Abstract
Context
The health and policy implications of regional variation in incidence and outcome of out-of-hospital cardiac arrest remain to be determined. The Resuscitation Outcomes Consortium conducts clinical research to improve outcomes after out-of-hospital cardiac arrest in 11 North American sites.
Objectives
To evaluate whether cardiac arrest incidence and outcome differ across geographic regions.
Design, Setting and Patients
Prospective multi-center observational study of all out-of-hospital cardiac arrests from May 1, 2006 to April 30, 2007 followed to hospital discharge. Cases were assessed by organized emergency medical services (EMS) personnel, did not have traumatic injury, and received attempts at external defibrillation, or chest compressions; or resuscitation was not attempted. Included data were available as of June 28, 2008. Excluded was one site with incomplete case capture. Census data were used to determine rates adjusted for age and gender.
Outcome Measures
Incidence rate, mortality rate, case fatality rate and survival to discharge for patients assessed by EMS, treated by EMS or with an initial rhythm of ventricular fibrillation.
Results
The total catchment population was 21.4 million. There were 20,520 arrests in 10 sites. Patients were aged 0-108 years. 61% were men. 11,898 (58.0% of total) had resuscitation attempted. 2,729 (22.9% of treated) had initial rhythm of ventricular fibrillation, ventricular tachycardia or were shockable by an automated external defibrillator. 954 (4.6%) were discharged alive. EMS-treated cardiac arrest per 100,000 population across sites was median 52.1 (interquartile range (IQR) 48.0, 70.1); survival ranged from 3.0% to 16.2%, median 8.5% (IQR 5.2%, 10.2%). Ventricular fibrillation incidence was median 12.6 (IQR 10.6, 15.2); survival ranged from 7.7% to 39.4%, median 21.8% (IQR 14.8%, 25.1%). All p-values for differences across sites were < 0.001.
Conclusions
We observed significant and important regional differences in out-of-hospital cardiac arrest incidence and outcome, which require additional investigation to improve public health.
It remains to be determined how often out-of-hospital cardiac arrest (OHCA) occurs. Recent sources state about 166,000 to 310,000 Americans per year have an OHCA.(1) However resuscitation is not attempted in many of these. The reported incidence of OHCA varies from 17 to 128.6 per 100,000 person years.(2) Reported survival to discharge after the onset of OHCA varies from 0% to 21%.(3, 4)
Accurate estimation of the burden of OHCA is essential to evaluate progress towards improving public health by reducing cardiovascular disease. Clinical trials often exclude patients at higher risk of poor outcomes, so estimation of the burden of illness based only on those enrolled in trials is subject to bias. Knowledge of regional variation in outcomes after cardiac arrest could guide identification of effective interventions that are used in some communities but have not been implemented in others. Potential interventions include culturally-appropriate public health initiatives, community support, and equitable access to high-quality prehospital emergency care.
The Resuscitation Outcomes Consortium (ROC) is a clinical research network conducting clinical research in the areas of cardiopulmonary arrest and severe traumatic injury. This network consists of 11 sites and one central coordinating center. The Resuscitation Outcomes Consortium (ROC) was established to evaluate the treatment of people with life-threatening injury or out-of-hospital cardiac arrest and conduct clinical trials of promising scientific and clinical advances so as to improve resuscitation outcomes. A registry (ROC Epistry-Cardiac Arrest) was created by this consortium including all cardiac arrests assessed or treated by emergency medical services (EMS) personnel in the participating geographic regions. We hypothesized that there would be significant regional variation in the incidence and outcome of OHCA.
METHODS
Design and Setting
The ROC Epistry-Cardiac Arrest is a prospective multicenter observational registry of OHCA in EMS agencies and receiving institutions in eight US sites and three Canadian sites.(5) These sites are participants in the ROC clinical research network. One site that self-reported incomplete case capture (San Diego, CA) was excluded from all analyses.
Population
The population of interest was all OHCA cases that occurred within the catchment area of a participating EMS agency, including infants, children and adults. The census tract of the location of the case was identified and recorded in order to assess the catchment population served by the agency using census data. Subgroups of the cohort included all EMS-assessed OHCA, EMS-treated OHCA, and cardiac arrests with an initial rhythm of ventricular fibrillation.
Inclusion and Exclusion Criteria
Included were cases of cardiac arrest that occurred outside the hospital, were evaluated by EMS personnel and: a) received attempts at external defibrillation (by lay responders or emergency personnel), or chest compressions by organized EMS personnel; or b) were pulseless but did not receive attempts to defibrillate or CPR by EMS personnel. This latter group included patients with a do not attempt resuscitation directive signed and dated by a physician, extensive history of terminal illness or intractable disease, or request from the patient's family. Excluded were cases that had traumatic injury.
Key Covariates
Arrests were classified as “obvious” cause when the circumstances and evidence clearly supported such an etiology (i.e., arrest in a patient with a known toxic ingestion). Etiology was classified as “no obvious cause” for arrests where the cause was uncertain or where there was evidence of a primary cardiac etiology.
Some patients were initially treated with a manual defibrillator capable of recording the patient's initial rhythm. Others were initially treated with an automated external defibrillator with a built-in computer algorithm capable of classifying the patient's initial rhythm as resembling ventricular fibrillation (i.e. shockable) or not (i.e. not shockable). Therefore initial rhythm was categorized as ventricular fibrillation, ventricular tachycardia pulseless electrical activity, asystole, shockable or not shockable. For the purpose of this analysis, ventricular fibrillation, ventricular tachycardia or shockable rhythms were grouped together.
Data Management and Quality Assurance
Each site used multiple strategies to identify consecutive OHCA cases. Examples of case identification strategies included telephone notification of each incident of defibrillator use or CPR by EMS providers, regular hand sorting through paper EMS charts, or electronic queries of EMS records by a variety of data fields; i.e., dispatch call type, vital signs, diagnosis, or a combination of these fields.
Data were abstracted from EMS and hospital records using standardized definitions for patient characteristics, EMS process and outcome at hospital discharge. Data were abstracted locally, coded without personal health information, and transmitted to the data coordinating center by web entry of individual cases or batch upload of multiple cases grouped together. Site-specific quality assurance included initial and continuing education of EMS providers in data collection. The data coordinating center assured the quality of the data by a) using range and logic checks in both the web-based data entry forms and the batch upload process, b) systematic review of data to uncover inconsistencies, c) review of randomly selected records to confirm accuracy of data entry, and d) annual site visits.
Outcome Measures
The annual incidence was calculated per 100,000 population for the 12 month period of May 1, 2006 to April 30, 2007. The incidence rate in persons of any age was adjusted for age and sex to those of the 2000 census for the United States and 2001 census for Canada. The mortality rate was calculated as the number of known deaths per population using similar methods. The case fatality rate was calculated as the number of known deaths divided by the total number of cases including those with missing final status. The survival rate was calculated as the number of known survivors divided by the total number of cases, including those with missing final vital status. Note that case fatality rate and survival rate would only sum to 100% if final vital status were known for all patients.
Survival to discharge was defined as discharge alive from hospital after the index OHCA. Patients who were transferred to another acute care facility (e.g. to undergo implantable cardioverter defibrillator placement) were considered to be still hospitalized. Patients transferred to a non-acute ward or facility were considered discharged.
Statistical Analysis
We were aware before study implementation that the use of the prehospital emergency care record to abstract data for inclusion in the study databases could be associated with incomplete data due to the need for rapid treatment in the field and consequent lack of time for EMS providers to complete the record. A common approach to accounting for such unobserved data is to use multivariate analysis to describe observed outcomes as a function of covariates based on cases with complete data. Then outcomes are estimated for cases with incomplete data. However, this method underestimates uncertainty.(6) Instead missing cases were accounted for by using multiple imputation methods.(7-9) Estimated expected cases for agency by month were determined by averaging observed cases in an agency by month based on Mar 2006 – Feb 2007 data. We assumed an agency was missing cases if the observed rate was “much” less than expected average (p value < 0.005), especially at the start of the enrollment period and at the end of the reporting year. A Poisson regression model was used within each site to estimate the expected incidence m for each month with underreported episodes for each agency. For each of 10 imputed datasets, a random draw from a Poisson distribution with mean m was used to impute the number of missing cases. For each such missing case, covariate values were then obtained through hot deck imputation using valid cases from months with good data at the corresponding agency.(9)
The baseline characteristics of EMS systems and the EMS performance on cases were summarized by using categorical and parametric or non-parametric descriptors as appropriate. These were reported by site. Imputation was performed by using Excel (Microsoft Corporation, Redmond, WA). Statistical analyses were performed by using S-PLUS 6.2 (Insightful Corporation, Seattle, WA). Differences in rates between sites were assessed with χ2 tests.
Dr. Nichol had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analyses. The funding organization had no role in the collection, management, analysis and interpretation of the data or the preparation of the manuscript. A representative of the National Institutes of Health reviewed and approved the manuscript.
Human Subjects
The study was approved under waiver of documented written consent under minimal risk criteria by 74 U.S. institutional review boards and 34 Canadian research ethics boards as well as 26 EMS institutional review boards. In addition, approval in the form of a memorandum of understanding was obtained from 24 hospitals and from 94 EMS systems.
RESULTS
Ten sites were included (Table 1). Some of these sites include the entire named region (e.g. Dallas) whereas others include several municipalities with the region (e.g. Alabama). These had a median catchment population of 1,709,049 (interquartile range (IQR) 958,960, 2,581,569). Median population density was 698 (IQR 405, 1,596) individuals per square mile. 211 of 225 EMS agencies participating in the Consortium transported patients included in this analysis to 227 of 268 receiving hospitals in the sites’ catchment area. These included a mix of fire-based and non-fire-based governmental and private agencies that provided basic or advanced life support and did or did not provide patient transport.
Table 1.
Characteristics of Included Sites
| Site | Service Area Population | Population density (residents/ sq mile) | Number of EMS agencies | Number of Hospitals |
|---|---|---|---|---|
| Alabama | 644,701 | 485 | 13 | 14 |
| Dallas, TX | 1,989,357 | 3,173 | 11 | 22 |
| Iowa | 1,015,347 | 388 | 19 | 19 |
| Milwaukee, WI | 940,164 | 3,885 | 16 | 16 |
| Ottawa, ON | 4,030,696 | 314 | 39 | 37 |
| Pittsburgh, PA | 935,967 | 396 | 6 | 38 |
| Portland, OR | 1,751,119 | 431 | 15 | 16 |
| Seattle, WA | 1,666,978 | 1,573 | 35 | 18 |
| Toronto, ON | 5,627,021 | 911 | 32 | 55 |
| Vancouver, BC |
2,779,373 |
1,604 |
39 |
33 |
| Total | 21,398,723 | 640 | 225 | 268 |
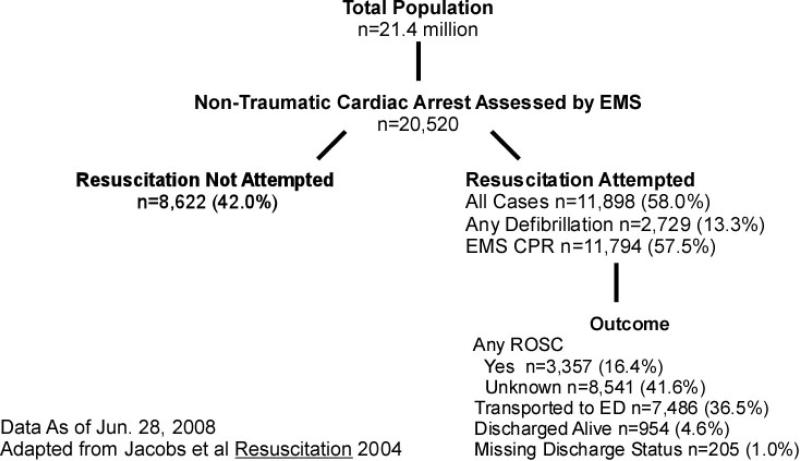
The total catchment population was 21.4 million (Figure 1). There were 20,520 cases of OHCA assessed by EMS. Of these, 19,920 (97.1% of total) were observed and 600 (2.9%) were imputed. These imputed cases were distributed among 12 agencies (5.7% of total) at 7 sites. Resuscitation was attempted in 11,898 (58.0% of total) of cases. 954 (4.6% of total) were known discharged alive.
Figure 1.
Patient Flow Based on Utstein Template for Out-of-Hospital Cardiac Arrest
The patient and EMS characteristics of all cases are shown in Table 2. EMS-assessed OHCA patients had a median age of 67 years (IQR 53, 80) (Table 2). 12,631 (61.6%) were men. 2,271 (11.1%) of these occurred in public locations. 2,730 (13.3%) had a first recorded rhythm of ventricular fibrillation, and 943 (4.6%) had missing or undetermined as first recorded rhythm. Among EMS-treated OHCA, 1,848 (15.5%) occurred in public locations. 4,410 (37.0%) were witnessed by bystanders, 1,074 (9.0%) by EMS personnel, 5,407 (45.4%) were unwitnessed and 1,007 (8.5%) had unknown witnessed status. 2,729 (22.9%) had a first-recorded rhythm of ventricular fibrillation; and 3,739 (31.4%) received bystander CPR. Time from call to arrival of first advanced life support was median 7:24 (IQR 5:13,10:43) minutes. Arrests with a first-recorded rhythm of ventricular fibrillation, ventricular tachycardia or reported shockable by AED were similar to EMS-treated arrests except that 29.2% occurred in public locations (p-value <0.001 compared to 15.5% of EMS-treated arrests).
Table 2.
Patient and EMS Characteristics of All Episodes
|
EMS-Assessed Cardiac Arrest N=20,520 |
EMS-Treated Cardiac Arrest N=11,898 |
Initial Rhythm VT/VF or Reported Shockable by AED N=2,729 |
Witnessed Initial Rhythm VT/VF N=1,850 |
EMS-Assessed but Not Treated N=8,622 |
|
|---|---|---|---|---|---|
| Age, Median (IQR) | 67 (53, 80) | 67 (53, 79) | 64.7 (54, 76) | 64.6 (54,76) | 68 (53, 81) |
| Unknown | 449 (2%) | 92 (1%) | 26 (1%) | 13 (1%) | 357 (4%) |
| Gender | |||||
| Male | 12,631 (61%) | 7,550 (64%) | 2,073 (76%) | 1,420 (77%) | 5,081 (59%) |
| Unknown | 183 (0.8%) | 27 (0.2%) | 5 (0.2%) | 4 (0.2%) | 156 (1.8%) |
| Location of arrest | |||||
| Public | 2,271 (11%) | 1,848 (16%) | 798 (29%) | 575 (31%) | 393 (5%) |
| Health Care | 274 (1%) | 231 (2%) | 46 (2%) | 40 (2%) | 43 (0%) |
| Home/Non-public | 17,455 (85%) | 9,773 (82%) | 1883 (69%) | 1,235 (67%) | 7,682 (89%) |
| Missing | 520 (3%) | 16 (0%) | 2 (0%) | 0 (0%) | 504 (6%) |
| First Recorded Rhythm | |||||
| VT/VF/Shockable | 2,730 (13%) | 2,729 (23%) | 2,729 (100%) | 1,850 (100%) | 1 (0%) |
| Not Shockable | 1,086 (5%) | 1,085 (9%) | n/a | n/a | 1 (0%) |
| Asystole | 4,793 (23%) | 4,792 (40%) | n/a | n/a | 1 (0%) |
| PEA | 2,350 (11%) | 2,349 (20%) | n/a | n/a | 1 (0%) |
| Not Determined | 551 (3%) | 549 (5%) | n/a | n/a | 2 (0%) |
| Unknown | 1,336 (7%) | 394 (3%) | n/a | n/a | 942 (11%) |
| No Analysis by EMS | 7,674 (37%) | n/a | n/a | n/a | 7,674 (89%) |
| Witnessed Status | |||||
| Bystander | 4,728 (23%) | 4,410 (37%) | 1,589 (58%) | 1,589 (86%) | 318 (4%) |
| EMS | 1,127 (5%) | 1,074 (9%) | 262 (10%) | 262 (14%) | 53 (1%) |
| Unwitnessed | 11,850 (58%) | 5,407 (45%) | 724 (26%) | n/a | 6443 (75%) |
| Missing | 2,815 (14%) | 1,007 (9%) | 155 (6%) | n/a | 1808 (21%) |
| Bystander CPR | |||||
| Performed | 3,989 (19%) | 3,739 (31%) | 1,091 (40%) | 827 (45%) | 249 (3%) |
| Missing | 3,910 (19%) | 1,289 (11%) | 222 (8%) | 262 (14%) | 2,620 (30%) |
| Time from call to first ALS arrival, median (IQR) | 7:00 (5:00,10:00) | 7:24 (5:13,10:43) | 7:15 (5:00,10:37) | 8:00 (5:31,11:42) | 6:11 (4:45, 8:37) |
| Missing | 3,995 (19%) | 897 (8%) | 195 (7%) | 141 (8%) | 3,098 (36%) |
| Time from call to first EMS rhythm analysis, median (IQR) | 9:31 (7:10,12:41) | 9:38 (7:18,12:49) | 8:58 (6:53,11:43) | 9:52 (7:34,12:55) | 8:04 (6:00,11:02) |
| Missing | 9,297 (45%) | 1,622 (14%) | 202 (7%) | 87 (5%) | 7,976 (90%) |
| Etiology of Arrest | |||||
| No Obvious Cause | 17,727 (86%) | 10,962 (92%) | 2,665 (98%) | 1,810 (98%) | 6,764 (78%) |
| Other Cause | 1,548 (8%) | 910 (8%) | 62 (2%) | 38 (2%) | 638 (7%) |
| Missing | 1,245 (6%) | 26 (0%) | 3 (0%) | 2 (0%) | 1,220 (14%) |
| 1st Arriving Rig Service Level | |||||
| BLS | 698 (3%) | 286 (2%) | 64 (2%) | 42 (2%) | 412 (5%) |
| BLS-D | 8,383 (41%) | 5,269 (44%) | 1,257 (46%) | 885 (48%) | 3,055 (35%) |
| BLS+ | 2,584 (13%) | 1,761 (15%) | 357 (13%) | 246 (13%) | 823 (10%) |
| ALS | 8,732 (43%) | 4,459 (37%) | 1,018 (37%) | 655 (36%) | 4,272 (50%) |
| Unknown | 183 (1%) | 123 (1% | 33 (1%) | 0 (1%) | 61 (1%) |
EMS-assessed OHCA incidence and outcome are described in Table 3. The Milwaukee site (801 treated cardiac arrests and 135 arrests with resuscitation not attempted) was excluded from this analysis because of self-reported incomplete data on patients in whom resuscitation was not attempted. There were 19,584 EMS-assessed OHCA during 20.5 million person-years of observation, resulting in an unadjusted incidence of EMS-assessed OHCA of 95.7 per 100,000 person years. The adjusted incidence per 100,000 census population ranged from 71.8 to 159.0 (median 96.8, IQR 77.5, 106.7). The adjusted mortality rate per 100,000 census population ranged from 68.9 to 153.2 (median 93.5, IQR 71.4, 103.3). The known case fatality rate ranged from 91.5% to 96.9% (median 96.0%, IQR 92.2%, 96.3%). The known survival to discharge ranged from 1.1% to 8.4% (median 3.5%, IQR 3.1%, 6.4%). The proportion of patients with vital status missing ranged from 0.1% to 2.0% (median 1.0%, IQR 0.4%, 1.4%). All p-values for differences across sites were < 0.001.
Table 3.
Incidence and Outcome of EMS-Assessed Out-of-Hospital Cardiac Arrest
| Site Identityd | Alabama N=715 |
Dallas N=2,462 |
Iowa N=1,028 |
Ottawa N=2,965 |
Pittsburgh N=1,217 |
Portland N=1,320 |
Seattle N=2,349 |
Toronto N=5,155 |
Vancouver N=2,373 |
Overall N=19,584 |
P Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted Incid. Rate, per 100,000 | 106.7 | 159.0 | 93.1 | 71.8 | 105.1 | 77.5 | 144.0 | 96.8 | 75.9 | 95.0 | <0.001 |
| Adjusted Mort. Rate, per 100,000 | 103.3 | 153.2 | 86.1 | 68.9 | 101.1 | 71.4 | 131.8 | 93.5 | 70.0 | 90.0 | <0.001 |
| Case Fatality Rate, % | 96.9 | 96.3 | 92.4 | 96.0 | 96.2 | 92.2 | 91.5 | 96.5 | 92.2 | 94.8 | <0.001 |
| Survival to Disch., % | 1.1 | 2.3 | 6.3 | 3.3 | 3.5 | 6.4 | 8.4 | 3.1 | 6.8 | 4.5 | <0.001 |
| Vital Status Missing, % | 2.0 | 1.4 | 1.2 | 0.7 | 0.3 | 1.4 | 0.1 | 0.4 | 1.0 | 0.8 | <0.001 |
Milwaukee was excluded from this analysis because of self-reported incomplete data on patients in whom resuscitation was not attempted.
EMS-treated OHCA is described in Table 4. The unadjusted incidence of EMS-treated OHCA was 55.6 per 100,000 person years. The adjusted incidence per 100,000 census population ranged from 40.3 to 86.7 (median 52.1, IQR 48.0, 70.1). The adjusted mortality rate per 100,000 census population ranged from 36.9 to 78.0 (median 47.0, IQR 42.8, 60.1). The known case fatality rate ranged from 83.6% to 94.1% (median 90.8%, IQR 87.7%, 92.9%).The known survival to discharge ranged from 3.0% to 16.2% (median 8.5%, IQR 5.2%, 10.2%). The proportion of patients with vital status missing ranged from 0.1% to 5.3% (median 1.3%, IQR 0.7%, 2.3%). All p-values for differences across sites were < 0.001.
Table 4.
Incidence and Outcome of EMS-Treated Out-of-Hospital Cardiac Arrest
| Site Identity | Alabama N=267 |
Dallas N=1,265 |
Iowa N=565 |
Milwaukee N=801 |
Ottawa N=1,836 |
Pittsburgh N=575 |
Portland N=793 |
Seattle N=1,170 |
Toronto N=2,992 |
Vancouver N=1,634 |
Overall N=11,898 |
P Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted Incid. Rate, per 100,000 | 40.3 | 82.9 | 51.3 | 86.7 | 45.1 | 51.1 | 47.0 | 74.4 | 57.0 | 52.8 | 56.0 | <0.001 |
| Adjusted Mort. Rate, per 100,000 | 36.9 | 77.2 | 44.4 | 78.0 | 42.3 | 47.1 | 41.0 | 62.3 | 53.6 | 46.9 | 50.9 | <0.001 |
| Case Fatality Rate, % | 91.7 | 93.2 | 86.5 | 90.0 | 93.6 | 92.2 | 87.3 | 83.6 | 94.1 | 88.8 | 90.9 | <0.001 |
| Survival to Disch., % | 3.0 | 4.2 | 11.3 | 9.9 | 5.3 | 7.1 | 10.4 | 16.2 | 5.2 | 9.8 | 7.9 | <0.001 |
| Vital Status Missing, % | 5.3 | 2.7 | 2.3 | 0.1 | 1.1 | 0.7 | 2.3 | 0.2 | 0.7 | 1.4 | 1.3 | <0.001 |
Ventricular fibrillation is described in Table 5. The unadjusted incidence of ventricular fibrillation was 12.8 per 100,000 person years. The adjusted incidence per 100,000 census population ranged from 9.3 to 19.0 (median 12.6, IQR 10.6, 15.2). The adjusted mortality rate per 100,000 census population ranged from 7.2 to 13.7 (median 10.1, IQR 8.9, 11.2). The known case fatality rate ranged from 60.2% to 89.2% (median 76.1%, IQR 72.3%, 83.4%). The known survival to discharge ranged from 7.7% to 39.4% (median 21.8%, IQR 14.8%, 25.1%). The proportion of patients with vital status missing ranged from 0% to 7.7% (median 2.2%, IQR 1.2%, 3.1%). All p-values for differences across sites were < 0.001.
Table 5.
Incidence and Outcome of Ventricular Fibrillation
| Site Identity | Alabama N=65 |
Dallas N=195 |
Iowa N=135 |
Milwaukee N=165 |
Ottawa N=429 |
Pittsburgh N=102 |
Portland N=249 |
Seattle N=297 |
Toronto N=614 |
Vancouver N=478 |
Overall N=2,729 |
P Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted Incid. Rate, per 100,000 | 9.9 | 12.8 | 12.4 | 18.7 | 10.4 | 9.3 | 15.1 | 19.0 | 11.4 | 15.2 | 12.8 | <0.001 |
| Adjusted Mort. Rate, per 100,000 | 8.8 | 10.7 | 8.9 | 13.7 | 8.6 | 7.2 | 11.3 | 11.5 | 9.5 | 10.9 | 9.8 | <0.001 |
| Adjusted Case Fatality Rate, % | 89.2 | 83.3 | 71.9 | 73.4 | 83.5 | 77.0 | 75.2 | 60.2 | 83.5 | 71.8 | 76.8 | <0.001 |
| Survival to Disch., % | 7.7 | 8.9 | 23.6 | 26.6 | 14.7 | 21.8 | 21.7 | 39.4 | 15.2 | 25.6 | 21.0 | <0.001 |
| Vital Status Missing, % | 3.1 | 7.7 | 4.5 | 0.0 | 1.9 | 1.1 | 3.1 | 0.4 | 1.4 | 2.6 | 2.3 | <0.001 |
DISCUSSION
In a large prospective multi-center observational study of out-of-hospital cardiac in regions dispersed throughout North America, 8.0% of treated arrests and 21.0% of ventricular fibrillation arrests survived to discharge. A minority of treated arrests received bystander CPR. Incidence, mortality, case fatality rate and survival to discharge of EMS-assessed, EMS-treated and ventricular fibrillation arrests differed significantly across geographic regions. Part of the regional differences in incidence could be attributable to differences in the completeness of case ascertainment and potential for undetected cases. However each site had or implemented approaches to ascertain arrests from all EMS agencies within their geographic area. This prospective approach in conjunction with statistical methods to account for missing cases provide the most robust resource to date for determining the public health magnitude of cardiac arrest. Thus the observed differences in incidence reflect differences in the underlying risk of OHCA as well as the local approach to organized emergency response and post-resuscitation care in hospital.
Others have reported regional variation in the incidence of out-of-hospital cardiac arrest.(10, 11) Such gradients are associated with socioeconomic and racial disparities in health. As a consequence of these gradients, cardiovascular disease is the leading cause of income-related differences in premature mortality in the United States,(12) and Canada.(13)
It is plausible that use of secondary prevention in patients with established cardiovascular disease is more common in some regions compared to others.(14) This would reduce the occurrence of out-of-hospital cardiac arrest if secondary prevention attenuated arrhythmic risk. Randomized trials of statin therapy,(15, 16) and secondary analyses of statin use in a trial of implantable defibrillators demonstrate that use of such medication reduces the risk of subsequent arrest.(17) Other studies demonstrate that beta-antagonists reduce the risk of death due to arrest in patients with heart failure.(18) But the magnitude of regional variation in medication use is much less than the magnitude of variation in cardiac arrest observed in the present study. Therefore differences in prevention do not fully explain our findings.
Also it is plausible that patients with symptoms of acute myocardial infarction have less delay in seeking care in some geographic regions compared to others.(19) This would reduce the occurrence of infarct-related ventricular fibrillation or shift it to the in hospital setting. If such differences in delay in care exist, it seems unlikely that they are due to differences in patient delay in reacting to symptoms of infarction since interventions to modify this delay have had limited success.(20) Instead such differences could reflect regional differences in care and outcome for patients with acute cardiovascular events.(14, 21) Such differences could be reduced by implementation of systems of care for such patients.(22) But we observed large regional variation in survival of all EMS-treated arrests as well as in the minority of arrests that were due to ventricular fibrillation and potentially associated with acute infarction. Therefore regional variation in care for acute cardiovascular events does not fully explain our findings.
Others have reported survival from 0%(23) to 21%(4) after treatment of out-of-hospital cardiac arrest. EMS agencies in large cities have special challenges in achieving good outcomes after cardiac arrest.(24, 25) Our analysis suggests that such differences do not reflect inter-study differences in inclusion criteria or outcome definition, as each site in the present study implemented uniform definitions of cardiac arrest and survival.
Instead, it seems likely that these differences reflect in part regional differences in the availability of emergency cardiac care.(26) These differences include: bystander CPR, lay responder defibrillation programs,(27) EMS factors such as experience of providers,(28) and types of interventions provided by EMS providers(29, 30) or treatments available at receiving hospitals.(31, 32) Some of these factors have been associated with differences in survival or quality of life after resuscitation,(3, 33-35) although no analysis has had adequate power to detect the independent effect of all of these factors.
Morbidity and mortality from most cardiovascular diseases has declined over the last 30 years.(36) The majority of this reduction has been attributed to risk factor modification.(37) Unfortunately, there has been little improvement in the incidence of OHCA survival over this same period of time.(38, 39) Experts have proposed that OHCA be designated a reportable event to facilitate monitoring and improvement of cardiovascular health.(40) The present study demonstrates that large regional differences in OHCA epidemiology exist, and are a prelude to further analysis to understand the causes of these variations as well as implementation of targeted interventions to reduce them. The discordance between case fatality rate and survival to discharge re-emphasize the importance of complete ascertainment of vital status as national, public reports of OHCA incidence and outcome become available.
Cardiovascular disease is the leading cause of death in the United States.(1) The Institute of Medicine has identified the need to improve funding for EMS operations.(41) Extrapolation of the mortality rate observed in the study regions to the total population of the United States suggests that there are 294,851 (quasi confidence intervals 236,063, 325,007) EMS-assessed OHCA cases annually in the United States.a Extrapolation of this study to the total population of Canada suggests that there are 32,160 (quasi confidence intervals 25,748, 35,450) EMS-assessed OHCA cases annually in Canada.b Collectively these estimates of burden imply that allocation of increased resources to EMS operations is necessary to achieve an important impact on cardiovascular health in either country.
If survival after OHCA treated by EMS could be increased from the study average of 7.9% to the maximum of 16.2% throughout North American, then the premature deaths of 15,500 individuals would be prevented each year.c Ongoing funding for fundamental, translational and clinical research related to emergency cardiovascular care is necessary to ensure that we are able to achieve such improvements in public health.
This study has several strengths compared to previous studies. Clinical trials often exclude patients at higher risk of poor outcomes so estimation of the burden of illness based only on those enrolled in trials is subject to bias. Existing OHCA registries do not contain the necessary information to determine which interventions are effective in the out-of-hospital setting. Several large regional registries have evaluated the effectiveness of out-of-hospital interventions upon outcomes after OHCA.(42, 43) However these underestimated the incidence of OHCA because they excluded individuals who are assessed but not treated by EMS personnel.
This study has some limitations. First, ROC sites were selected by a competitive process emphasizing regional sites with well organized EMS systems and associated investigators, so results observed in participating ROC sites may not be representative of the community at large. However the catchment population of participating communities includes approximately 10% of the North American population and has diverse geographic and socioeconomic characteristics. To the best of our knowledge, this population is larger than that of any other ongoing out-of-hospital cardiac arrest registry. Second, it is plausible that incidence, structure, process and outcome data reported by each site are subject to ascertainment bias since not all responses are audited. However all sites agreed to the data elements before study initiation, trained data collection personnel, and altered existing paper or electronic data capture to increase the likelihood of data capture. As well, our use of timely episode reporting by sites facilitates quick feedback from the coordinating center to sites and to responders to reduce incomplete data. Another limitation is that the expected number of OHCA cases was not observed for some agencies during specific time intervals within the sampling period. Multiple imputation was used to account for such missing data. This approach allows us to better estimate variability of the data, and ensure appropriately proportionate weight for each agency. This method assumes that the cases we randomly impute, which in our case were from the same agency in a different time period, have the same patient, EMS process and outcome characteristics as the missing data. Registry data have not shown any significant time trends that would bias this imputation process.(44) Furthermore only a small proportion of the total cases were imputed in this study, so it seems unlikely that this imputation would reduce its validity.
We were unable to assess the effect of hospital-based post-resuscitation care on outcomes after out-of-hospital cardiac arrest due to our lack of patient-specific data about processes of care delivered in hospital. In-hospital therapeutic hypothermia improves outcomes after out-of-hospital cardiac arrest.(31) A small trial suggested that hemofiltration to reduce inflammation after out-of-cardiac arrest offers additional benefit.(45) Observational studies suggest that early percutaneous coronary intervention improves outcomes as well.(46, 47) Therefore, future assessments of regional variation in outcome after out-of-hospital cardiac arrest should assess the relative impact of out-of-hospital and hospital-based care.
Also we were unable to describe neurologic outcome at discharge. Assessment of Cerebral Performance Category (CPC) at discharge is a recommended part of resuscitation outcome studies.(48) However CPC has limited discrimination between mild and moderate brain injury. A small study with incomplete follow-up of survivors demonstrated only moderate correlation with other measures of health-related quality of life.(49) Although a larger study demonstrated a better correlation between CPC and a generic measures of health-related quality of life, CPC should not be considered a substitute for reliable and valid measures of the latter.(50) Nonetheless previous studies demonstrate that resuscitation interventions that are associated with better survival are also associated with better quality of life.(34, 35)
These findings have implications for prehospital emergency care. The four-fold variation in survival after EMS-treated cardiac arrest and seven-fold variation in survival after ventricular fibrillation demonstrate that cardiac arrest is a treatable condition. But only 31.4% of treated arrests (84.8% of bystander witnessed) received bystander CPR. Therefore ongoing efforts are necessary to encourage lay people to be ready, willing and able to provide CPR when necessary. Further improvements in outcome could be achieved by reducing the time to ALS arrival by improving early detection of cardiac arrest, dispatch protocols, deployment of existing vehicles, the number of vehicles available to respond, the quality of CPR, and real-time or post-event quality assurance.
CONCLUSIONS
OHCA is common and lethal. There are significant and important regional variations in the incidence and outcome of cardiac arrest. OHCA should be designated a reportable event to facilitate monitoring and improvement in cardiovascular health. Additional investigation is necessary to understand the causes of this variation in an effort to better understand implications for allocation of resources to prehospital emergency care clinical practice, and translational cardiac arrest research to reduce the magnitude of this variation and improve cardiovascular health.
Acknowledgments
This study was supported by a cooperative agreement (5U01 HL077863, HL077881; HL077871; HL077873; HL077872; HL077866; HL077908; HL077867; HL077885; HL077885) with the National Heart, Lung and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, the Canadian Institutes of Health Research (CIHR)-Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the American Heart Association and the Heart and Stroke Foundation of Canada.
Footnotes
The authors reported the following conflicts of interest:
Nichol
Employment- University of Washington; Membership- American Heart Association ACLS Subcommittee; Medic One Foundation Board of Directors; Grants- Resuscitation Outcomes Consortium (NIH U01 HL077863) 2004-2009; Randomized Trial of CPR Training Aid; Equipment (Asmund S. Laerdal Foundation for Acute Medicine); Equipment donation- Mannequins to support overseas medical mission (Laerdal Inc.); Monitor/defibrillator to support overseas medical mission (Medtronic Physio-Control Inc.). Travel Expenses- Single trip in 2006 (INNERcool Inc.); Single trip in 2006 (Radiant Inc.); Consultant- Northfield Laboratories Inc.
Thomas
None declared.
Callaway
Employment- University of Pittsburgh and UPMC Health System; Membership- American Heart Association ACLS Subcommittee; Grants- Resuscitation Outcomes Consortium (NIH U01 HL077871) 2004-2009; Hypothermia and Gene Expression after Cardiac Arrest (NIH R01 N5046073) 2002-2006; American Heart Association Grant-in-Aid (0755359U) Cerebrovascular Effects of Thrombin Activation after Cardiac Arrest; Equipment donation- Hypothermia device to support laboratory research (Medivance Inc.). Patents- Co-inventor on patents related to ventricular fibrillation waveform analysis.
Hedges
None declared.
Powell
None declared.
Aufderheide
Employment- Medical College of Wisconsin; Membership- American Heart Association BLS Subcommittee; Grants- Resuscitation Outcomes Consortium (NIH U01 HL077866) 2004-2009; Neurological Emergency Treatment Trials (NETT) Network (NIH U01 NS058927; Immediate trial (NIH R01 HL077821); ResQTrial (NIH 2-R44 HL65851); Consultant- Take Heart America; JoLife; Medtronic.
Lowe
None declared.
Brown
Employment- University of Alabama; Grants- Resuscitation Outcomes Consortium (NIH U01 77881) 2004-2009; Protocolized Care for Early Septic Shock (Alabama Dept of Public Health and Centers for Disease Control); “Blast Injuries” course throughout Alabama; Equipment Loan- Medtronic Physio-Control Inc. for ROC.
Rea- Employment- University of Washington; Membership- American Heart Association ACLS Subcommittee; Grants- Resuscitation Outcomes Consortium (NIH U01 HL077867) 2004-2009;
Dreyer
None declared.
Davis
Employment- University of San Diego; Grants- Resuscitation Outcomes Consortium (NIH U01 77908) 2004-2009; Gene expression in neuronal ischemic preconditioning (NIH); Air medical neurological and perfusion monitoring (Unrestricted grant from ZOLL); Cardiovascular effects of ventilation (Unrestricted grant from Cardinal Health).
Idris
None declared.
Stiell
None declared.
96.8 per 100,000 × 304,598,626 U.S. population (www.census.gov accessed on July, 14, 2008).
96.8 per 100,000 × 33,223,840 Canadian population (http://www.statcan.ca/menu-en.htm accessed on July 14, 2008).
52.1 per 100,000 × (304,598,626 + 33,223,840) × (16.2% - 7.9%).
REFERENCES
- 1.Lloyd-Jones D, Flegal K, Friday G, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.107.187998. In Press. [DOI] [PubMed] [Google Scholar]
- 2.Rea TD, Pearce RM, Raghunathan TE, et al. Incidence of out-of-hospital cardiac arrest. Am J Cardiol. 2004 Jun 15;93(12):1455–60. doi: 10.1016/j.amjcard.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Nichol G, Stiell IG, Laupacis A, et al. A Cumulative Metaanalysis Of The Effectiveness of Defibrillator-Capable Emergency Medical Services For Victims Of Out-Of-Hospital Cardiac Arrest. Annals of Emergency Medicine. 1999;34(4):517–25. [PubMed] [Google Scholar]
- 4.Grmec S, Krizmaric M, Mally S, et al. Utstein style analysis of out-of-hospital cardiac arrest--bystander CPR and end expired carbon dioxide. Resuscitation. 2007 Mar;72(3):404–14. doi: 10.1016/j.resuscitation.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Morrison LJ, Nichol G, Rea TD, et al. Rationale, Development and Implementation of the Resuscitation Outcomes Consortium Epistry–Cardiac Arrest. Resuscitation. 2007 doi: 10.1016/j.resuscitation.2008.02.020. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham JW, Schafer JL. On the Performance of Multiple Imputation for Multivariate Data with small sample size. In: Hoyle RH, editor. Statistical Strategies for Small Sample Research. SAGE Publications; London: 1999. pp. 1–32. [Google Scholar]
- 7.Little RJA, Rubin DB. Statistical Analysis with Missing Data. John Wiley and Sons; New York: 1987. [Google Scholar]
- 8.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991 Apr;10(4):585–98. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 9.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; New York: 1987. [Google Scholar]
- 10.Becker LB, Han BH, Meyer PM, et al. Racial differences in the incidence of cardiac arrest and subsequent survival. The CPR Chicago Project. N Engl J Med. 1993 Aug 26;329(9):600–6. doi: 10.1056/NEJM199308263290902. [DOI] [PubMed] [Google Scholar]
- 11.Galea S, Blaney S, Nandi A, et al. Explaining racial disparities in incidence of and survival from out-of-hospital cardiac arrest. Am J Epidemiol. 2007 Sep 1;166(5):534–43. doi: 10.1093/aje/kwm102. [DOI] [PubMed] [Google Scholar]
- 12.Singh GK, Siahpush M. Increasing inequalities in all-cause and cardiovascular mortality among US adults aged 25-64 years by area socioeconomic status, 1969-1998. Int J Epidemiol. 2002 Jun;31(3):600–13. doi: 10.1093/ije/31.3.600. [DOI] [PubMed] [Google Scholar]
- 13.Wilkins R, Berthelot J-M, Ng E. Trends in mortality by neighbourhood income in urban Canada from 1971 to 1996. Health Rep. 2002;13S:45–72. doi: 10.1503/cmaj.1031528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menon V, Rumsfeld JS, Roe MT, et al. Regional outcomes after admission for high-risk non-ST-segment elevation acute coronary syndromes. Am J Med. 2006 Jul;119(7):584–90. doi: 10.1016/j.amjmed.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Scandinavian Simvastatin Survival Study Group Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). The Lancet. 1994;344:1383–89. [PubMed] [Google Scholar]
- 16.Anonymous Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998 Nov 5;339(19):1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell LB, Powell JL, Gillis AM, et al. Are Lipid-Lowering Drugs Also Antiarrhythmic Drugs? An Analysis of the Antiarrhythmics Versus Implantable Defibrillators (AVID) Trial. J Am Coll Cardiol. 2003;42(1):81–7. doi: 10.1016/s0735-1097(03)00498-4. [DOI] [PubMed] [Google Scholar]
- 18.Domanski MJ, Krause-Steinrauf H, Massie BM, et al. A comparative analysis of the results from 4 trials of beta-blocker therapy for heart failure: BEST, CIBIS-II, MERIT-HF, and COPERNICUS. J Card Fail. 2003 Oct;9(5):354–63. doi: 10.1054/s1071-9164(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 19.Govindarajan A, Schull M. Effect of socioeconomic status on out-of-hospital transport delays of patients with chest pain. Ann Emerg Med. 2003 Apr;41(4):481–90. doi: 10.1067/mem.2003.108. [DOI] [PubMed] [Google Scholar]
- 20.Luepker RV, Raczynski JM, Osganian S, et al. Effect of a community intervention on patient delay and emergency medical service use in acute coronary heart disease: The Rapid Early Action for Coronary Treatment (REACT) Trial. Jama. 2000;284(1):60–7. doi: 10.1001/jama.284.1.60. [DOI] [PubMed] [Google Scholar]
- 21.O'Connor GT, Quinton HB, Traven ND, et al. Geographic variation in the treatment of acute myocardial infarction: the Cooperative Cardiovascular Project. Jama. 1999 Feb 17;281(7):627–33. doi: 10.1001/jama.281.7.627. [DOI] [PubMed] [Google Scholar]
- 22.Ting HH, Rihal CS, Gersh BJ, et al. Regional systems of care to optimize timeliness of reperfusion therapy for ST-elevation myocardial infarction: the Mayo Clinic STEMI Protocol. Circulation. 2007 Aug 14;116(7):729–36. doi: 10.1161/CIRCULATIONAHA.107.699934. [DOI] [PubMed] [Google Scholar]
- 23.Gray AJ, Redmond AD, Martin MA. Use of the automatic external defibrillator-pacemaker by ambulance personnel: the Stockport experience. BMJ. 1987;294:1133–5. doi: 10.1136/bmj.294.6580.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lombardi G, Gallagher J, Gennis P. Outcome of out-of-hospital cardiac arrest in New York City. The Pre-Hospital Arrest Survival Evaluation (PHASE) Study. JAMA. 1994;271(9):678–83. [PubMed] [Google Scholar]
- 25.Dunne RB, Compton S, Zalenski RJ, et al. Outcomes from out-of-hospital cardiac arrest in Detroit. Resuscitation. 2007 Jan;72(1):59–65. doi: 10.1016/j.resuscitation.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Eisenberg MS, Horwood BT, Cummins RO, et al. Cardiac arrest and resuscitation: a tale of 29 cities. Ann Emerg Med. 1990 Feb;19(2):179–86. doi: 10.1016/s0196-0644(05)81805-0. [DOI] [PubMed] [Google Scholar]
- 27.Anonymous Public-access defibrillation and survival after out-of-hospital cardiac arrest. N Engl J Med. 2004 Aug 12;351(7):637–46. doi: 10.1056/NEJMoa040566. [DOI] [PubMed] [Google Scholar]
- 28.Soo LH, Gray D, Young T, et al. Influence of ambulance crew's length of experience on the outcome of out-of-hospital cardiac arrest. Eur Heart J. 1999;20(7):535–40. doi: 10.1053/euhj.1998.1334. [DOI] [PubMed] [Google Scholar]
- 29.Kudenchuk PJ, Cobb LA, Copass MK, et al. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N Engl J Med. 1999;341(12):871–8. doi: 10.1056/NEJM199909163411203. [DOI] [PubMed] [Google Scholar]
- 30.Dorian P, Cass D, Schwartz B, et al. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med. 2002 Mar 21;346(12):884–90. doi: 10.1056/NEJMoa013029. [DOI] [PubMed] [Google Scholar]
- 31.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. New England Journal of Medicine. 2002;346(8):557–63. doi: 10.1056/NEJMoa003289. [comment] [DOI] [PubMed] [Google Scholar]
- 32.Anonymous Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. The Hypothermia after Cardiac Arrest Study Group. New England Journal of Medicine. 2002;346(8):549–56. doi: 10.1056/NEJMoa012689. [erratum appears in N Engl J Med 2002 May 30;346(22):1756].
- 33.van der Hoeven J, de Koning J, can der Weyden P, et al. Improved outcome for patients with a cardiac arrest by supervision of the emergency medical services system. Netherlands J of Med. 1995;46:123–30. doi: 10.1016/0300-2977(94)00106-j. [DOI] [PubMed] [Google Scholar]
- 34.Bergner L, Bergner M, Hallstrom AP, et al. Service factors and health status of survivors of out-of-hospital cardiac arrest. Am J Emerg Med. 1983;1(3):259–63. doi: 10.1016/0735-6757(83)90101-8. [DOI] [PubMed] [Google Scholar]
- 35.Frandsen F, Nielsen JR, Gram L, et al. Evaluation of Intensified Prehospital Treatment in Out-of-Hospital Cardiac Arrest: Survival and Cerebral Prognosis. The Odense Ambulance Study. Cardiology. 1991;79:256–64. doi: 10.1159/000174888. [DOI] [PubMed] [Google Scholar]
- 36.Rosamond WD, Chambless LE, Folsom AR, et al. Trends in the Incidence of Myocardial Infarction and in Mortality Due to Coronary Heart Disease, 1987 to 1994. N Engl J Med. 1998;339:861–7. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 37.Tillinghast SJ, Doliszny KM, Gomez-Marin O, et al. Change in survival from out-of-hospital cardiac arrest and its effect on coronary heart disease mortality—Minneapolis-St. Paul: the Minnesota Heart Survey. Am J Epidemiol. 1991;134(8):851–61. doi: 10.1093/oxfordjournals.aje.a116160. [DOI] [PubMed] [Google Scholar]
- 38.Rea TD, Eisenberg MS, Becker LJ, et al. Temporal trends in sudden cardiac arrest: a 25-year emergency medical services perspective. Circulation. 2003 Jun 10;107(22):2780–5. doi: 10.1161/01.CIR.0000070950.17208.2A. [DOI] [PubMed] [Google Scholar]
- 39.Herlitz J, Bang A, Gunnarsson J, et al. Factors associated with survival to hospital discharge among patients hospitalised alive after out of hospital cardiac arrest: change in outcome over 20 years in the community of Goteborg, Sweden. Heart. 2003 Jan;89(1):25–30. doi: 10.1136/heart.89.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nichol G, Rumsfeld J, Eigel B, et al. Essential Features of Designating Out-of-Hospital Cardiac Arrest as a Reportable Event. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.107.189472. [DOI] [PubMed] [Google Scholar]
- 41.Anonymous . Emergency Medical Services At the Crossroads. Institute of Medicine; Washington, DC: 2006. [Google Scholar]
- 42.Stiell IG, Wells GA, Spaite DW, et al. The Ontario Prehospital Advanced Life Support (OPALS) Study: Rationale and Methodology for Cardiac Arrest Patients. Ann Emerg Med. 1998;32(2):180–90. doi: 10.1016/s0196-0644(98)70135-0. [DOI] [PubMed] [Google Scholar]
- 43.Ekstrom L, Herlitz J, Wennerblom B, et al. Survival after cardiac arrest outside hospital over a 12-year period in Gothenberg. Resuscitation. 1994;27:181–7. doi: 10.1016/0300-9572(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 44.Brooks SC, Schmicker RH, Rea TD, et al. Evidence for Circadian Variability in the Frequency of Out-of-Hospital Cardiac Arrest. Circulation. 2007;116(II):934. [Google Scholar]
- 45.Laurent I, Adrie C, Vinsonneau C, et al. High-volume hemofiltration after out-of-hospital cardiac arrest: a randomized study. J Am Coll Cardiol. 2005 Aug 2;46(3):432–7. doi: 10.1016/j.jacc.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 46.Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997 Jun 5;336(23):1629–33. doi: 10.1056/NEJM199706053362302. [DOI] [PubMed] [Google Scholar]
- 47.Knafelj R, Radsel P, Ploj T, et al. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation. 2007 Aug;74(2):227–34. doi: 10.1016/j.resuscitation.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation. 2004 Nov 23;110(21):3385–97. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 49.Hsu JWY, Callaham M, Madsen CD. Quality-of-Life and Formal Functional Testing of Survivors of Out-of-Hospital Cardiac Arrest Correlates Poorly with Traditional Neurological Outcome Scales. Ann Emerg Med. 1996;28:597–605. doi: 10.1016/s0196-0644(96)70080-x. [DOI] [PubMed] [Google Scholar]
- 50.Stiell IG, Nesbitt LP, Nichol G, et al. Comparison of the Cerebral Performance Category Score and the Health Utilities Index for Survivors of Cardiac Arrest. Ann Emerg Med. 2008 Apr 30; doi: 10.1016/j.annemergmed.2008.03.018. [DOI] [PubMed] [Google Scholar]