adopt are strongly related to the physical state of the lipid membrane hosting them. It has already been shown that, due to the hydrophobic matching mechanism, a change in bilayer thickness can induce a change in the orientation of the protein transmembrane α-helices. In the work at issue, we demonstrate that also the thermodynamical and mechanical properties of the lipid bilayer affect the protein conformations and functions. Protein dynamics has been already considered as a key factor to understand protein function.12 Moreover, the solvent is a major determinant for driving the characteristic dynamics of proteins in a “slaved” form.13 Solvent properties fluctuations can be coupled with protein fluctuations. This is usually studied for soluble proteins, but we showed earlier in reference 8, that for KcsA an increase of fluctuations of the lipid bilayers at the phase transition induces a variation in the characteristic times of the ion channel functionality. Moreover, an increase in single channel conductivity in the phase transition region of the bilayer is most probably the result of a variation of the prevalent conformation of the pore region of the channel favored by a change in the mechanical properties of the surrounding lipids. This behavior can be interpreted assuming that the open channel state can be subdivided in many substates and the lipid environment mechanical properties (viscosity, elasticity, compressibility) can affect the distribution among those possible substates. The selected substate can so determine the conductivity across the channel. An approach to the dynamics of membrane proteins can allow understanding how their activity can be affected by the presence of any stimulus altering the distribution among all the possible substates.
References
- 1.Tinoco I, Jr, Bustamante C. The effect of force on thermodynamics and kinetics of single molecule reactions. Biophys Chem. 2002;101:513–533. doi: 10.1016/s0301-4622(02)00177-1. [DOI] [PubMed] [Google Scholar]
- 2.Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature. 2009;459:379–385. doi: 10.1038/nature08147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagatolli LA, Ipsen JH, Simonsen AC, Mouritsen OG. An outlook on organization of lipids in membranes: searching for a realistic connection with the organization of biological membranes. Prog Lipid Res. 2010;49:378–389. doi: 10.1016/j.plipres.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Cantor RS. The inf luence of membrane lateral pressures on simple geometric models of protein conformational equilibria. Chem Phys Lipids. 1999;101:45–56. doi: 10.1016/s0009-3084(99)00054-7. [DOI] [PubMed] [Google Scholar]
- 5.McIntosh TJ, Simon SA. Roles of bilayer material properties in function and distribution of membrane proteins. Annu Rev Biophys Biomol Struct. 2006;35:177–198. doi: 10.1146/annurev.biophys.35.040405.102022. [DOI] [PubMed] [Google Scholar]
- 6.Andersen OS, Koeppe RE. Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 7.Pollack GH, Chin WC, editors. Phase Transitions in Cell Biology. Berlin: Springer; 2008. [Google Scholar]
- 8.Seeger HM, Aldrovandi L, Alessandrini A, Facci P. Changes in single K+ channel behavior induced by a lipid phase transition. Biophys J. 2010;99:3675–3683. doi: 10.1016/j.bpj.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundbaek JA. Lipid bilayer-mediated regulation of ion channel function by amphiphilic drugs. J Gen Physiol. 2008;131:421–429. doi: 10.1085/jgp.200709948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee AG. The effects of lipids on channel function. J Biol. 2009;8:86. doi: 10.1186/jbiol178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeger HM, Di Cerbo A, Alessandrini A, Facci P. Supported lipid bilayers on mica and silicon oxide: comparison of the main phase transition behavior. J Phys Chem B. 2010;114:8926–8933. doi: 10.1021/jp1026477. [DOI] [PubMed] [Google Scholar]
- 12.Frauenfelder H. In: The Physics of Proteins. Chan SS, Winnie S, editors. Berlin: Springer; 2010. p. 1. [Google Scholar]
- 13.Fenimore PW, Frauenfelder H, McMahon BH, Parak FG. Slaving: solvent fluctuations dominate protein dynamics and function. Proc Natl Acad Sci USA. 2002;99:16047–16051. doi: 10.1073/pnas.212637899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seeger HM, Bortolotti CA, Alessandrini A, Facci P. Phase-transition-induced protein redistribution in lipid bilayers. J Phys Chem B. 2009;113:16654–16659. doi: 10.1021/jp907505m. [DOI] [PubMed] [Google Scholar]