Abstract
The glucocorticoid hormone cortisol has been shown to impair episodic memory performance. The present study examined the effect of two doses of hydrocortisone (synthetic cortisol) administration on autobiographical memory retrieval.
Healthy volunteers (n=66) were studied on two separate visits, during which they received placebo and either moderate-dose (0.15 mg/kg IV; n=33) or high-dose (0.45 mg/kg IV; n=33) hydrocortisone infusion. From 75 to 150 min post-infusion subjects performed an Autobiographical Memory Test and the California Verbal Learning Test (CVLT).
The high-dose hydrocortisone administration reduced the percent of specific memories recalled (p = 0.04), increased the percent of categorical (nonspecific) memories recalled, and slowed response times for categorical memories (p <0.001), compared to placebo performance (p < 0.001). Under moderate-dose hydrocortisone the autobiographical memory performance did not change significantly with respect to percent of specific or categorical memories recalled or reaction times. Performance on the CVLT was not affected by hydrocortisone.
These findings suggest that cortisol affects accessibility of autobiographical memories in a dose-dependent manner. Specifically, administration of hydrocortisone at doses analogous to those achieved under severe psychosocial stress impaired the specificity and speed of retrieval of autobiographical memories.
Keywords: Cortisol, Autobiographical memory, Human, Declarative memory, Depression
1. Introduction
Behavioral studies have suggested that a curvilinear relationship exists between glucocorticoid concentrations and memory consolidation across species, such that extremely low and high levels impair episodic memory performance, whereas moderate levels enhance performance (Lupien & McEwen, 1997; Herbert et al., 2006). In humans, dose-dependent effects are reported, with low doses of hydrocortisone improving memory, and high dose impairing performance on word lists (Abercrombie, Kalin, Thurow, Rosenkranz, & Davidson 2003) and paragraph recall (Newcommer et al., 1999). This effect in humans predominantly affects the episodic memory system. Hydrocortisone administration generally does not affect performance on many non-episodic memory tasks including serial addition, vigilance, line orientation, or delayed match to sample (Newcommer, Craft, Hershey, Askins, & Bardgett, 1994), but does dose-dependently affect performance on working memory tasks (Lupien, Gillin, & Hauger, 1999), and alters emotional biases in tests of spatial attention (Putman, Hermans, & Van Honk, 2010). Several studies failed to find effects of cortisol administration on episodic memory for word lists (Hsu, Garside, Massey & McAllister-Williams, 2003; Alhaj, Massey, & McAllister-Williams, 2008); however these studies employed recognition tests (involving old/new decisions) while those finding effects employed free recall tests. The data suggest that cortisol exerts dose-dependent effects on the consolidation of episodic information when administered prior to learning.
In contrast, studies in which corticosteroids were administered after learning but before retrieval have not established whether variable doses of glucocorticoid hormone administration differentially alters retrieval of episodic information from long-term memory (reviewed in de Quervain, Aerni, Schelling & Roozendaal, 2009). In humans, a single oral administration of 25mg hydrocortisone impaired recall of words or word pairs learned 24 hours earlier (de Quervain, Roozendaal, Nitsch, McGaugh & Hock, 2000; de Quervain et al., 2003), and 30mg oral hydrocortisone administered four hours after learning impaired recall of word pairs (Kuhlmann, Kirschbaum & Wolf, 2005). These studies assessed the effects of only single doses of hydrocortisone and tested recall within one day of learning.
Autobiographical memory is a subsystem of episodic memory in which the remembered events are specific to one's past experiences within their temporal and historical context (Tulving, 2002). This episodic memory system is unique because of its self-referential nature and because it shows enhancement by the emotional salience or arousal associated with the remembered event (Conway, 2003). Therefore, it might be hypothesized that this memory system would exhibit differential performance at varying levels of cortisol secretion. Additionally, autobiographical memories consist of material encoded during the entire life span, therefore allowing examination of cortisol's effects on material encoded beyond one day prior to administration.
To date, three studies have investigated the effect of varying cortisol levels on autobiographical memory performance. Barnhofer, Kuehn, and de Jong-Meyer (2005) did not find a correlation between endogenous cortisol levels and the number of specific memories recalled on the Autobiographical Memory Test (AMT; Williams & Scott, 1988). However, the change in cortisol levels over the 40-min study period correlated positively with the specificity of memories recalled in response to neutral cue words in depressed men. Buss, Wolf, Witt, and Hellhammer (2004) found that oral administration of 10mg of hydrocortisone to healthy men one hour prior to testing resulted in a significant reduction in the proportion of specific autobiographical memories generated in response to neutral cue words relative to the placebo condition. Schlosser et al. (2010) found that 10mg of hydrocortisone orally administered one hour prior to performing the AMT decreased the number of specific memories recalled in healthy controls without significantly altering performance in depressed participants.
The purpose of the present study was to extend the findings of Buss et al. (2004) and Schlosser et al. (2010) in healthy humans by investigating the effects of multiple doses of hydrocortisone on autobiographical memory recall, using hydrocortisone administered via the intravenous rather than the oral route. During two sessions, subjects received placebo and either a moderate- or high-dose of hydrocortisone and then recalled autobiographical memories in response to positive, negative, and neutral cue words. Based upon the effects of differing cortisol concentrations on delayed recognition and paragraph recall reviewed above, we hypothesized that autobiographical memory performance would exhibit dose-dependent effects of hydrocortisone administration, with the high-dose group showing the most impaired memory performance.
2. Methods
2.1 Subjects
Sixty-six psychiatrically and medically healthy, right-handed, non-smoking subjects (32 males) participated. Subjects were recruited from the community via advertisements and evaluated in the outpatient clinic of the NIH Clinical Center. Volunteers underwent medical history, physical examination, and laboratory screening that included assessments of thyroid, hematological, electrolyte function, HIV and urine drug testing. Exclusion criteria included major medical or neurological disorders (including peptic ulcer disease), and exposure to any medication likely to influence CNS function, metabolism, or endocrine status within three weeks of entry. Subjects also were screened for personal or family history of psychiatric disorders using an unstructured interview with a psychiatrist, the Structured Clinical Interview for DSM-IV Disorders (SCID; First, Spitzer, Miriam & Williams, 1995), and the Family Interview for Genetic Studies (FIGS; Maxwell, 1992). Volunteers with a personal or family history of a major psychiatric disorder, individuals who met DSM-IV criteria for alcohol and/or substance abuse within one year prior to screening, or had a lifetime history of substance dependence were excluded. Menopausal status and presence of estrogen supplementation were exclusionary criteria for females. Women taking oral contraceptives were also excluded. Because the cortisol response to stress varies in women across the menstrual cycle, females were tested only during the luteal phase, defined as beginning after the 16th day after the start of menstruation until the beginning of the following menstruation. After receiving a complete explanation of the study procedures, all participants provided written informed consent as approved by the NIMH-IRB. Subjects received financial compensation for their participation.
2.2 Materials, Design and Procedure
Testing was conducted individually in a private room at the day hospital of the National Institute of Health Clinical Center. Subjects were randomly assigned to moderate-dose (0.15 mg/kg; mean total dose=10.9mg, SD=2.05) or high-dose (0.45 mg/kg; mean total dose=31.8mg, SD=8.74) hydrocortisone groups. In two testing sessions separated by a one to two week interval, participants received either placebo or hydrocortisone under double-blind conditions. The order of infusion (hydrocortisone versus placebo) was randomized across subjects. The infusions lasted two min and were performed between 11:30am and 12:00pm. Cognitive testing began 75 min post-infusion and lasted up to 75 min. This timing is informative, as glucocorticoid induced elevation in activity of the basolateral nucleus of the amygdala (BLA) is thought to underlie the enhancement of memory consolidation and recall for emotionally arousing events (Roozendaal & McGaugh, 1997). In rodents, the intrinsic excitability of neurons in the BLA increases significantly between 60 and 120 minutes post corticosterone administration (Duvarci & Pare, 2007), and increased electrophysiological activity in the BLA persists for at least two hours post-injection (Kavushansky & Richter-Levin, 2006).
Blood (10ml per draw) was sampled to assay cortisol and corticosteroid binding globulin (CBG) pre-infusion and at +15 min, +30 min, +45 min, immediately prior to neuropsychological testing session at +75 min, and immediately following neuropsychological testing at + 150 min. Blood samples were stored at −70° C until assay. Assays were performed using the Corticosteroid Binding Globulin IRIA (Radioimmunoassay) Kit (Biosource, Nivelles, Belgium) for CBG, and Nichols Advantage® Specialty System (Nichols Institute Diagnostics, San Clemente, CA) for total cortisol. Free cortisol was then calculated from the CBG and total cortisol assays using the formula U2 K (1+N) + U (1+N+K) –C = 0, where U is the molar concentration of unbound cortisol, C the molar concentration of total cortisol, K is the affinity of transcortin for cortisol at 37°, and N is the proportion of albumin-bound to unbound cortisol. Measurements were converted to nmol/l.
The Autobiographical Memory Test (AMT; Williams & Broadbent, 1986) is a cued memory test in which subjects are presented with positively, negatively, and neutrally valenced cue words and instructed to recall one specific autobiographical memory following each cue word. Two different cue word sets were administered randomly on the two test days using words equivalent in valence and salience (Williams & Scott, 1988). Our version of the AMT consisted of 18 cue words presented orally, with six each of neutral, positive, and negative valence. Subjects were presented with three practice words given examples of correct and incorrect memories. No time limit on memory retrieval was imposed. If a subject recalled a memory, the response time was recorded with a stopwatch and was defined as the latency to the first word of each response (a standard measure of response time used in AM studies; e.g., Williams & Scott, 1988).
Memories were then coded according to their level of specificity using conventional definitions for coding autobiographical memories (e.g. Williams & Scott 1988; Williams et al., 2007; Anderson, Goddard & Powell, 2010). A specific memory was defined as memory for an event that took place at an identified place and did not last longer than one day. That is, a memory was called specific if the subject was able to give a date, day of the week, or time of day when the episode occurred. A categorical memory was defined as a memory referring to a category of events containing a number of specific episodes, without reference to one specific event. An extended memory was defined as a memory that referred to an extended period of time without reference to a specific event within the time period (e.g., a week long vacation). All responses were rated by a single rater (KY), and an independent rater scored 38.5% of responses; interrater reliability agreement was 88.7% (Cohen's Kappa of 0.83).
The California Verbal Learning Test (CVLT; Delis, Kramer, Kaplan & Ober 1987) was also administered to assess the specificity of the results to the autobiographical memory system. This test involved oral presentation of 16 words over five immediate recall trials. The list contained four words from each of four categories. Adjacent words on the lists were from different categories. Following the five trials, a second “interference” list was presented for one trial. Free and category cued recall of the first list was then tested (short term delay). After a 20-min interval during which the AMT was administered, free recall and cued recall of the first list were again assessed (long delay). Two distinct standardized versions of the CVLT were administered randomly on the two test days.
2.3 Statistical Analysis
The Shapiro-Wilks test confirmed that the data were normally distributed (Ws>0.97, ps > 0.11) and Levine's test for equality of variances confirmed that the variances were equal across groups (Fs(3,62) < 1.921, ps > 0.135), supporting the use of parametric analysis. Using SPSS, repeated measure ANOVAs were used to analyze free cortisol concentrations, the percent of memories recalled, and response time to retrieve a memory. Difference scores for the memory variables were created by subtracting measures obtained following hydrocortisone from those obtained following placebo (e.g., positive differences indicated an increase from placebo).
Repeated measures for plasma cortisol analysis were Time and Condition; repeated measures for the AM test were Memory Type and Valence. Between subjects variables were Sex and Dose. To determine if the difference from placebo was significant, one sample t-tests were conducted. To determine if differences between dose or sex were significant, independent samples t-tests were conducted. The post-hoc tests were corrected for multiple comparisons (Bonferroni).
The CVLT was scored using the CVLT-II Comprehensive Scoring System. This resulted in 23 variables measuring memory performance for each free and cued recall attempt during the short and long delay conditions. A 2×2 ANOVA was used to analyze whether Dose or Sex significantly affected any of these variables.
3. Results
Subject characteristics appear in Table 1. The groups assigned to each dose did not differ on age or BMI as evidenced by no significant main effect or interaction in a Dose × Sex ANOVA (Fs(1, 60) < 1.21, ps > 0.28). The effect of infusion order (PL-HC vs. HC-PL) on the dependent variables was assessed by comparing the subgroups that received placebo first versus those who received hydrocortisone first using independent samples t-tests. This analysis was performed separately for the high and low dose groups. No significant difference was found, suggesting that the infusion order did not affect the results (ts(22) < 1.61, ps > 0.12).
Table 1.
Age and BMI (SD) for the moderate and high dose hydrocortisone groups for males and females.
| Males | Females | |||
|---|---|---|---|---|
| Moderate Dose | High Dose | Moderate Dose | High Dose | |
| Age | 26.5 (6.72) | 29.9 (9.13) | 29.2 (4.71) | 28.8 (6.59) |
| BMI | 25.7 (2.62) | 26.1 (3.63) | 23.8 (4.09) | 24.9 (4.38) |
3.1 Cortisol Measurements
The results of the repeated measures ANOVA of the within subjects factor of Time (baseline, 0, 15, 30, 45, 75, and 150 min post infusion) and Condition (placebo or hydrocortisone), and the between subjects factors of Dose (high or moderate) and Sex for free cortisol levels appear in table 2. Analyses of total cortisol and CBG appear in the Supplemental Materials.
Table 2.
Mean (SD) free cortisol levels in nmol/l as a function of dose, condition, sex, and time post hydrocortisone infusion.
| Male | Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Moderate Dose | High Dose | Moderate Dose | High Dose | ||||||
| Placebo | Drug | Placebo | Drug | Placebo | Drug | Placebo | Drug | ||
| Time (minutes) | Baseline | 17.4 (19.6) | 10.8 (10.4) | 7.92 (4.96) | 11.0 (6.85) | 16.5 (36.1) | 17.7 (24.7) | 17.1 (17.6) | 19.9 (15.2) |
| 0 | 14.1 (10.1) | 102 (83.0) | 9.41 (6.11) | 312 (207) | 99.7 (263) | 89.2 (158) | 14.6 (13.5) | 488 (549) | |
| 15 | 11.1 (6.04) | 63.4 (47.8) | 9.06 (7.08) | 285 (130) | 51.3 (94.5) | 129 (180) | 12.1 (11.0) | 475 (421) | |
| 30 | 15.7 (21.5) | 41.7 (27.2) | 10.2 (9.64) | 185 (99.8) | 53.2 (102) | 106 (193) | 14.7 (15.0) | 341 (341) | |
| 45 | 16.1 (18.2) | 35.7 (31.4) | 11.9 (16.5) | 132 (68.4) | 35.8 (60.7) | 102 (188) | 15.8 (17.9) | 394 (385) | |
| 75 | 18.1 (20.6) | 26.1 (17.0) | 9.85 (8.12) | 75.0 (35.0) | 30.3 (49.2) | 80.2 (150) | 17.8 (21.4) | 195 (240) | |
| 150 | 12.8 (14.3) | 9.85 (6.82) | 16.0 (22.6) | 28.5 (16.0) | 27.2 (67.5) | 17.2 (15.6) | 21.5 (33.5) | 69.5 (110) | |
The free cortisol levels showed a significant effect of passage of Time (F(6,57)=12.0, p<0.001), Dose (F(1,62) = 12.1, p < 0.001), Condition (F(1,62) = 44.9, p < 0.001)), and a Time by Dose by Condition interaction (F(1,62) = 18.2, p < 0.001). The cortisol levels in the high-dose group remained significantly higher following hydrocortisone than those following placebo (ts(32) > 2.40, ps < 0.02). For the moderate-dose group, cortisol levels following hydrocortisone and at the beginning of neuropsychological testing (75 min post-infusion) showed a trend toward remaining higher than under placebo (ts(32) > 1.76, ps < 0.08). However, by the end of neuropsychological testing (150 min post-infusion), free cortisol in the moderate-dose group was no longer significantly elevated relative to placebo (t(32) = 0.93, p = 0.36). The cortisol levels remained significantly higher following hydrocortisone infusion in the high dose group than in the moderate dose group (ts(64) > 2.55, ps < 0.02). Under placebo the cortisol levels did not change significantly following infusion (ts(32) < 1.47, ps > 0.15). Additionally, the baseline cortisol levels did not differ significantly between the placebo and hydrocortisone days for either the moderate (t(32) = 1.74, p = 0.10) or high (t(32) = 1.55, p = 0.13) dose groups.
There was a main effect of Sex (F(1,62) = 7.06, p = 0.01), and a Sex by Condition interaction (F(1,62) = 3.82, p = 0.05). At all times examined during the hydrocortisone condition, females had higher free cortisol levels than males (ts(64) > 1.89, ps < 0.03). This sex effect also was significant for the baseline measure obtained for the hydrocortisone session (t(64) = 2.09, p = 0.04) and trended towards significance for that obtained on the placebo day (t(64) =1.65, p = 0.10). Women showed higher cortisol levels than men regardless of dose or time (the Sex by Time interaction was not significant; Fs(1,62) < 2.33, ps > 0.14).
Because we observed sex differences in total and free cortisol levels, and pervious studies have observed sex differences in glucocorticoid effects (Stark et al., 2006) and during autobiographical memory testing (Friedman & Pines, 1991; Bauer, Stennes & Haight, 2003), we examined whether sex differences emerged on Autobiographical Memory Test performance.
3.2 Specificity of Memories
A Repeated Measures ANOVA was conducted on the within-subjects factors of Valence (positive, negative, neutral) and Memory Type (specific, categorical), and on the between-subjects factors of Dose (moderate, high) and Sex. The dependent variables were Percent of memories recalled and Response Time to recall specific and categorical memories. As the mean number of “no memory” (0.29 +/- 0.77), and extended memory responses (0.69 +/- 1.07) was less than one (< 3% of total responses) we did not include these memory types in the analysis.
The percent of memories recalled under placebo and hydrocortisone did not show main effects of Sex (F(1,62) = 0.01, p = 0.93), Dose (F(1,62) = 0.16, p = 0.69), Valence (F(1,62) = 0.71, p = 0.72), or Memory Type (F(1,62) = 0.14, p = 0.72). The only significant interaction was for Memory Type by Dose (F(1,62) = 2.95, p = 0.01), with opposite effects observed for specific and categorical memories (Figure 1A). For the percent of specific memories recalled, the change from placebo observed in the moderate-dose group significantly differed from that in the high-dose group following hydrocortisone (t(57) = 2.16, p = 0.05). The percent of specific memories recalled did not change significantly following moderate-dose hydrocortisone (t(31) = 0.64, p = 0.53), but decreased following high-dose hydrocortisone (t(26) = 2.28, p = 0.04).
Figure 1.
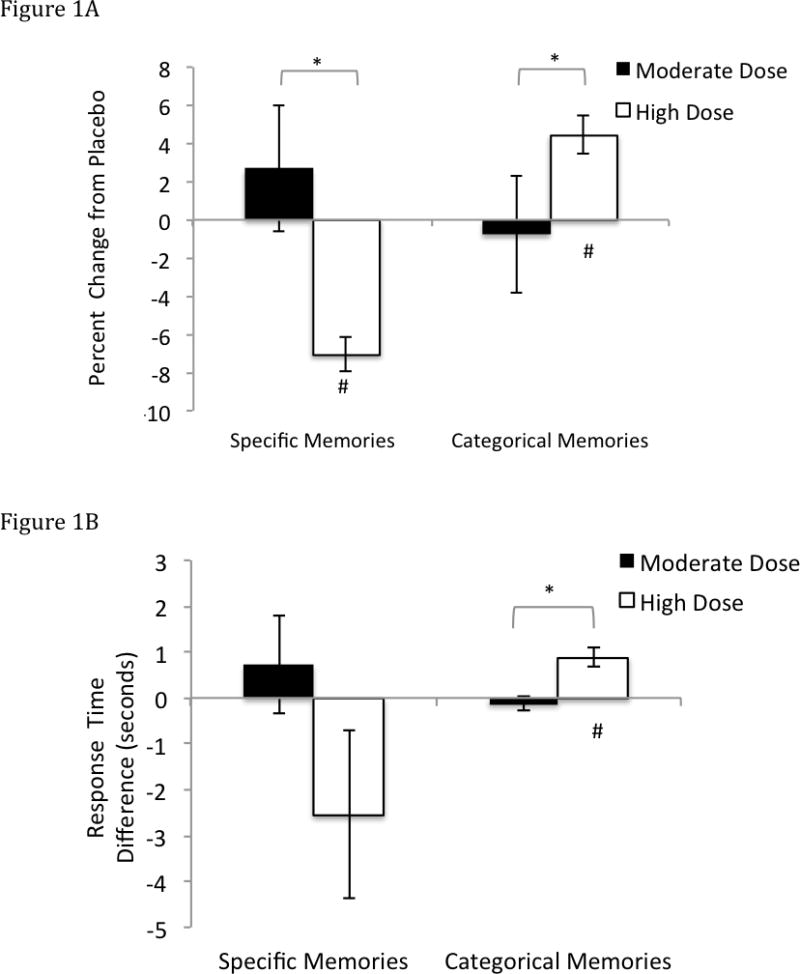
(A) Difference in the percent of specific and categorical memories retrieved between placebo and hydrocortisone infusion for moderate and high doses of hydrocortisone. Error bars indicate one standard error of the mean. # = significant difference from 0 at p < 0.05 * indicates a significant difference between doses at p < 0.05.
(B) Difference in the response times for specific and categorical memories retrieved between placebo and hydrocortisone infusion for low and high doses of hydrocortisone. Error bars indicate one standard error of the mean. # indicates significant difference from 0 at p < 0.05, * indicates a significant difference between doses at p < 0.05.
For the percent of categorical memories recalled, the changes seen in the moderate-dose group also differed from those in the high-dose group (t(57) = 3.84, p = 0.001), but in a direction opposite to that seen for specific memories. Following moderate dose infusion the percent of categorical memories recalled showed a non-significant trend toward decreasing relative to placebo (t(31) = 1.83, p = 0.08), while under the high-dose infusion, the percent of categorical memories increased versus placebo (t(29) = 4.56, p = 0.001).
3.3 Retrieval Response Time
No significant main effects emerged for changes in retrieval response time (Dose (F(1, 58) = 2.52, p = 0.12); Sex (F(1, 58) = 0.18, p = 0.67); Valence (F(2,57) = 0.32, p = 0.73); Memory Type (F(1,58) = 2.37, p = 0.13)).
A Memory Type by Dose interaction on response retrieval times was evident (F(1,58) = 5.92, p = 0.02; Figure1b). This interaction was driven by changes in retrieval times for categorical memories; moderate dose hydrocortisone infusion did not significantly alter the response times (t(30)= 0.90, p = 0.38), but high dose hydrocortisone infusion prolonged response times for these memories (t(30) = 4.37, p < 0.001). The difference in the change in response time for categorical memories between the high and moderate dose groups was significant (t(60) = 4.08, p < 0.001). In contrast, the response times for specific memories did not change significantly following either moderate (t(32) = 0.68, p = 0.50) or high dose administration (t(32) = 1.39, p = 0.17), and the response times to recall specific memories did not differ between high and moderate dose groups (t(64) = 0.67, p = 0.51).
A Dose by Sex interaction on memory retrieval time was evident (F(1,58) = 4.33, p = 0.04), which was accounted for by response times to recall any memory increasing following moderate-dose and decreasing following high-dose hydrocortisone in males but not females (mean RT differences in males and females, respectively were 1.46s +/- 5.64s and 0.09 +/- 7.17s for moderate dose and -3.20s +/- 8.03s and -0.06 +/- 4.31s for high dose). The difference between high and low dose RT changes was significant for the males (p = 0.03). No other difference was present (ps > 0.5).
3.4 CVLT
Difference scores calculated by subtracting performance under placebo from that under hydrocortisone (see Supplemental Materials) showed no main effect of Dose (Fs(1,52) < 2.77, ps > 0.11) or Sex (Fs(1,52) < 2.57, ps > 0.12), and no Dose by Sex interaction (Fs(1,52) < 2.55, ps > 0.12) for any variable examined. Exploratory one sample t-tests were also not significant (ts(57) < 1.42, ps > 0.16 for high and moderate doses combined, ts(28) < 1.20, ps > 0.24 for moderate dose; ts(29) < 1.64, ps > 0.12 for high dose) indicating no change in performance under either placebo or hydrocortisone.
4. Discussion
Hydrocortisone infusion affected autobiographical memory recall in a dose-dependent manner. The moderate-dose hydrocortisone infusion did not alter significantly either the percent of specific versus categorical memories recalled, or the response times for retrieving such memories versus placebo. In contrast, under the high-dose condition the percent of specific memories recalled decreased while both the percent of non-specific categorical memories recalled and the reaction times to recall categorical memories increased. These data indicate that an elevation of plasma cortisol concentrations to the range expected during severe stress (Chernow et al., 1987), interfered with both the ability to retrieve autobiographical memories and the retrieval speed. These data converge with those from pervious studies to support the hypothesis that higher cortisol levels result in dose-dependent impairment of episodic retrieval.
The significant reduction in the proportion of specific memories recalled which we observed following IV administration of hydrocortisone (0.45 mg/kg) appeared consistent with reductions in the percent of specific memories reported by Schlosser et al. (2010) and Buss et al. (2004) following oral administration of hydrocortisone (10mg). Despite the methodological differences between Buss et al.'s (2004) study and the current study (e.g., including route of administration and the latency between administration and testing [60 min versus 75 min]) the levels of free cortisol reported in the Buss et al. (2004) study at the time of the autobiographical memory task (99.1 +/- 20.4 nmol/l) were only modestly smaller than those achieved under the high dose hydrocortisone condition in our study (140 +/- 32.5nmol/l for males and females combined). In contrast, under the moderate dose condition used herein the free cortisol concentration was 52 +/- 18.6nmol/l at testing. Schlosser et al. (2010) did not report cortisol levels in their study; therefore comparisons with the levels obtained in the current study were unclear.
Our finding that the response time for retrieving categorical memories is slower under high-dose hydrocortisone infusion is novel, however, as previous studies examining the relationship between cortisol and autobiographical memories evaluated effects on the proportion of specific memories recalled but did not assess effects on response time.
Despite the findings that the moderate-dose group had significantly higher levels of cortisol at the start of the neuropsychological testing compared to placebo, by the end of the testing session, cortisol levels in this group did not differ significantly between the hydrocortisone and placebo conditions, potentially accounting for the non-significant behavioral results from this group. Nevertheless, the extent to which the decreasing levels of cortisol during the testing period influenced our behavioral results remains unclear, as the rapid genomic effects of cortisol would likely persist after plasma levels return to baseline (Falkenstein, Tillman, Christ, Feuring & Wehling, 2000). Early gene effects of rising cortisol levels occur within 15 minutes and persist for hours (Makara & Haller, 2001). Additionally, non-significant effects on autobiographical memory recall were reported by Tollenaar, Elzinga, Spinhoven and Everaerd (2009) following stress induced elevation of cortisol to levels comparable to those seen following moderate dose administration in the current study. Nevertheless, our study design did not allow assessment of whether the performance patterns observed in the moderate-dose group might have been more pronounced had testing occurred temporally closer to the infusion.
The negative results from the CVLT data suggest that high-dose hydrocortisone's effects on cognitive performance may be relatively specific to autobiographical memory retrieval. During the CVLT both learning and recall of the words occurred after hydrocortisone administration; we thus assessed consolidation as well as retrieval of information from long-term memory. Although studies have found performance on word list recall to be affected by stress (e.g. Almela, Hidalgo, Villada, Espin, Gomez-Amor & Salvador, 2010; Smeets, Giesbrecht, Jelicic & Merckelbach, 2007), the effects of glucocorticoid administration on CVLT performance had not previously been examined. Because the CVLT uses semantically related words that can be grouped together, the CVLT is not a pure test of episodic learning and memory, but instead measures repetition learning, semantic organization, and proactive interference (Delis, Fridland, Kramer & Kaplan, 1988). The lack of a hydrocortisone effect on the CVLT suggests that the impairment in autobiographical memory retrieval seen following high-dose hydrocortisone does not reflect a general impairment of memory retrieval. Additionally, these results are consistent with Roozendaal's (2003) hypothesis that the acute effects of glucocorticoids are different for memory consolidation and memory retrieval.
Our results suggest a mechanism for the autobiographical memory deficits seen in major depressive disorder (MDD), which is associated with both impaired access to specific memories (van Vreeswijk & de Wilde, 2004) and abnormally increased cortisol secretion (Gibbons & McHugh, 1962). Autobiographical memory impairments in depression conceivably may be due, in part, to glucocorticoid effects on retrieval. Nevertheless, the chronic hypercortisolemia associated with depression may not be represented by acutely increased cortisol levels in healthy volunteers.
Women were studied during the luteal phase of their menstrual cycle, when stressed cortisol responses appear similar to those of men (Kirschbaum, Kudielks, Gaab, Schommer & Hellhammer, 1999). Nevertheless, women showed higher levels free cortisol concentrations than men at all time-points examined following infusion. Compatible with these data, women studied in the luteal phase also show higher free cortisol concentrations following exogenous ACTH stimulation compared to men (Kirschbaum et al., 1999). Gender differences also were evident for the behavioral results; the response times for memory retrieval did not differ between low and high dose conditions in women, in contrast to men. These sex differences may reflect the interactions between ovarian steroids, glucocorticoid receptor function, and hypothalamic-pituitary-adrenal axis function characterized in preclinical studies (Lesniewska, Miskowiak, Nowak & Malendowicz, 1990; Burgess & Handa, 1992).
Previous studies examining the effects of glucocorticoid administration on autobiographical memory did not specifically investigate sex effect. Buss et al. (2004) included only males. Schlosser et al. (2009) reported, “overall, women showed better memory performance” without giving additional detail. Interestingly, Barnhofer et al., (2005) observed that correlations between cortisol levels and memory specificity in depressed subjects were significant only for males, potentially in line with our finding that differential effects of cortisol on overall memory response times were evident only in men.
A limitation of our study design that merits comment is that the same subjects were not tested in all three conditions (placebo, moderate-, and high-dose hydrocortisone) because elevated cortisol concentrations can alter the expression of multiple genes within the CNS, and it is unclear how long such changes may persist (Datson, Marsink, Meijer & de Kloet, 2008). Thus, to avoid the possibility that the high-dose infusion may alter the results of testing in subsequent study sessions each subject received only one hydrocortisone dose. This unpaired design may have reduced sensitivity to detect differences in memory performance between the moderate- and high-dose conditions.
In summary, autobiographical memory is affected by elevations in cortisol levels in a dose-dependent manner. When cortisol levels are increased to those reached during severe stress (Drevets et al., 2002; Chatterton, Vogelsong, Lu & Hudgens, 1997; Parker et al., 1985), memory for autobiographical events is impaired, although this effect may not extend to conditions in which cortisol is elevated to levels seen during mild-to-moderate stress (Kirschbaum, Pirke & Hellhammer, 1993; Chernow et al., 1987). The successful recall of autobiographical memories can play a role in generating adaptive responses in novel circumstances. From a heuristic perspective, however, it might be argued that when stressors become severe or life-threatening, maintaining the focus of attention toward instinctive or reflexive fight, flight, or fright responses takes precedence.
Supplementary Material
Acknowledgments
Funding for this study was provided by NIMH Z01-MH002814; the DIRP within NIMH provided critical peer review for the study design and approval of manuscript submission, NIMH had no further role in the collection, analysis and interpretation of data, or in the writing of the report. We would like to acknowledge the support of the NIMH Intramural Research Program, and Joan Williams and Michele Drevets for assistance with recruitment and clinical assessment of the participants.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behavioral Neuroscience. 2003;117:505–516. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- Alhaj HA, Massey AE, McAllister-Williams RH. Effects of cortisol on the laterality of the neural correlates of episodic memory. Journal of Psychiatric Research. 2008;42:971–981. doi: 10.1016/j.jpsychires.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Almela M, Hidalgo V, Villada C, Espin L, Gomez-Armor J, Salvador A. The impact of cortisol reactivity to acute stress on memory: Sex differences in middle-aged people. Stress. 2010 doi: 10.3109/10253890.2010.514671. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Goddard L, Powell JH. Reduced specificity of autobiographical memory as a moderator of the relationship between daily hassles and depression. Cognition and Emotion. 2010;24:702–709. [Google Scholar]
- Barnhofer T, Kuehn E, de Jong-Meyer R. Specificity of autobiographical memories and basal cortisol levels in patients with major depression. Psychneuroendocrinology. 2005;30:403–411. doi: 10.1016/j.psyneuen.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Stenns L, Haight JC. Representation of the inner self in autobiography: Women's and men's use of internal states language in personal narratives. Memory. 2003;11:27–42. doi: 10.1080/741938176. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocortiocotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Buss C, Wolf OT, Witt J, Hellhammer DH. Autobiographical memory impairment following acute cortisol administration. Psychoneuroendocrinology. 2004;29:1093–1096. doi: 10.1016/j.psyneuen.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Vogelsong KM, Lu Y, Hudgens GA. Hormonal response to psychological stress in men preparing for skydiving. Journal of Clinical Endocrinology and Metabolism. 1997;82:2503–2509. doi: 10.1210/jcem.82.8.4133. [DOI] [PubMed] [Google Scholar]
- Chernow B, Alexander HR, Smallridge RC, Thompson WR, Cook D, Beardsley D, Fink MP, Lake CR, Fletcher JR. The hormonal responses to surgical stress. Archives of Internal Medicine. 1987;147:1273–1278. [PubMed] [Google Scholar]
- Conway MA. Commentary: Cognitive-affective mechanisms and processes in autobiographical memory. Memory. 2003;11:217–224. doi: 10.1080/741938205. [DOI] [PubMed] [Google Scholar]
- Datson NA, Marsink MC, Meijer OC, de Kloet ER. Central corticosteroid actions: Search for gene targets. European Journal of Pharmacology. 2008;583:272–289. doi: 10.1016/j.ejphar.2007.11.070. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Fronteirs in Neuroendocrinology. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Henke K, Aerno A, Treyer V, McGaugh JL, Berthold T, Nitsch RM, Buck A, Roozendaal B, Hock C. Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. European Journal of Neuroscience. 2003;17:1296–302. doi: 10.1046/j.1460-9568.2003.02542.x. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nature Neuroscience. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: Construct validation of the California Verbal Learning Test. Journal of Consulting and Clinical Psychology. 1988;56:123–130. doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Obar BA. CVLT: California Verbal Learning Test. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacology, Biochemistry, and Behavior. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. The Journal of Neuroscience. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein E, Tillman HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones – a focus on rapid, nongenomic effects. Pharmacological Reviews. 2000;52:513–556. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams J. Structured clinical interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0) New York State Psychiatric Institute: Biometrics Research Department; 1995. [Google Scholar]
- Friedman A, Pines A. Sex differences in gender-related childhood memories. Sex Roles. 1991;25:25–32. [Google Scholar]
- Gibbons JL, McHugh PR. Plasma cortisol in depressive illness. Journal of Psychiatric Research. 1962;1:162–171. doi: 10.1016/0022-3956(62)90006-7. [DOI] [PubMed] [Google Scholar]
- Herbert J, Goodyer IM, Grossman AB, Hastings MH, de Kloet ER, Lightman SL, Lupien SJ, Roozendaal B, Seckl JR. Do corticosteroids damage the brain? Journal of Neuroendocrinology. 2006;18:393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Garside MJ, Massey AE, McAllister-Williams RH. Effects of a single dose of cortisol on the neural correlates of episodic memory and error processing in healthy volunteers. Psychopharmacology. 2003;167:431–442. doi: 10.1007/s00213-003-1413-2. [DOI] [PubMed] [Google Scholar]
- Kavushansky A, Richter-Levin G. Effects of stress and corticosterone on activity and plasticity in the amygdala. Journal of Neuroscience Research. 2006;84:1580–1587. doi: 10.1002/jnr.21058. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress response in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kuhlman S, Kirschbaum C, Wolf OT. Effects of oral cortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiology of Learning and Memory. 2005;83:158–162. doi: 10.1016/j.nlm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Lesniewska B, Miskowiak B, Nowak M, Malendowicz LK. Sex differences in adrenocortical structure and function. XXVII. The effect of either stress on ACTH and cortisterone in intact, gonadectomized, and testosterone- or estradiol-replaced rats. Research in Experimental Medicine. 1990;190:95–103. doi: 10.1007/pl00020011. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gillin CJ, Hauger RL. Acute effects of corticosteroids: A dose-response study in humans. Behavioral Neuroscience. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen B. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Research Reviews. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Makara GB, Haller J. Non-genomic effects of glucocorticoids in the neural system: evidence, mechanisms and implications. Progress in Neurobiology. 2001;65:367–390. doi: 10.1016/s0301-0082(01)00012-0. [DOI] [PubMed] [Google Scholar]
- Maxwell ME. Family Interview for Genetic Studies (FIGS): manual for FIGS. National Institute of Mental Healthy, Bethesda, MD: Clinical Neurogenetics Branch, Intramural Research Program; 1992. [Google Scholar]
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid-induced impairments in declarative memory performance in adult humans. Journal of Neuroscience. 1994;14:2047–2053. doi: 10.1523/JNEUROSCI.14-04-02047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Melson A, Hershey T, Craft S, Richards K, Alderson M. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Archives of General Psychiatry. 1999;56:527–33. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- Parker L, Eugene J, Farber D, Lifrak E, Lai M, Juler G. Dissociation of adrenal androgen and cortisol levels in acute stress. Hormone and Metabolic Research. 1985;17:209–212. doi: 10.1055/s-2007-1013493. [DOI] [PubMed] [Google Scholar]
- Putman P, Hermans EJ, van Honk J. Cortisol administration acutely reduces threat-selective spatial attention in healthy young men. Physiology and Behavior. 2010;99:294–300. doi: 10.1016/j.physbeh.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Progress in neuro-psychopharmacology and biological psychiatry. 2003;27:1213–1223. doi: 10.1016/j.pnpbp.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiology of Learning and Memory. 1997;67:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- Schlosser N, Wolf OT, Fernanco SC, Riedesel K, Otte C, Muhtz C, Beblo T, Driessen M, Lowe B, Wingfeld K. Effects of acute cortisol administration on autobiographical memory in patients with major depression and healthy controls. Psychoneuroendocrinology. 2010;35:316–320. doi: 10.1016/j.psyneuen.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Smeets T, Giesbrecht T, Jelicic M, Merckelbach H. Context-dependent enhancement of declarative memory performance following acute psychosocial stress. Biological Psychiatry. 2007;76:116–123. doi: 10.1016/j.biopsycho.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, Schienle A, Vaitl D. Influence of the stress hormone cortisol on fear conditioning in humans: Evidence for sex differences in the response of the prefrontal cortex. Neuroimage. 2006;32:1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Tollenaar M, Elzinga B, Spinhoven P, Everaerd W. Autobiographical memory after acute stress in healthy young men. Memory. 2009;17:301–310. doi: 10.1080/09658210802665845. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- van Vreeswijk MF, de Wilde EJ. Autobiographical memory specificity, psychopathology, depressed mood and the use of the Autobiographical Memory Test: a meta-analysis. Behaviour Research and Therapy. 2004;42:731–743. doi: 10.1016/S0005-7967(03)00194-3. [DOI] [PubMed] [Google Scholar]
- Williams JM, Barnhofer T, Crane C, Hermans D, Raes F, Watkins E, Dalgleish T. Autobiographical memory specificity and emotional disorder. Psychological Bulletin. 2007;133:122–148. doi: 10.1037/0033-2909.133.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Broadbent K. Autobiographical memory in attempted suicide patients. Journal of Abnormal Psychology. 1986;95:144–149. doi: 10.1037//0021-843x.95.2.144. [DOI] [PubMed] [Google Scholar]
- Williams JM, Scott J. Autobiographical memory in depression. Psychological Medicine. 1988;18:689–695. doi: 10.1017/s0033291700008370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


