Abstract
Recent work from our interdisciplinary research group has revealed the emergence of inter-brain synchronization across multiple frequency bands during social interaction.1 Our findings result from the close collaboration between experts who study neural dynamics and developmental psychology. The initial aim of the collaboration was to combine knowledge from these two fields in order to move from a classical one-brain neuroscience towards a novel two-body approach. A new technique called hyperscanning has made it possible to study the neural activity of two individuals simultaneously. However, this advanced methodology was not sufficient in itself. What remained to be found was a way to promote real-time reciprocal social interaction between two individuals during brain recording and analyze the neural and behavioral phenomenon from an inter-individual perspective. Approaches used in infancy research to study nonverbal communication and coordination, between a mother and her child for example, have so far been poorly applied to neuroimaging experiments. We thus adapted an ecological two-body experiment inspired by the use of spontaneous imitation in preverbal infants. Numerous methodological and theoretical problems had to be overcome, ranging from the choice of a common time-unit for behavioral and brain recordings to the creation of algorithms for data processing between distant brain regions in different brains. This article will discuss the underlying issues and perspectives involved in elucidating the pathway from individual to social theories of cognition.
Key words: social neuroscience, developmental psychology, hyperscanning, non-linear dynamics, synchrony, top-down control, interpersonal coordination, consciousness
Is Neuroscience One-Brain Bound?
The major focus in neuroscience remains the isolated brain whereby hypotheses are drawn about the neurophysiologic mechanisms underlying our socio-cognitive abilities. Such “stand-alone” or “self-sufficient” approach to brain dynamics is in line with early computational cognitive sciences inherited from information theory. The later connectionist approach, however, introduced a more dynamic and holistic perspective.
While Gibsonian theories pointed out the importance of the environment in cognition, only a small community of neuroscientists moved away from the “one brain-bound” perspective inscribed in the computer metaphor. For instance, sensorimotor theories of cognition such as enaction2 and dynamical system approaches3–5 have started to show that the dynamical coupling of cognitive agents with their environment is an inherent part of the cognitive mechanisms.6 Indeed, the structure of the organism and the perception-action loops with the surrounding environment create statistical regularities that shape the information structure of the nervous system.7 Thus, perception of the world is seen as an exploratory process that cannot be reduced to merely an internal representation or reconstruction of the world.8 For instance, perception of space relies on intertwined neural spatial maps and motor functional networks.9 As Husserl proposed: “Our way to perceive space seems not to be given but rather built up by the experience of exploring the space itself.”10 Moreover, this perception varies between organisms according to the intrinsic properties of their body, including their sensory organs.11
Similarly, in spite of our cultural propensity to interact with others, our ability to socialize is neither a given nor fixed once and for all. Development provides numerous examples of transitory adaptations to both the physical and social environment. For instance, several studies have shown that imitation is crucial for infants to communicate before verbal language.12 Later, our daily life interactions even start before any words have been pronounced: before they even speak to each other, people look at each other and try to guess what each other's moods are from their facial expressions, posture and movements. Now imagine two adults who can not speak to each other, who can see only each other's hands via a double video system, and who are told to move their hands and imitate whenever they feel like it: will they come back to this nonverbal imitative method as used by young children? Will they imitate each other and take turns as imitator and model? In addition to successfully demonstrating that they do this, our experimental design provided simultaneous dual recordings of their brain activity.1 However, the question still remains, how should we relate the brain recordings of the two partners and what can such data tell us?
A Neurodynamic Approach to Social Interactions
The human brain not only integrates but coordinates information at multiple scales in space and time.5 The brain's complexity guarantees a balance between local specificity and connectivity, between functional segregation and integration.13 However, how information generated by multiple brain areas is integrated and coordinated across the brain is still a matter of debate. Several mechanisms have been proposed, including neural phase synchronization, which is based on the fact that brain information processing relies strongly on oscillatory activity.14–16 The Phase Locking Value (PLV) is a practical method for the quantification of neural synchronization between two neuroelectric signals in a specific frequency band.17 The fact that the perception-action loops of the two participants were intertwined in our experiment leads us to hypothesize that neural synchronizations, as measured by PLV, may exist between their two brains during periods in which the two subjects imitated one another reciprocally. Rather than using the classical PLV used to measure synchrony in the individual brain, we measured synchrony between two separated brains using a hyper-phase locking value (h-PLV). What can this h-PLV measure?
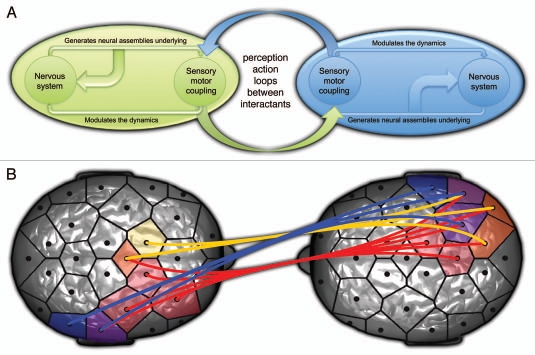
First, it is important to notice that despite inter-individual variability, the first hyperscanning studies done in fMRI have demonstrated strong anatomical and functional similarities between different human brains embedded in the same natural perceptual context.18 However, these similarities were observed at the timescale of seconds. In the case of reciprocal imitation, in which we used in our experiment, both perceptual and motor contexts are shared between the two participants on a milliseconds timescale. Taking into account that the phases of oscillatory activity in both sensory and motor areas have been linked to low-level information such as visual motion19 or hand velocity,20 in the case of reciprocal imitation, this low-level sensory-motor information has to flow inside and between the two brains (see Fig. 1A). Thus, the h-PLV could reflect information being dynamically shared through an interindividual sensory-motor loop. These loops emerge from a bi-directional coupling between the participants, with the behavior of each one influencing the other's behavior, and inter-brain synchronizations reflecting their perception-action entanglement. This synchronization may facilitate the transmission of information between two interacting brains in a similar fashion to synchronization within a single brain mediating communication between various brain regions.14,15
Figure 1.
(A) Schematic view of dyadic social interaction taken as a dynamical sensorimotor coupling. Inspired by Rudrauf 2003.33 (B) Inter-brain synchronization in alpha (blue), beta (orange) and gamma (red) frequency bands related to interactional synchrony during spontaneous imitation of hand movements. Inspired by Dumas et al. 2010.1
Interestingly, this dynamical phenomenon is purely emergent and asymmetric (see Fig. 1B). It cannot be reduced to the activities of each brain taken separately and is not only caused by the similarity of action and perception of the two players. Although previous studies have cued the subjects to make movements synchronously,21 in our study both participants movements converge through a mutual coordination to the state of interactional synchrony. “Turn-taking” is another emergent phenomena that can be observed during these real-time reciprocal interactions. Interestingly, the alternation roles between model and imitator cannot be integrally tackled through classically-induced contexts and one-brain recordings. Although we observed “turn-taking” during the experiment, the real-time constraints prevented us from investigating neural dynamics potentially linked to this phenomenon. It shows the extreme complexity of creating balanced paradigms that permit both real reciprocal interactions while providing sufficient control over specific emergent behaviors. Further hyperscanning studies should find well-designed experiments that can provide innovative ways to tackle such transient dynamics, which are strongly linked to inter-individual coordination.
Open questions also remain regarding the strong anatomical-functional similarities observed between humans18 and their potential links with our social abilities. For instance, to what extent could alterations of the distributed neural networks that underlie sensorimotor couplings between self and others lead to dynamic perturbations and cause social disturbances? If the Hebbian perspective of social cognition proposed by Keysers and Perrett22 suggests a more dynamical alternative to the classical mirror hypothesis, further conceptual jumps remain to be performed. For example, most approaches of the neural basis of social cognition adopt a symmetrical standpoint from which the brain activities of one person are either mirroring, or simulating, others actions and thoughts. The hyperscanning methodology could help develop paradigms that also capture the inherent asymmetries between people interacting, a phenomenon that remains unexplored.
Beyond Individual Consciousness?
We have seen that moving from a onebrain to a two-body perspective provides both new methodological and theoretical spaces where neuroscience can tackle everyday reciprocal social interactions.23,24 But this shift can do more than answering this issue and providing new tools, it may also allow us to address entirely new questions. For instance, key issues such as the influence of structural constraints on dynamics can, for instance, be tackled at both the behavioral and the neural level throughout development so as to better understand how they are intertwined. The opportunity to record two individuals interacting could also give new observations towards the study of multiscale dynamics and discover general laws governing complex systems and their interactions.
From a theoretical standpoint, the investigation of inter-brain relationships questions also the link between objective measures and behaviors. The vast problem behind neuroimaging is often expressed in the study of consciousness where the neural correlates of consciousness can not be disentangled from consciousness itself.25 This strongly questions the whole field and new alternatives may emerge for escaping this dualism26 and extending the definition of consciousness.27 For instance, recent studies have demonstrated that a form of collective intelligence can be measured.28–30 Theoretical approaches such as the Integrated Information Theory proposed by Tononi31 give also rise to a novel kind of formalism of consciousness and be extended to a social group. This announces for the coming years a renewed interest in cognitive science for the concept of “collective consciousness” designed by Durkheim more than a century ago.32 Hence, in addition to offering another take at the “hard problem”, nascent two-body neuroscience also has the potential to reshape our existing definitions of consciousness itself.
References
- 1.Dumas G, Nadel J, Soussignan R, Martinerie J, Garnero L. Inter-brain synchronization during social interaction. PLoS One. 2010;5:278–288. doi: 10.1371/journal.pone.0012166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson E, Varela F. Radical embodiment: neural dynamics and consciousness. Trends Cogn Sci. 2001;5:418–425. doi: 10.1016/s1364-6613(00)01750-2. [DOI] [PubMed] [Google Scholar]
- 3.Kelso J. Dynamic patterns: The self-organization of brain and behavior. The MIT Press; 1995. [Google Scholar]
- 4.Freeman W, Holmes M, West G, Vanhatalo S. Fine spatiotemporal structure of phase in human intracranial EEG. Clinic Neurophysiol. 2006;117:1228–1243. doi: 10.1016/j.clinph.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Le Van Quyen M, Bragin A. Analysis of dynamic brain oscillations: methodological advances. Trends Neurosci. 2007;30:365–373. doi: 10.1016/j.tins.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Clark A. An embodied cognitive science? Trends Cogn Sci. 1999;3:345–351. doi: 10.1016/s1364-6613(99)01361-3. [DOI] [PubMed] [Google Scholar]
- 7.Sporns O, Tononi G, Kotter R. The human connectome: a structural description of the human brain. PLoS Comput Biol. 2005;1:42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noé A. Action in perception. The MIT Press; 2004. [Google Scholar]
- 9.Rizzolatti G, Riggio L, Sheliga B. Space and selective attention. Attention and performance XV: Consc Nonconsc Inform Process. 1994:2319265. [Google Scholar]
- 10.Nagel T. What is it like to be a bat? Philosoph Review. 1974;83:435–450. [Google Scholar]
- 11.Husserl E. Grundlegende Untersuchungen zum phänomenologischen Ursprung der Räumlichkeit der Natur. In: Farber M, editor. Philosophical Essays in Memory of Edmund Husserl. Cambridge, MA: Harvard University Press; 1940. pp. 307–325. [Google Scholar]
- 12.Nadel-Brulfert J, Baudonniere P. The social function of reciprocal imitation in 2-year-old peers. Int J Behav Dev. 1982;5:95. [Google Scholar]
- 13.Tononi G, Sporns O, Edelman G. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc Natl Acad Sci USA. 1994;91:5033. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varela F, Lachaux J, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 15.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Canolty R, Ganguly K, Kennerley S, Cadieu C, Koepsell K, Wallis J, et al. Oscillatory phase coupling coordinates anatomically dispersed functional cell assemblies. Proc Natl Acad Sci USA. 2010;107:17356. doi: 10.1073/pnas.1008306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lachaux J, Rodriguez E, Martinerie J, Varela F. Measuring phase synchrony in brain signals. Human Brain Mapping. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303:1634. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- 19.Cosmelli D, David O, Lachaux J, Martinerie J, Garnero L, Renault B, et al. Waves of consciousness: ongoing cortical patterns during binocular rivalry. Neuroimage. 2004;23:128–140. doi: 10.1016/j.neuroimage.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Jerbi K, Lachaux J. Coherent neural representation of hand speed in humans revealed by MEG imaging. Proc Natl Acad Sci USA. 2007;104:7676. doi: 10.1073/pnas.0609632104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindenberger U, Li S, Gruber W, Mller V. Brains swinging in concert: cortical phase synchronization while playing guitar. BMC Neurosci. 2009;10:22. doi: 10.1186/1471-2202-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hari R, Kujala M. Brain basis of human social interaction: From concepts to brain imaging. Physiol Rev. 2009;89:453. doi: 10.1152/physrev.00041.2007. [DOI] [PubMed] [Google Scholar]
- 23.De Jaegher H. Social understanding through direct perception? Yes, by interacting. Conscious Cogn. 2009;18:535–542. doi: 10.1016/j.concog.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Keysers C, Perrett D. Demystifying social cognition: a Hebbian perspective. Trends Cogn Sci. 2004;8:501–507. doi: 10.1016/j.tics.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Chalmers D. The hard problem of consciousness. The Blackwell companion to consciousness. 2007:225–235. [Google Scholar]
- 26.Schilbach L. A second-person approach to other minds. Nature Reviews Neuroscience. 2010;11:449. doi: 10.1038/nrn2805-c1. [DOI] [PubMed] [Google Scholar]
- 27.Allen M, Williams G. Consciousness, plasticity, and connectomics: the role of intersubjectivity in human cognition. Front Psychol. 2011:2. doi: 10.3389/fpsyg.2011.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernst MO. Decisions Made Better. Science. 2010;329:1022–1023. doi: 10.1126/science.1194920. [DOI] [PubMed] [Google Scholar]
- 29.Woolley A, Chabris C, Pentland A, Hashmi N, Malone T. Evidence for a collective intelligence factor in the performance of human groups. Science. 2010 doi: 10.1126/science.1193147. [DOI] [PubMed] [Google Scholar]
- 30.Bahrami B, Olsen K, Latham PE, Roepstorff A, Rees G, Frith CD. Optimally Interacting Minds. Science. 2010;329:1081–1085. doi: 10.1126/science.1185718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tononi G. Consciousness as integrated information: A provisional manifesto. The Biological Bulletin. 2008;215:216. doi: 10.2307/25470707. [DOI] [PubMed] [Google Scholar]
- 32.Durkheim E. The Division of Labor in Society (1893) New York: Free Press; 1997. [Google Scholar]
- 33.Rudrauf D, Lutz A, Cosmelli D, Lachaux JP, Le Van Quyen M. From autopoiesis to neurophenomenology: Francisco Varela's exploration of the biophysics of being. Biol Res. 2003;36:27–65. doi: 10.4067/s0716-97602003000100005. [DOI] [PubMed] [Google Scholar]