Abstract
Background and Objectives
A noninvasive approach to vasectomy may eliminate male fear of complications related to surgery (e.g. hematoma, infection, acute and chronic pain, sterilization failure) and increase its acceptance. Noninvasive laser thermal occlusion of the canine vas deferens has recently been reported. In this study, high-frequency ultrasound is used to confirm successful laser thermal coagulation and scarring of the vas in a short-term canine model.
Materials and Methods
Bilateral noninvasive laser coagulation of the vas was performed in a total of 9 dogs using a laser wavelength of 1075 nm, incident power of 9.0 W, pulse duration of 500 ms, pulse rate of 0.5 Hz, and 3-mm-diameter spot. Cryogen spray was used to cool the scrotal skin surface and prevent burns during the procedure. A clinical ultrasound system with a 13.2-MHz high-frequency transducer was used to image the vas before after the procedure. Burst pressure measurements were performed on excised vasa to confirm thermal occlusion.
Results
Day 0 and 28 burst pressures averaged 291 ± 31 mmHg and 297 ± 26 mmHg, respectively, significantly greater than ejaculation pressures of 136 ± 29 mmHg. Ultrasound showed a hyperechoic vas segment after thermal coagulation (Day 0) and scarring (Day 28). Doppler ultrasound showed normal blood flow through the testicular artery, indicating no collateral thermal damage to proximal structures.
Conclusions
High-frequency ultrasound may be used as a noninvasive diagnostic tool to assist in determining successful short-term laser thermal coagulation and scarring of the vas.
Keywords: coagulation, laser, male sterilization, noninvasive, ultrasound, vasectomy
INTRODUCTION
Approximately 500,000 vasectomies are performed in the United States per year, making it the most common urological procedure in the U.S. [1]. Although vasectomy is more effective and less likely to have complications than tubal ligation, the number of men undergoing surgical sterilization is approximately three times less than women [2–4]. Fear of complications related to surgical vasectomy (e.g. hematoma, infection, acute and chronic pain, and sterilization failure) is a major factor in a couples’ choice of surgical sterilization [5,6]. A noninvasive technique for male sterilization may improve acceptance of vasectomy.
The experimental use of therapeutic focused ultrasound for noninvasive vasectomy in a canine model has been previously reported [7,8]. However, complications included inconsistent vas occlusion and scrotal skin burns.
As a more promising alternative, our laboratory is currently developing a noninvasive vasectomy technique utilizing near-infrared laser irradiation in conjunction with cryogen spray cooling of the scrotal skin surface for thermal occlusion of the vas. A laser-based approach offers several advantages compared to therapeutic focused ultrasound. First, the laser energy is delivered to the tissue in non-contact mode without the need for a coupling medium. This technique allows a conventional vasectomy approach to be taken for separating and isolating the vas under the skin prior to vasectomy using a standard vasectomy ring clamp. It also preserves the surgeon’s field-of-view so the skin surface can be visually monitored during the procedure, and the formation of scrotal skin burns can be prevented. During previous preliminary noninvasive laser vasectomy studies, we reported consistent thermal occlusion and scarring of the vas while minimizing scrotal skin burns in ex vivo and in vivo canine models [9–11].
High-frequency diagnostic ultrasound has recently been used to measure the anatomy of the normal vas deferens [12]. In this study, high-frequency ultrasound is introduced as a noninvasive diagnostic method for confirming successful thermal occlusion and scarring of the canine vas after noninvasive laser vasectomy. This study also builds upon previous acute studies, by demonstrating, for the first time, successful noninvasive laser coagulation and occlusion of the canine vas in a short-term, in vivo model without any adverse complications such as scrotal skin burns.
METHODS
Animal Studies
All procedures were conducted under an animal protocol approved by the Johns Hopkins Animal Review Committee. Noninvasive thermal occlusion of the vas was performed bilaterally in a total of 9 dogs. Vasa were harvested and used for burst pressure measurements at Day 0 (3 dogs) and Day 28 (6 dogs). The animals were monitored on a daily basis for signs of distress during the study and then neutered and adopted out to caring homes at the completion of the study.
Laser Parameters
A compact, tabletop, 50-Watt Ytterbium fiber laser (Model TLR1075-50, IPG Photonics, Oxford, MA) emitted near-infrared radiation with a wavelength of 1075 nm which was focused with a lens into a 400-µm-core fiber optic patch-cord. Another lens at the end of the patch-cord delivered a collimated laser beam to the tissue surface. The laser radiation was delivered in long-pulse mode, with average power of 9.0 W, 500-ms pulse duration, 0.5 Hz pulse rate, and 3-mm-diameter spot at the scrotal skin surface.
Cooling Parameters
A Dynamic Cooling Device (DCD, Candela Laser Corporation, Wayland, MA) was used to deliver cryogen (halocarbon 134a, 1,1,1,2-tetrafluoroethane, boiling point = − 26 °C) to the scrotal skin surface through a solenoid valve to prevent overheating and formation of skin burns during the procedure. Three cryogen pulses were applied to pre-cool the skin prior to laser irradiation. During irradiation, cryogen spray was delivered intermittently between laser pulses with a 60-ms pulse duration, pulse rate of 0.25 Hz, and a 2-cm-diameter spot concentric with the laser spot. A cryogen mask was used to thermally insulate surrounding scrotal skin from cryogen spray to avoid superficial freeze burns. Details of the experimental setup have been previously reported [11].
Ultrasound Parameters
A clinical ultrasound console (SSD-Alpha 7, Aloka, Wallingford, CT) with 13.2 MHz high-frequency linear array transducer (UST-5411, Aloka) was used to image the native vas at Day 0, the thermally coagulated vas at Day 0, and the scarred vas at Day 28. This transducer provided an axial image resolution of approximately 200 µm and an imaging depth of 2 cm. Simultaneous application of Doppler US at a frequency of 6.15 MHz helped to distinguish between the vas and spermatic cord. Doppler US was also used to indicate whether there was normal blood flow through the testicular artery after the procedure and confirm the absence of collateral thermal damage to proximal tissue structures.
Indicators of Vas Occlusion
Burst pressure measurements were performed to quantify the degree of vas closure at Days 0 and 28. A 3-way stopcock was connected to a 50 mL syringe (filled with saline), pressure transducer, and hypodermic needle. The vas was attached to a 27G hypodermic needle and clamped with hemostats. A pressure analyzer unit was calibrated. Then saline from the syringe was slowly pumped into the vas lumen using a syringe pump at a rate of 0.01 ml/s, elevating the vas pressure until it burst open. Details of this experimental setup have been previously reported [11]. The burst pressure measurements were recorded as a mean ± standard deviation for each group of acute and chronic studies.
RESULTS
Day 0 burst pressures (291 ± 31 mmHg) for the thermally coagulated vas did not differ significantly from Day 28 burst pressures (297 ± 26 mmHg) for the scarred vas. However, both of these vas burst pressures were significantly higher than burst pressures that the canine vas typically experiences during ejaculation (136 ± 29 mmHg) [13], thus demonstrating strong vas occlusion.
High-frequency ultrasound images of the vas were taken at Day 0 before and after the procedure to confirm successful thermal coagulation of the vas, and again at Day 28 to identify the scarred region of the vas (Figure 1). These images showed an increased, hyperechoic signal and a disruption of the vas structure in the laser-treated region. The sonographic length of the coagulated vast at Day 0 measured 4.1 ± 0.7 mm, and the length of the scarred vas measured 3.7 ± 0.5 mm. These measurements roughly correspond to the 3-mm-diameter laser spot used, with a slightly larger increase in coagulated vas length due to both light scattering and thermal diffusion that occurs in the tissue during the procedure.
Figure 1.
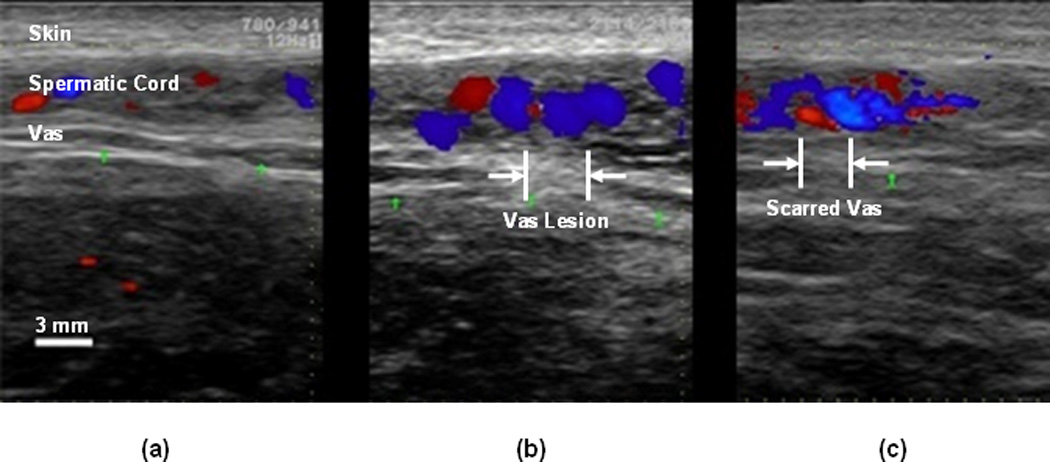
Representative high-frequency ultrasound images of the canine vas deferens: (a) At Day 0 before the procedure; (b) At Day 0 immediately after the procedure; and (c) At Day 28 at the completion of the study. Note the disruption of the vas deferens structure in the region that was targeted by the laser. The blue and red colors represent Doppler imaging of blood flow through the testicular artery in the spermatic cord.
The thermally coagulated and scarred vas was excised for examination to verify the ultrasound images (Figure 2). The thermally coagulated vas segment was identified by several indicators including blanching and shrinkage of the vas wall. The scarred vas, although less distinct, also showed discoloration and shrinkage in the treated area. Both the thermally coagulated and scarred vas segments tended to bulge, indicative of blockage at the treatment site, during burst pressure studies.
Figure 2.
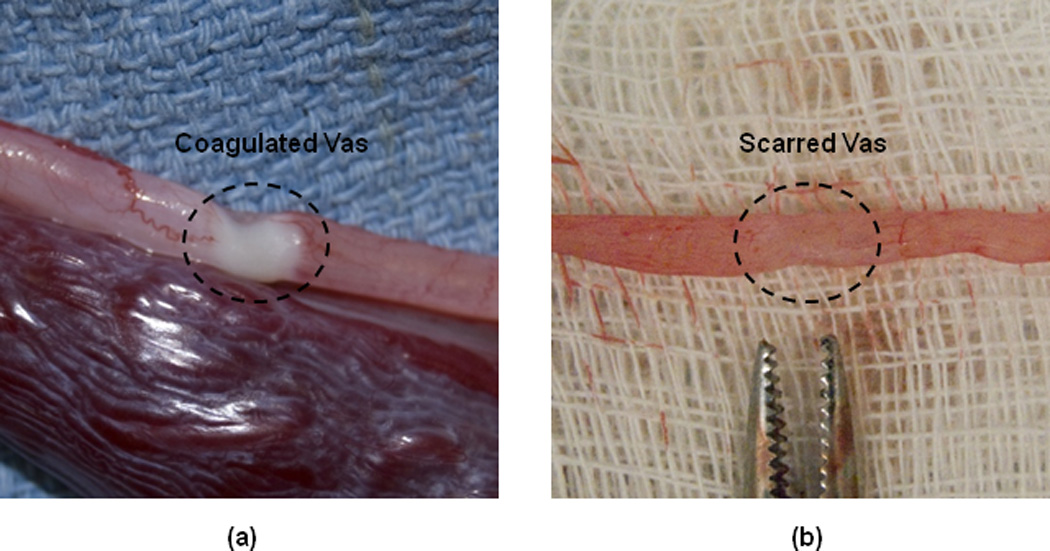
Representative images of the excised canine vas: (a) At Day 0 immediately after the procedure, showing the thermally coagulated zone; and (b) At Day 28, showing the scarred region of the vas.
While our previous noninvasive laser vasectomy studies have noted the formation of minor scrotal skin burns with the use of higher laser powers [10], there was no evidence of skin burns in this study. Figure 3 shows the scrotal skin surface at Day 0 before and after the procedure and at Day 28. Only temporary skin irritation and reddening was observed due to the blunt trauma of the vasectomy ring clamp tips on the skin. Such trauma caused by the vas ring clamp is normal for the conventional vasectomy technique as well. This irritation disappeared approximately 15 minutes after the clamp was removed and the procedure completed. One dog experienced some melena and diarrhea, unrelated to the laser procedure, which was successfully treated with medication. Otherwise, the dogs were monitored on a daily basis and showed no visual signs of skin damage, or discomfort with the treatment site in the scrotal region.
Figure 3.
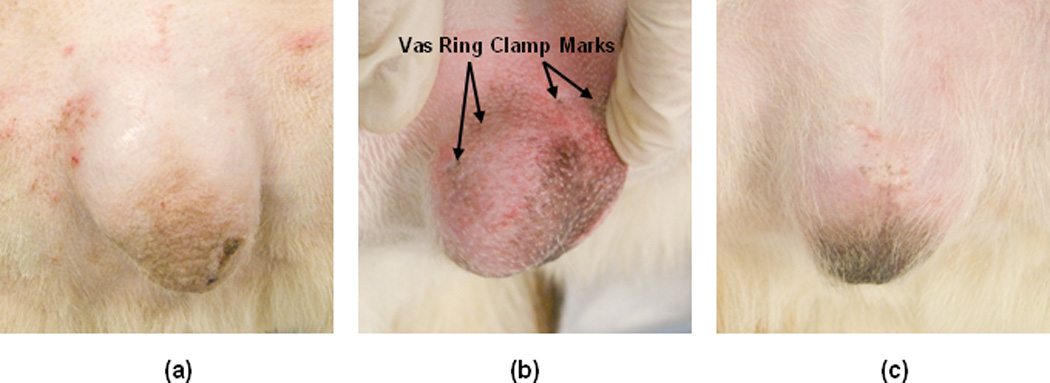
Representative images of the canine scrotal skin surface: (a) At Day 0 before the procedure; (b) At Day 0 immediately after the procedure; and (c) At Day 28 at the completion of the study. Note that much of the skin irritation observed immediately after the procedure is due to the vasectomy ring clamp used to isolate the vas underneath the skin. This irritation is temporary and disappears soon after the procedure.
DISCUSSION
Vasectomy is a safe, simple, effective, and inexpensive surgical procedure for male sterilization. However, despite the low morbidity of this procedure, societal pressures, psychological factors (such as the perception of the loss of “manhood’), and male fear of surgery are reasons frequently cited by couples choosing other forms of contraception. The development of a noninvasive laser vasectomy technique may eliminate many of these concerns and increase male acceptance of vasectomy. During previous studies, we have reported successful laser thermal occlusion and scarring of the vas while minimizing scrotal skin injury in ex vivo and in vivo canine models [9–11].
The results of this study demonstrate that the canine vas becomes scarred and remains occluded, confirmed by the burst pressure measurements, in a short-term study. High-frequency ultrasound served as a useful noninvasive diagnostic tool for qualitatively confirming successful thermal occlusion and scarring of the vas, indicated by a hyperechoic signal and disrupted vas structure in the laser treated region.
It may be possible to further improve the ultrasound resolution (at the expense of shallower imaging depth) by using a higher frequency ultrasound transducer than the 13.3 MHz transducer used in this study. However, this transducer was chosen initially because it is the highest frequency transducer used with a standard clinical ultrasound system commonly available to physicians.
While the use of burst pressure measurements is not the definitive indicator of a successful vasectomy, it does provide a reliable quantitative source of feedback to determine the degree of vas closure with comparison to typical vas ejaculation pressures. Our results show that the vas burst pressures after both thermal coagulation at Day 0 and scarring at Day 28 were over twice that of previously reported canine vas ejaculation pressures, thus demonstrating a robust, short-term closure of the vas in this canine model. However, once the noninvasive laser vasectomy system and treatment parameters have been fully optimized, it will be necessary to perform more definitive and longer term chronic canine studies. These studies will include examining recanalization and azospermia rates, with comparison of surgical vasectomy and noninvasive laser vasectomy, prior to clinical studies.
It should also be noted that our 100% success rate for thermal occlusion and scarring of the vas, with no adverse complications, is promising, considering that the canine model is a more difficult model than the human for vasectomy in several respects. First, the canine vas is more difficult to isolate with the ring clamp than the human vas, making successful laser targeting more challenging. Second, canine scrotal skin appears to be significantly thicker than human scrotal skin, making it more difficult for the laser radiation to penetrate the skin and reach the vas without creating skin burns. Third, the canine scrotal skin also contains more hair follicles which may serve as absorption and burn sites for the laser radiation. Some of these current challenges may not be present when this technique is translated into clinical studies.
Finally, although this was not intended to be a cost comparison study, it is worth considering some of the costs involved with noninvasive laser vasectomy versus conventional surgical vasectomy. It is well known that the cost of a conventional surgical vasectomy is relatively low (approximately $500–1000 per procedure). For noninvasive laser vasectomy, there are significant capital costs associated with the purchase of the laser and cryogen systems, although it should be noted that laser rentals are a viable and popular option for laser surgical procedures. The ultrasound system, if used in a clinical setting, is portable and readily available for shared use, and therefore may not add significant cost to the procedure. The cost of the cryogen spray used for cooling the scrotal skin during the procedure was calculated to be almost negligible (adding less than $2 per procedure). Additionally, some supplies currently used during surgical vasectomy (e.g. surgical tray, clips, sutures,..etc) would not be needed for noninvasive laser vasectomy. It is also possible that a patient would be willing to pay a slightly higher fee associated with a noninvasive vasectomy compared to surgical vasectomy.
CONCLUSIONS
High-frequency ultrasound may be used as a diagnostic tool to assist in determining successful laser thermal coagulation and scarring of the vas during noninvasive laser vasectomy.
ACKNOWLEDGMENTS
This project is supported by a research grant from the NIH, National Institute of Child Health and Human Development, and the United States Agency for International Development, through a subcontract from Family Health International (Durham, NC). The authors thank Dawn Ruben and Laurie Pipitone for assisting with the animal studies, James Hsia of the Candela Corporation (Wayland, MA) for providing the cryogen cooling system, and John Walsh and Lyn Pugh of Aloka (Wallingford, CT) for providing the ultrasound system and probes.
REFERENCES
- 1.Barone MA, Hutchinson PL, Johnson CH, Hsia J, Wheeler J. Vasectomy in the United States, 2002. J Urol. 2006;176:232–236. doi: 10.1016/S0022-5347(06)00507-6. [DOI] [PubMed] [Google Scholar]
- 2.Mosher WD, Martinez GM, Chandra A, Abma JC, Wilson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data. 2004;350:1–36. [PubMed] [Google Scholar]
- 3.Chandra A, Martinez GM, Mosher WD, Abma JC, Jones C. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005;25:1–160. [PubMed] [Google Scholar]
- 4.Martinez GM, Chandra A, Abma JC, Jones J, Mosher WD. Fertility, contraception, and fatherhood: data on men and women from cycle 6 (2002) of the 2002 National Survey of Family Growth. Vital Health Stat. 2006;26:1–142. [PubMed] [Google Scholar]
- 5.Miller WB, Shain RN, Pasta DJ. Tubal sterilization or vasectomy: how do married couples make the choice. Fertil Steril. 1991;56:278–284. doi: 10.1016/s0015-0282(16)54485-9. [DOI] [PubMed] [Google Scholar]
- 6.Shain RN, Miller WB, Holden AE. Factors associated with married women’s selection of tubal sterilization and vasectomy. Fertil Steril. 1985;43:234–244. [PubMed] [Google Scholar]
- 7.Fried NM, Sinelnikov YD, Pant B, Roberts WW, Solomon SB. Noninvasive vasectomy using a focused ultrasound clip: Thermal measurements and simulations. IEEE Trans Biomed Eng. 2001;48:1453–1459. doi: 10.1109/10.966604. [DOI] [PubMed] [Google Scholar]
- 8.Roberts WW, Chan DY, Fried NM, Wright EJ, Nicol T, Jarrett TW, Kavoussi LR, Solomon SB. High intensity focused ultrasound ablation of the vas deferens in a canine model. J Urol. 2002;167:2613–2617. [PubMed] [Google Scholar]
- 9.Cilip CM, Jarow JP, Fried NM. Noninvasive laser vasectomy: preliminary ex vivo tissue studies. Lasers Surg Med. 2009;41:203–207. doi: 10.1002/lsm.20744. [DOI] [PubMed] [Google Scholar]
- 10.Cilip CM, Ross AE, Jarow JP, Fried NM. Noninvasive laser coagulation of the canine vas deferens, in vivo. Proc SPIE. 2010;7548:75481D:1–75481D:5. doi: 10.1117/1.3463009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cilip CM, Ross AE, Jarow JP, Fried NM. Application of an optical clearing agent during noninvasive laser coagulation of the canine vas deferens. J Biomed Opt. 2010;15:055012. doi: 10.1117/1.3463009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Middleton WD, Dahiya N, Naughton CK, Teefey SA, Siegel CA. High-resolution sonography of the normal extrapelvic vas deferens. J Ultrasound Med. 2009;28:839–846. doi: 10.7863/jum.2009.28.7.839. [DOI] [PubMed] [Google Scholar]
- 13.Shafik A. Electrovasogram: a canine study of the electromechanical activity of the vas deferens. Urology. 1995;46:692–696. doi: 10.1016/S0090-4295(99)80303-3. [DOI] [PubMed] [Google Scholar]


