Abstract
Objective
Evaluate the effect of vocal fold surface dehydration on mucosal wave amplitude and frequency.
Study Design
Controlled test-retest.
Setting
Larynges were mounted on an excised larynx phonation system and attached to a pseudolung in a triple-walled sound-attenuated room that eliminated background noise and maintained a stabilized room temperature and humidity level.
Subjects and Methods
High-speed video was recorded for eight excised canine larynges during exposure to dehumidified air at 20 cm H2O. Control trials consisted of high-speed videos recorded for two excised canine larynges during exposure to humidified air at the same pressure.
Results
In the majority of larynges, increased levels of dehydration were correlated with decreased amplitude and frequency. The slope of the linear regression fitted to the change in amplitude (p=0.003) as well as the percent change (p<0.001) between the initial and final trials were significantly decreased in dehydrated larynges. These measurements with respect to the change in frequency were also significantly decreased in dehydrated larynges (p<0.001; p=0.027).
Conclusion
Vocal fold surface dehydration caused a decrease in mucosal wave amplitude and frequency. This study provides objective, quantitative support for the mechanism of voice deterioration observed after extreme surface dehydration.
INTRODUCTION
During phonation, the passage of air from the lungs through the glottis causes oscillation of the vocal folds at a certain frequency for a given glottal configuration. This transduction of energy from pulmonary airflow into vocal fold vibration in turn results in pulsatile airflow that is the sound source. The mucosal wave during normal phonation is best characterized as a symmetrical transverse wave along the mucosal margin in the sagittal plane. It propagates from the inferior margin of the folds and subsequently travels superiorly to the superior margin of the folds1. Changes in the mass, viscosity, length, or tension of the lamina propria may cause abnormalities in the propagation of the mucosal wave2,3. Previous studies have manipulated the viscosity of the lamina propria by applying artificial mucous to the vocal folds, resulting in quantitative changes in the mucosal wave. Increased viscosity was correlated with increased contact phase of the glottic cycle and decreased vibratory frequency, amplitude, and open-quotient of the glottic cycle4,5.
Stiffness and viscosity of the lamina propria can also increase with dehydration of the vocal folds6. Changes in phonatory parameters observed after vocal fold dehydration are similar to those observed after the application of viscous fluid to the vocal fold; therefore, voice deterioration following dehydration has been attributed to changes in biomechanical properties such as viscosity7–11. The amount of energy dissipated due to friction in the oscillating vocal folds increases with viscosity, thus more aerodynamic energy is required to maintain the same phonatory conditions in dehydrated vocal folds12. Presumably by causing increased viscosity of the vocal fold mucosa, dehydration has been shown to increase phonation threshold pressure (PTP) and phonation threshold flow (PTF), both of which indicate the “ease of phonation”9,10. These trends have been observed at the systemic, tissue, and mucosal surface levels of dehydration7,9,12–15.
The effects of increased viscosity on mucosal wave characteristics have been quantified using electroglottography (EGG), laryngostroboscopy, and x-ray stroboscopy4,5. EGG represents mucosal wave as a waveform of electrical impedance that describes the time-varying relative vocal fold contact area patterns within the glottal cycle16. However, EGG waveforms are easily confounded by normal variations, such as mucus that spans the width of the glottis, so it may provide inconsistent measurements of frequency and glottis cycle17,18. Measurements of the mucosal wave using stroboscopy may not accurately characterize an aperiodic mucosal wave because this technique creates a composite image of the mucosal wave averaged over several cycles19. Among other causes, abnormal vibrations may be associated with extreme changes in lamina propria physiology induced by dehydration. The advent of high-speed digital imaging (HSDI) has provided a more accurate method of mucosal wave visualization because it provides real-time visualization at frequencies much higher than those of phonation20. HSDI has been found to produce significantly more accurate and interpretable images of the mucosal waves of pathological larynges than stroboscopy20–22.
By inducing dehydration in the lamina propria of the vocal folds, this study contributes quantitative support for inferred mechanisms of voice deterioration due to dehydration and may provide reference for clinical evaluation. With the increased use of high-speed video to visualize the mucosal wave, measurement and analysis of vocal fold oscillation is necessary to evaluate and refine current theories of voice production23. This study provides pertinent estimates of the mucosal wave for patients suffering from dehydration.
METHODS
Larynges
In this excised study, the sample population consisted of 10 excised canine larynges. The larynges were obtained immediately postmortem from canines that died from causes unrelated to this study. Approval from an institutional review board was not necessary as no humans were involved, and approval from an animal care and use committee was not required as the animals were not sacrificed for the present study. The larynges were excised according to the procedure described by Jiang and Titze24, and they were subsequently examined to ensure the absence of diseased tissue or lesions. The larynges were immediately placed in a 0.9% saline solution and quickly frozen for later use, whereupon they were thawed slowly.
Apparatus
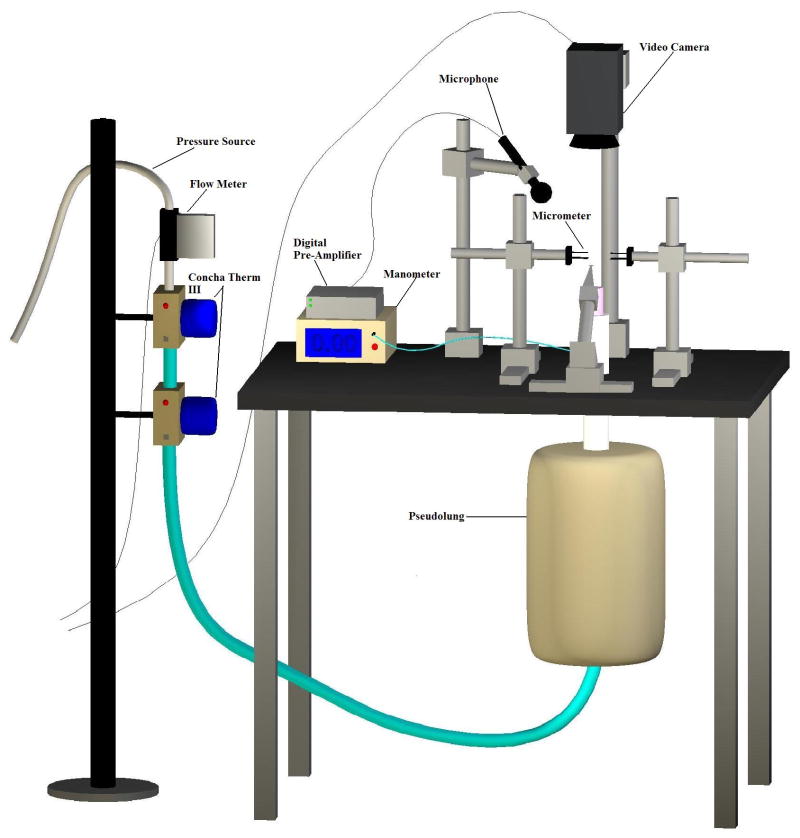
The epiglottis, cuneiform cartilages, corniculate cartilages, and ventricular folds were dissected away to expose the vocal folds immediately prior to experimentation. The superior cornua and the superior portions of the thyroid cartilage were also removed to facilitate the insertion of lateral micrometers into the arytenoid cartilages during the trials. The larynges were mounted on an excised larynx phonation system, as is shown in Figure 1. A small segment of the trachea inferior to the larynx was clamped to a pipe using a hose clamp. The pipe was connected in series to two ConchaTherm III heater-humidifiers (Fisher & Paykel Healthcare Inc., Laguna Hills, CA) and an Ingersoll Rand (type 30) air compressor controlled sub-glottal pressure. Two three-pronged micrometers were inserted into the lateral aspects of the arytenoids, controlling vocal fold adduction and abduction at the point of glottal closure. The elongation of the vocal folds was controlled by another micrometer system sutured to the anterior commissure of the thyroid cartilage. The length of the vocal folds was measured for calibration purposes. The elongation of the vocal folds remained constant throughout all trials.
Figure 1.
Schematic of excised larynx bench apparatus.
All measurements were taken in a triple-walled sound-attenuated room that eliminated background noise and maintained a stabilized room temperature and humidity level. A high-speed digital camera (Fastcam-ultima APX) was mounted on a track system above the vocal folds. This camera recorded the vocal fold vibrations at a rate of 4,000 frames per second and at a resolution of 256 × 512 pixels.
Experimental Procedure
For the 8 dehydration trials, warm, non-humidified air between 36° and 38° C and 25 ± 3% relative humidity was directed through the vocal folds at a constant pressure of 20 cm H2O until the larynges ceased to phonate. No saline solution was applied to the larynges during the trials. During phonation, the camera was automated to take 768 frames of high-speed video, initially every 60 seconds. When phonation became more irregular than its initial quality, the camera’s automation was adjusted to record every 10 seconds. Videos were recorded until the larynx ceased to phonate at 20 cm H2O.
Control Trials
During the 2 control trials, warm, humidified air of about 36° to 38° C and 100% relative humidity was directed through the vocal folds at a constant pressure of 20 cm H2O for 30 minutes. The larynges were kept hydrated throughout the 30 minutes of phonation with applications of 0.9% saline solution at 30 second intervals. During the 30 minutes of phonation, the camera was automated to take 800 frames of high-speed video every 60 seconds.
Data Analysis
The HSDI recordings of the mucosal wave were analyzed using a custom MATLAB program (The MathWorks, Natick, MA), and the mucosal wave characteristics of each of the four vocal fold lips (right-upper, right-lower, left-upper, left-lower) were quantified via digital videokymography (VKG). Threshold-based edge detection, manual wave segment extraction, and non-linear least squares curve fitting using a Fourier Series were applied to the VKG to determine the most closely fitting sinusoidal curve. The coefficients of the wave function for this curve were used to derive the amplitude and frequency of each vocal fold lip in the control and dehydration trials.
The slope of the linear regression fitted to the changes in amplitude and frequency over time as well as the percent change for these parameters were calculated for each larynx. Mann-Whitney Rank Sum tests were applied to the data to determine whether statistically significant differences existed between the percent changes for amplitude and frequency in the dehydrated larynges and those in the control larynges. The Pearson product-moment correlation coefficients were also calculated for the mucosal wave data for each larynx to determine the existence and direction of correlations between dehydration level and amplitude and frequency, respectively.
RESULTS
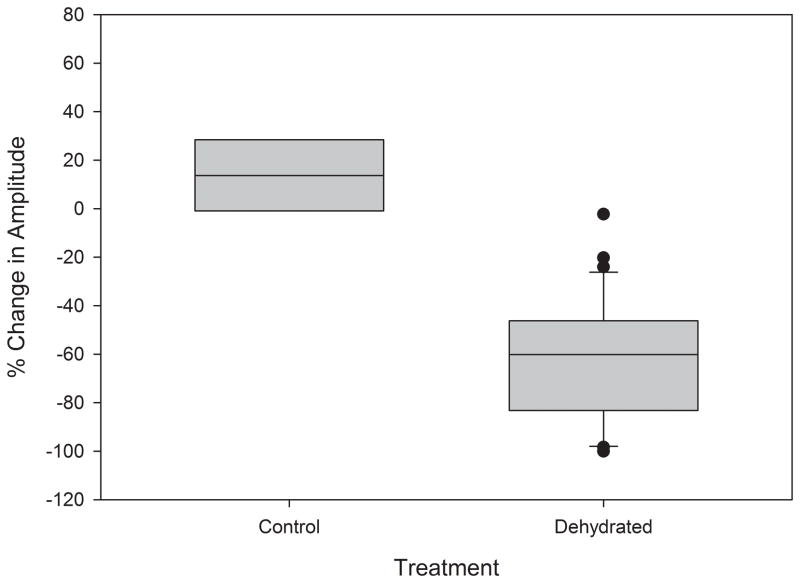
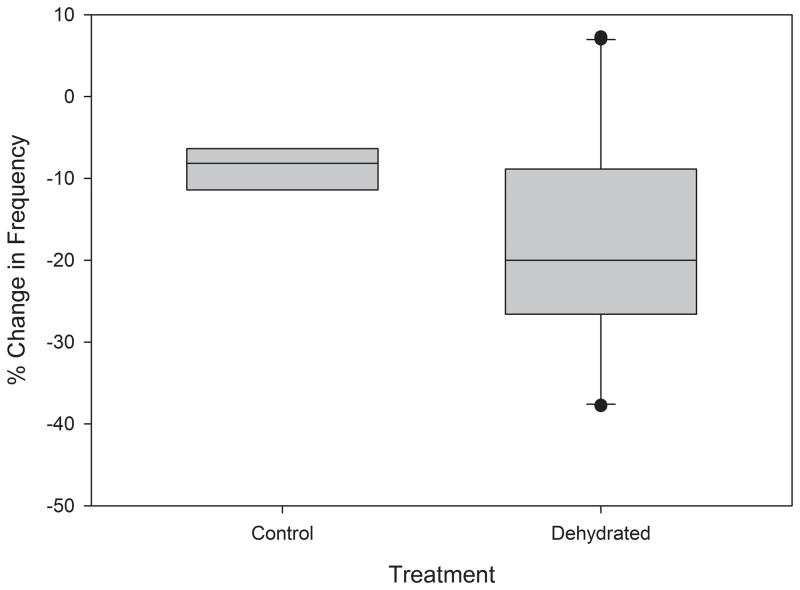
The results of the Mann-Whitney Rank Sum tests indicated the existence of a statistically significant difference between the mean slopes of the control and dehydration treatment linear regressions fitted to the changes in amplitude (p=0.003) and frequency (p<0.001) over time. The difference between mean percent changes in amplitude (p<0.001) and frequency (p=0.027) over time of the control and dehydrated groups was also statistically significant, as illustrated in Figures 2 and 3. These results are summarized in Table 1.
Figure 2.
Effect of dehydration on mucosal wave amplitude. Aggregate amplitude data obtained from excised larynges in control and experimental treatments. Lower and upper edges of box represent 25th and 75th percentile, respectively. Line contained within box represents median, whiskers represent maximum and minimum, and outliers are represented by dots.
Figure 3.
Effect of dehydration on mucosal wave frequency. Aggregate frequency data obtained from excised larynges in control and experimental treatments. Lower and upper edges of box represent 25th and 75th percentile, respectively. Line contained within box represents median, whiskers represent maximum and minimum, and outliers are represented by dots.
Table 1.
Summary of changes in means of mucosal wave parameters between initial and final phonation cycles. The value following + indicates the SD of the sample population.
| Parameter | Control | Dehydrated | p-value |
|---|---|---|---|
|
Amplitude
| |||
| % Change | 0.17±0.24 | −0.62±0.25 | <0.001* |
| Slope | 4.1E-5±4.2E-5 | −4.1E-3±0.010 | 0.003* |
|
| |||
|
Frequency
| |||
| % Change | −0.088±2.6E-2 | −0.18±0.13 | 0.027* |
| Slope | −0.013±0.45 | −0.20±0.15 | <0.001* |
Asterisk denotes significant p-value.
In the control treatment, the Pearson product-moment correlation coefficients indicated the existence of a slightly positive correlation between trial time and amplitude and a slightly negative correlation between trial time and frequency. Although changes were observable, they were small compared to those in the dehydrated larynges. The Pearson product-moment correlation coefficient between dehydration time and amplitude indicated that changes in this mucosal wave parameter also conformed to a negative linear trend in 7 out of 8 larynges. The Pearson product-moment correlation coefficient between dehydration time and frequency was negative in all of the dehydrated larynges. The Pearson product-moment correlation coefficients for amplitude and frequency in both control and dehydrated larynges are provided in Table 2. Qualitatively, decreases in the parameters of amplitude and frequency were observable between kymographic images of the control and dehydrated larynges (Figure 4).
Table 2.
Summary of Pearson product-moment correlation coefficients between initial and final phonation cycles for individual larynges.
| Larynx | Amplitude | Frequency | ||
|---|---|---|---|---|
| R-value | p-value | R-value | p-value | |
|
Control
| ||||
| 1 | 0.255 | 0.005* | −0.150 | 0.102 |
| 2 | 0.132 | 0.150 | −0.225 | 0.014* |
|
| ||||
|
Dehydrated
| ||||
| 3 | −0.154 | 0.046* | −0.338 | <0.001* |
| 4 | −0.442 | <0.001* | −0.483 | <0.001* |
| 5 | −0.646 | <0.001* | −0.581 | <0.001* |
| 6 | −0.597 | <0.001* | −0.855 | <0.001* |
| 7 | 0.0406 | 0.784 | −0.560 | <0.001* |
| 8 | −0.807 | <0.001* | −0.784 | <0.001* |
| 9 | −0.703 | <0.001* | −0.607 | <0.001* |
| 10 | −0.742 | <0.001* | −0.710 | <0.001* |
Asterisk denotes significant p-value. R-value refers to Pearson product-moment correlation coefficient.
Figure 4.
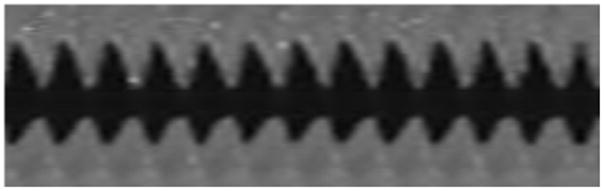
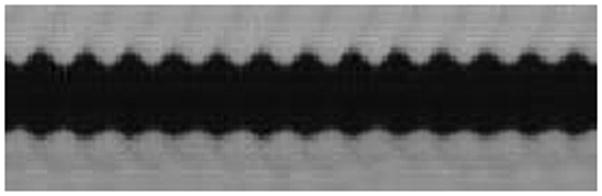
Kymographic images of excised larynges while hydrated (A) and after dehydration (B).
DISCUSSION
The present study demonstrates the effects of dehydration on the mucosal wave parameters of amplitude and frequency. In the majority of larynges, increased levels of dehydration, modeling longer periods of air flow, were correlated with decreased amplitude and frequency. Previous studies identified the level of vocal fold hydration as a variable affecting voice quality and the aerodynamic parameters of PTP and PTF, but the relationships between dehydration and vibratory parameters have not been previously quantified9,12,13. The results of the study by Chan and Tayama established a link between hydration and tissue rheology of the lamina propria; dehydration was directly related to vocal fold tissue stiffness and viscosity6. The results of this study provide support for the inverse relationship between vocal fold tissue viscosity and mucus viscosity and mucosal wave amplitude and frequency4,5.
In most of the dehydrated larynges, a statistically significant negative correlation between the level of dehydration and mucosal wave amplitude was observed. The slope of the linear regression fitted to the change in amplitude over time as well as the percent change between the amplitudes of the initial and final mucosal waves were significantly larger in the dehydrated condition than in the control condition. This decrease in amplitude during dessicating phonation may be due to changes in the viscoelastic and biomechanical properties of the larynx6. Functionally, PTP is an index of minimum energy required to initiate and vibration of the vocal folds25. The energy supplied to the vocal folds by the glottal airstream at this pressure is slightly larger than the energy dissipated during phonation due to tissue damping; therefore, the oscillation of the vocal folds can become self-sustained26. Dehydration is positively correlated with PTP; as the viscosity of the lamina propria increases, which can be caused by dehydration, the amount of energy lost due to internal friction in the folds also increases12,13. At a constant pressure, an increasingly greater portion of the energy provided by the glottal airstream is dissipated by friction as the level of dehydration increases. Consequently, the portion of energy expended as vocal fold oscillation decreases. Prolonged flow of dry air across the vocal folds also increases the viscosity of the mucus on the vocal fold surfaces. Ayache et al. found that the application of artificial mucus to the vocal fold surface of excised porcine larynges decreased vibratory frequency and increased the duration of the contact phase, both as an absolute value and as a proportion of the duration of the total glottal cycle4. Adhesion between the vocal folds may increase PTP. Nakagawa et al. also observed a similar decrease in mucosal wave amplitude after the application of artificial mucus to the vocal folds similarly increased the viscosity of the lamina propria surface5. The decrease in amplitude was attributed to an increase in vocal fold superficial tension, resulting in a reduction of the velocity of the mucosal wave. The combined factors of increased vocal fold tissue viscosity and mucus viscosity in the dehydrated excised canine larynges result in impaired aerodynamic parameters. In the present study, dehydration of the surface epithelia of the lamina propria was induced by exposing the vocal folds to constant dry airflow at a constant pressure of 20 cmH2O. As the level of dehydration increased, it is likely that PTP also increased until it reached and surpassed 20 cmH2O. The decrease in the energy expended in sustaining vocal fold oscillation resulted in continuously decreasing mucosal wave amplitude.
A statistically significant negative correlation was also observed between the level of dehydration and mucosal wave frequency across all dehydrated larynges included in this study. Ayache et al. determined that vibratory frequency decreased after the application of viscous artificial mucus to the vocal fold surface4. The decrease in frequency associated with high viscosity was also correlated with an increase in contact time between the vocal folds during the glottal cycle. Similarly, increased viscosity of the lamina propria due to dehydration appears to result in increased superficial tension, which causes prolonged contact between the vocal folds.
The decreases observed in mucosal wave amplitude and frequency during the present study are consistent with those of previous studies in which the viscosity of the mucosal wave was increased by the application of artificial mucus4,5. In the present study, the viscosity of the lamina propria was similarly increased by superficial dehydration. The literature and present study suggest that the effects of increased tissue viscosity on mucosal wave characteristics appear to be independent of the method of dehydration. Although small changes were observed in amplitude and frequency observed in the control trials, these may have been due to slight alterations in glottal configuration during prolonged exposure to airflow or some minimal level of tissue desiccation. Glottal configuration may be affected by changes in the volume of the vocal folds that result from dehydration. Linear inverse relationships exist between the level of dehydration and the tissue composition parameters of the liquid mass and volume fractions and the liquid:solid mass and volume ratios27. Dehydration reduces the liquid content of the vocal folds, thus decreasing their volumes. These changes would have similarly affected the dehydrated larynges.
This is the first study evaluating the effect of dehydration on amplitude and frequency using HSDI. The relationship between mucosal wave parameters and hydration has potential clinical impact, as deviations from normal values may be an indicator of possible laryngeal dysfunction. The inability to maintain normal fluid balance has been associated with several laryngeal pathologies, including lesions, nodules and polyps28, 29. The empirical measurements of changes in mucosal wave parameters during the dehydration process provide quantitative support for the biomechanical basis of voice deterioration. Although the results of this study suggest that the dehydration of epithelial cells in ex vivo larynges causes a decrease in the amplitude and frequency of mucosal wave, future studies could measure these parameters in human subjects with the aim of quantitatively describing mucosal waves associated with different types and degrees of dehydration. The effects of rehydration on vocal fold mucosal wave parameters may also be useful in evaluating the efficacy of different hydration treatments.
CONCLUSION
Dehydration is negatively correlated with mucosal wave amplitude and frequency. This study provides objective, quantitative support for the mechanism of voice deterioration observed after extreme surface dehydration. Clinically, these relationships may be used to objectively determine the extent of dehydration due to desiccation or to underlying laryngeal pathologies.
Acknowledgments
This study was funded by NIH grant numbers R01 DC008850 and R01 DC005522 from the National Institute on Deafness and other Communicative Disorders.
References
- 1.Matsushita H. The vibratory mode of the vocal folds in the excised larynx. Folia Phoniatr (Basel) 1975;27:7–18. doi: 10.1159/000263963. [DOI] [PubMed] [Google Scholar]
- 2.Svec JG, Sram F, Schutte HK. Videokymography in voice disorders: what to look for? Ann Otol Rhinol Laryngol. 2007;116:172–80. doi: 10.1177/000348940711600303. [DOI] [PubMed] [Google Scholar]
- 3.Qiu Q, Schutte HK, Gu L, et al. An automatic method to quantify the vibration properties of human vocal folds via videokymography. Folia Phoniatr Logop. 2003;55:128–36. doi: 10.1159/000070724. [DOI] [PubMed] [Google Scholar]
- 4.Ayache S, Ouaknine M, Dejonkere P, et al. Experimental study of the effects of surface mucus viscosity on the glottic cycle. J Voice. 2004;18:107–15. doi: 10.1016/j.jvoice.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa H, Fukuda H, Kawaida M, et al. Lubrication mechanism of the larynx during phonation: an experiment in excised canine larynges. Folia Phoniatr Logop. 1998;50:183–94. doi: 10.1159/000021460. [DOI] [PubMed] [Google Scholar]
- 6.Chan RW, Tayama N. Biomechanical effects of hydration in vocal fold tissues. Otolaryngol Head Neck Surg. 2002;126:528–37. doi: 10.1067/mhn.2002.124936. [DOI] [PubMed] [Google Scholar]
- 7.Fisher KV, Ligon J, Sobecks JL, et al. Phonatory effects of body fluid removal. J Speech Lang Hear Res. 2001;44:354–67. doi: 10.1044/1092-4388(2001/029). [DOI] [PubMed] [Google Scholar]
- 8.Hemler RJ, Wieneke GH, Dejonckere PH. The effect of relative humidity of inhaled air on acoustic parameters of voice in normal subjects. J Voice. 1997;11:295–300. doi: 10.1016/s0892-1997(97)80007-0. [DOI] [PubMed] [Google Scholar]
- 9.Verdolini K, Titze IR, Fennell A. Dependence of phonatory effort on hydration level. J Speech Hear Res. 1994;37:1001–7. doi: 10.1044/jshr.3705.1001. [DOI] [PubMed] [Google Scholar]
- 10.Witt RE, Regner MF, Tao C, et al. Effect of dehydration on phonation threshold flow in excised canine larynges. Ann Otol Rhinol Laryngol. 2009;118:154–9. doi: 10.1177/000348940911800212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang J, Ng J, Hanson D. The effects of rehydration on phonation in excised canine larynges. J Voice. 1999;13:51–9. doi: 10.1016/s0892-1997(99)80061-7. [DOI] [PubMed] [Google Scholar]
- 12.Finkelhor B, Titze I, Durham P. The effect of viscosity changes in the vocal folds on the range of oscillation. J Voice. 1988;1:320–5. [Google Scholar]
- 13.Jiang J, Verdolini K, Aquino B, et al. Effects of dehydration on phonation in excised canine larynges. Ann Otol Rhinol Laryngol. 2000;109:568–75. doi: 10.1177/000348940010900607. [DOI] [PubMed] [Google Scholar]
- 14.Hemler RJ, Wieneke GH, Lebacq J, et al. Laryngeal mucosa elasticity and viscosity in high and low relative air humidity. Eur Arch Otorhinolaryngol. 2001;258:125–9. doi: 10.1007/s004050100321. [DOI] [PubMed] [Google Scholar]
- 15.Sivasankar M, Erickson E, Schneider S, et al. Phonatory effects of airway dehydration: preliminary evidence for impaired compensation to oral breathing in individuals with a history of vocal fatigue. J Speech Lang Hear Res. 2008;51:1494–506. doi: 10.1044/1092-4388(2008/07-0181). [DOI] [PubMed] [Google Scholar]
- 16.Baer T, Lofqvist A, McGarr NS. Laryngeal vibrations: a comparison between high-speed filming and glottographic techniques. J Acoust Soc Am. 1983;73:1304–8. doi: 10.1121/1.389279. [DOI] [PubMed] [Google Scholar]
- 17.Childers DG, Krishnamurthy AK. A critical review of electroglottography. Crit Rev Biomed Eng. 1985;12:131–61. [PubMed] [Google Scholar]
- 18.Childers DG, Hicks DM, Moore GP, et al. Electroglottography and vocal fold physiology. J Speech Hear Res. 1990;33:245–54. doi: 10.1044/jshr.3302.245. [DOI] [PubMed] [Google Scholar]
- 19.Bless DM, Hirano M, Feder RJ. Videostroboscopic evaluation of the larynx. Ear Nose Throat J. 1987;66:289–96. [PubMed] [Google Scholar]
- 20.Patel R, Dailey S, Bless D. Comparison of high-speed digital imaging with stroboscopy for laryngeal imaging of glottal disorders. Ann Otol Rhinol Laryngol. 2008;117:413–24. doi: 10.1177/000348940811700603. [DOI] [PubMed] [Google Scholar]
- 21.Bonilha H, Deliyski D. Period and glottal width irregularities in vocally normal speakers. J Voice. 2008;22:699–708. doi: 10.1016/j.jvoice.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Bonilha H, Deliyski D, Gerlach T. Phase asymmetries in normophonic speakers: visual judgments and objective findings. Am J Speech-Lang Path. 2008;17:367. doi: 10.1044/1058-0360(2008/07-0059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doellinger M, Berry DA. Visualization and quantification of the medial surface dynamics of an excised human vocal fold during phonation. J Voice. 2006;20:401–13. doi: 10.1016/j.jvoice.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Jiang JJ, Titze IR. A methodological study of hemilaryngeal phonation. Laryngoscope. 1993;103:872–82. doi: 10.1288/00005537-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Chan RW, Titze IR. Dependence of phonation threshold pressure on vocal tract acoustics and vocal fold tissue mechanics. J Acoust Soc Am. 2006;119:2351–62. doi: 10.1121/1.2173516. [DOI] [PubMed] [Google Scholar]
- 26.Lucero JC. A theoretical study of the hysteresis phenomenon at vocal fold oscillation onset-offset. J Acoust Soc Am. 1999;105:423–31. doi: 10.1121/1.424572. [DOI] [PubMed] [Google Scholar]
- 27.Hanson KP, Zhang Y, Jiang JJ. Parameters quantifying dehydration in canine vocal fold lamina propria. Laryngoscope. 2010;120:1363–9. doi: 10.1002/lary.20927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verdolini K, Titze IR, Fennell A. Dependence of phonatory effort on hydration level. J Speech Hear Res. 1994;37:1001–7. doi: 10.1044/jshr.3705.1001. [DOI] [PubMed] [Google Scholar]
- 29.Verdolini K. Guide to Vocology. Iowa City, IA: National Center for Voice and Speech; 1998. [Google Scholar]