Abstract
Background
Care of patients with locally recurrent rectal cancer (LRRC) requires careful patient selection. While curative resection offers survival benefits, significant trade-offs exist for the patient. Knowledge of patient-reported outcomes will help inform treatment decisions.
Methods
Quality of life (QOL) and pain were prospectively assessed in 105 patients treated for LRRC at a single-institution, using the validated the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) and Brief Pain Inventory (BPI) questionnaires. In 54 patients enrolled and followed from diagnosis of LRRC, relationship between pre-treatment pain, QOL and overall survival (OS) were examined.
Results
Patients underwent curative surgical resection(C, 59%), non-curative surgery (NC, 12%), or nonsurgical treatment (NS, 28%). Median OS was 7.1, 1.4 and 1.9 years respectively(C vs. NC: p<0.001; C vs. NS: p=0.006; NC vs. NS: p=0.261). The physical well-being QOL differed over time (p=0.042) with greatest difference between C and NC surgery patients (p=0.049). The remaining QOL domain scores and pain scores demonstrated no significant time or treatment effect. For the 54 patients assessed from diagnosis, median OS was independently predicted by treatment group (C, NC, NS: 4.3, 1.7, vs. 2.4 years; p<0.001) and pain intensity (score ≤ 4vs. >4: 3.8vs. 2.0 years; p=0.001).
Conclusion
Curative surgery offered prolonged survival, but significant pain exists among long-term survivors and should be a focus of survivorship care. Noncurative surgery did not offer apparent advantages over nonsurgical palliation. Patient’s baseline pain has prognostic value, and should be assessed, treated and considered in treatment decisions.
Introduction
Recent advances in multimodality therapy and wide acceptance of total mesorectal excision (TME) have significantly improved the care of patients with rectal cancer. [1, 2] Rates of local pelvic failure after primary curative therapy has declined from 20–30%, to only 6–11% in the setting of clinical trials and specialized centers. [3, 4] However, locally recurrent rectal cancer (LRRC) remains a challenging problem. Local pelvic failure not only shortens patient’s life expectancy, but also decreases their quality of life (QOL). [5] Common cancer-related morbidities include severe pelvic pain, bleeding, obstruction, fistula, orchronic pelvic sepsis. [6] While the expected median survival is often measured in months with supportive care alone [7, 8], treatment options are often limited by previous pelvic irradiation, surgery and/or systemic therapy. [7] Indeed, care for patients with LRRC requires careful consideration of both patient-and tumor-related factors, in deciding which patients are suitable candidates for curative resection, surgical palliation, or nonsurgical treatments.
Current literature regarding LRRC has been predominated by reported outcomes of surgical salvage with curative intent. In these highly selected patients, a survival advantages has been demonstrated after margin-negative resection, with reported 5-year overall survival (OS) rates of 9–43%. [3, 9] However, significant trade-offs exist. Peri-operative morbidity and mortality are substantial; bowel, urinary and sexual functions as well as life style can be permanently altered; and prolonged multi-dimensional rehabilitation is needed after hospital discharge. [10] Although survival rates and short-term morbidities have been described, little is known about patient-reported outcomes such as functional lifestyles, symptoms, and QOL. Furthermore, outcomes of patients who undergo non-curative surgery or nonsurgical treatment have not been well-investigated. Fulfilling these gaps in knowledge would more fully inform the surgeon and the patient when challenged with these difficult treatment decisions. Therefore, a single-institutional prospective survey study of patients treated for LRRC was conducted with the following aims: 1) compare the overall survival (OS) of patients undergoing curative surgery(C), non-curative or palliative surgery (NC), or nonsurgical treatments (NS); 2) measure the QOL and pain symptoms over time in patients undergoing different interventions; and 3) explore the relationship between baseline QOL and pain symptoms with OS.
Methods
Study cohort and outcome measures
After approval from the Institutional Review Board, 105 eligible and consenting adult patients (≥ 18 years of age) with LRRC were prospectively enrolled to the study protocol between August 1997 and March 2007. LRRC was defined as histologically-proven recurrent rectal adenocarcinoma in the pelvis, at least 3 months after completion of primary curative therapy. Patients must have command of the English language for survey research, not have pre-existing chronic pain syndrome or constipation, and agree to survey completion. In this study, patients who underwent surgical exploration with complete resection of gross tumor were considered to have had a curative resection (C); those who underwent a non-curative exploration with residual gross tumor, either because of unexpected intra-operative findings or because of intent to palliate symptoms only were considered to have had a non-curative or palliative resection (NC); and the remainder did not have surgical intervention (NS).
Patients were enrolled to the study protocol at various time points after diagnosis of LRRC. A subgroup of 54 patients was enrolled prior to treatment initiation, while the remainder was enrolled after treatment for their LRRC. Patients reported the medical intervention for pain that they were receiving at the time of study enrollment, while subsequent pain management was per the discretion of the treating physicians and referral to specialist pain service was made as needed. Quality of life and pain symptoms were surveyed by self-administered questionnaires at enrollment and then every 3 months, until death or last follow-up. Survey results were blinded to the treating physicians.
Instruments for QOL and pain assessment
The Functional Assessment of Cancer Therapy-Colorectal (FACT-C) is a disease-specific self-report QOL instrument and a part of the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement Quality of Life System.[11][12] The FACT-C contains four domains and the colorectal subscale (CCS). According to the instrument, patients were instructed to answer based on their experience over the past 7 days. The physical well-being domain (PWB) addressed energy, nausea, family needs, pain, treatment toxicity, sickness feeling, and time in bed; the social well-being domain (SWB) addressed distance from friends, emotional support, general support, illness acceptance, family communication, partner relationship and sexual satisfaction; the emotional well-being domain (EWB) addressed sadness, self pride, hope, nervousness, and worry about death; and the functional well-being domain (FWB) addressed work, life enjoyment, illness acceptance, sleep, leisure, and overall contentment with QOL. The CCS elicited symptoms of abdominal cramping, weight loss, bowel control, digestion, diarrhea, appetite, and body image. Each item was scored from 0 (not at all) to 4 (very much). The maximal possible scores of PWB, SWB, EWB, FWB, CCS are: 28, 28, 20, 28, and 28(version 2.0), while FACT-C is a summation of these scores (maximum: 132). A higher score denotes better QOL. The FACT-C has demonstrated internal consistency, reliability and concurrent validity. It can discriminate between patient groups of different functional status and disease extent, and is sensitive to changeover time.[12]
The Brief Pain Inventory (BPI)-short form is one of the most widely used measurement tools for assessing clinical pain.[13, 14] According to a recent consensus panel, key measures of pain are pain intensity (severity) and pain interference (impact of pain on functioning).[15, 16] The pain intensity score is the average of the “worst,” “least,” “average,” and “now” pain scores on a scale from 0 (no pain) to 10 (pain as bad as you can imagine). The pain interference score is the average of the interference scores given to 7 daily activities (i.e. general activity, walking, work, mood, enjoyment of life, relations with others, and sleep) on a scale from 0 (does not interfere) to 10 (completely interferes). The BPI has been extensively validated and has proven internal consistency, reliability, and sensitivity to change.[13, 14, 17]
Statistical analysis
The distribution of continuous variables was summarized by median and interquartile range (IQR), while that of categorical variable as frequency and percent (%). Overall survival (OS), defined as the time from the date of LRRC diagnosis to date of death from any cause, was estimated by the Kaplan-Meier method censoring patients who were alive at last follow-up. Difference in OS by treatment group was compared using log rank test. The QOL and pain scores were calculated according to standard guidelines.[17, 18] To assess changes over time by different treatments, linear mixed effect models were constructed after adjusting for treatment group and after accounting for repeated measures. Fixed effects considered were treatment, time, and the interaction between time and treatment. When an overall linear trend over time was observed, interaction between time and treatment was examined by comparing different estimates of slope over time. Estimates of intercept and slope over time were compared between treatment groups using t-test, after adjusting p-values for multiple pair-wise comparisons (Bonferroni method for a p-value of 0.017). To explore the relationship between baseline QOL or pain score and OS, Cox proportional hazard regression model was constructed. In multivariate analysis, non-significant variables from univariate analyses were eliminated in a step-down fashion using a cut-off p-value of 0.10. A p-value of 0.05 denoted statistical significance unless otherwise noted. All analyses were performed using SAS version 9.1 (Cary, NC).
Results
Clinical outcomes
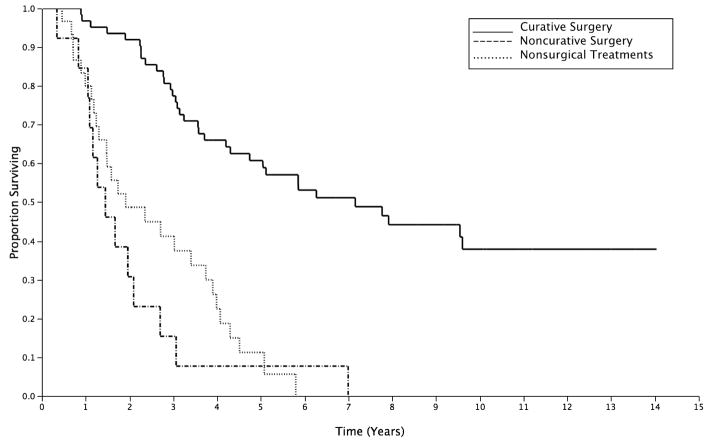
Curative surgical resection (C) was undertaken in 62 (59%) patients, while 13 (12.4%) and 30 (28.5%) patients underwent NC and NS treatments respectively. Curative surgical resection procedures included: repeat rectal resection (low anterior resection, coloanal anastomosis, or abdominoperineal resection) in 21 patients (33.9%), multi-visceral resection with bone resection in 25 patients (40.3%), without bone resection 9 (14.5%) and extra-rectal pelvic tumor excision in 7 (11.3%). Non-curative surgery included exploratory laparotomy with biopsy of extra-pelvic disease in 6 patients, intestinal bypass or diversion in 5 patients, transanal local excision in 1 patient and segmental resection for contained perforation in 1 patient. Nonsurgical management included chemoradiation in 21 patients, brachytherapy in 2 patients, and no intervention in the remainder. The median age of the study cohort was 51 years (IQR: 59–67) and the gender distribution was nearly equal (female: 53 patients, 50.3%). Patients did not significantly differ in age (p=0.08) or gender (p=0.64) across treatment groups. Chemoradiation was given as a component of the overall multimodality salvage treatment in 81.8%, 88.9% and 91.3% of the patients in C, NC and NS groups (p=0.21). From the diagnosis of LRRC, the median OS after C was significantly longer than that after NC or NS: 7.1 years vs. 1.4 or 1.9 years, respectively (C vs. NC: p<0.001; C vs. NS: p=0.006; and NC vs. NS: p=0.261; Figure 1).
Figure 1.
Overall survival of patients from diagnosis of LRRC by different treatment groups. Curative surgery (C): solid line; Noncurative surgery (NC): hashed line; Nonsurgical treatment (NS): dotted line.
Quality of life and pain in different treatment groups over time
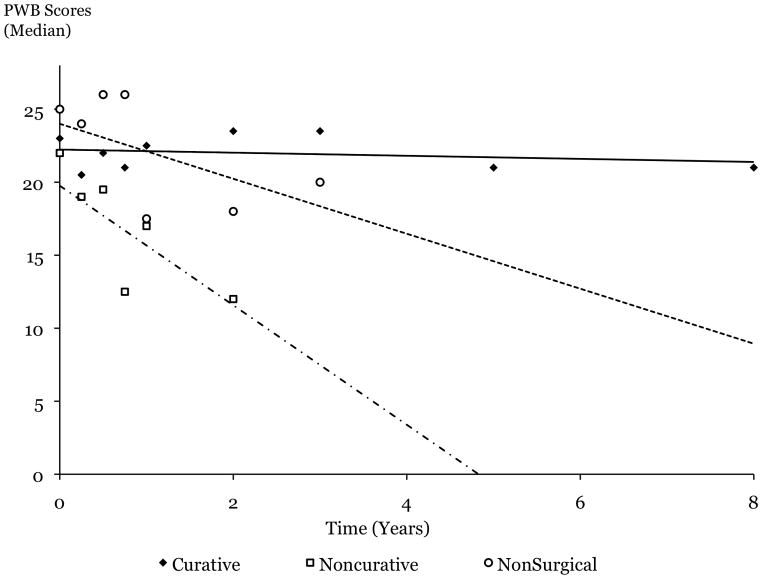
Among patient-reported QOL scores, only scores in the PWB domain exhibited significant impact of treatment, time and their interaction, as assessed by the mixed effects model (Table 1). To closely examine how time trends differed by treatment group, the slopes of the decline in QOL over time were compared among treatment groups (Figure 2). While PWB was largely preserved over time after C, it rapidly declined after NC or NS, reaching borderline significance when C and NC were compared (p=0.049, Figure 2).
Table 1.
Patient-reported quality of life scores as measured by FACT-C.
| Baseline | 3 mo | 6 mo | 9 mo | 1 year | 2 year | 3 year | 5 year | 8 year | P treatment | P time | P treatment * time | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. patients | ||||||||||||
| C | 31 | 20 | 18 | 22 | 26 | 28 | 14 | 12 | 5 | |||
| NC | 11 | 6 | 4 | 2 | 1 | 1 | ||||||
| NS | 12 | 11 | 7 | 5 | 2 | 2 | 1 | |||||
| PWB | ||||||||||||
| C | 23 (19, 26) | 20.5 (18, 23.8) | 22 (20, 25) | 21 (17.8, 25.3) | 22.5 (17, 26) | 23.5 (15.3, 26) | 23.5 (14, 27) | 21 (16.3, 26) | 21 (14, 25.5) | 0.13 | 0.01 | 0.04 |
| NC | 22 (12, 24) | 19 (14.5, 24) | 19.5 (14.3, 21) | 12.5 (8, 17) | 17 | 12 | ||||||
| NS | 25 (20.3, 27.8) | 24 (23, 26) | 26 (17, 27) | 16 (22.5, 28) | 17.5 (15, 20) | 18 (16, 20) | 20 | |||||
| FACT-C | ||||||||||||
| C | 102 (90, 116) | 101.5 (84.5, 104.8) | 98.6 ( 94, 113) | 94.7 (85.5, 108.8) | 96 (82, 115) | 105 (80.5, 112.8) | 99.5 (69, 116.8) | 95 (71.3, 114) | 103. 7 (65, 114) | 0.09 | 0.08 | 0.36 |
| NC | 94 (77, 107) | 81 (73.3, 101.1) | 92.3 (84.8, 99.1) | 70.4 (53, 87.8) | 87.8 | 83.8 | ||||||
| NS | 110 (90.7, 113) | 98 (97, 110) | 102 (97, 121) | 107.6 (104, 115.5) | 87.8 (78, 97.6) | 92.2 (88.4, 96) | 100 |
Scores are reported as median (interquartile range).
PWB=physical well-being; FACT-C=summation score.
Mo=months
No significant impact of treatment, time, or treatment*time interaction seen in other subdomains: SWB=social well-being; EWB=emotional well-being; FWB=functional well-being; CCS=colorectal subscale (data not shown).
Figure 2.
Patient-reported physical well-being (PWB) domain scores (median) as measured by the FACT-C questionnaire.
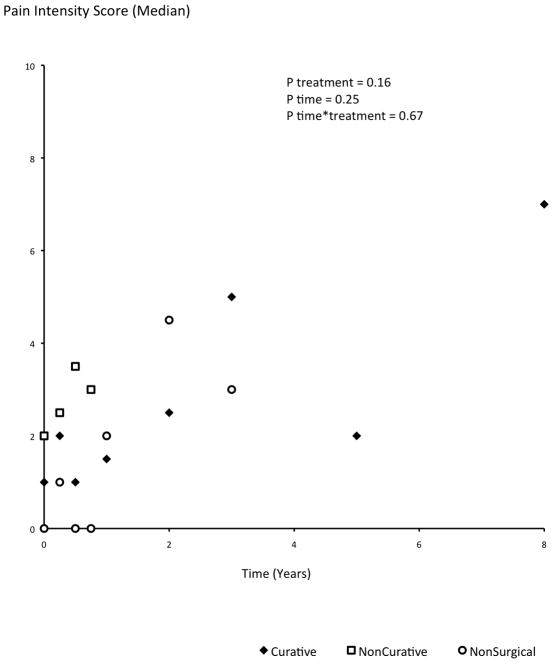
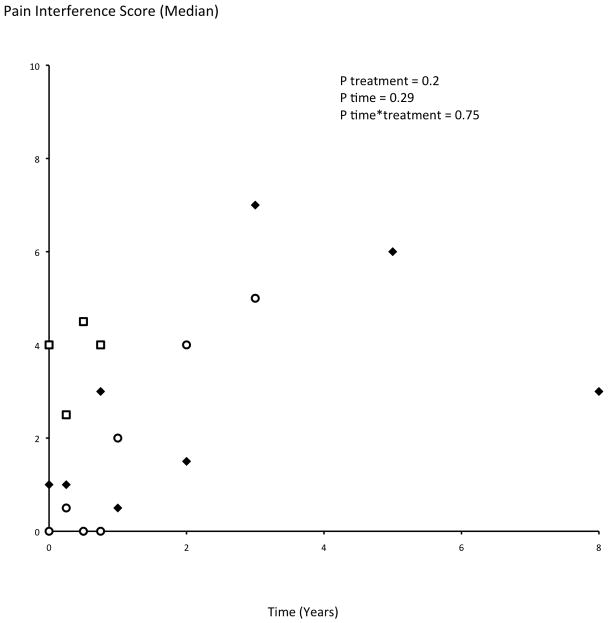
Reported pain intensity and pain interference did not demonstrate significant differences among treatment groups (pain intensity: p=0.16; pain interference: p=0.20), over time (p=0.25 and p=0.29 respectively), or by their interaction (p=0.67 and p=0.75 respectively; Figure 3a, b). Patient-reported treatments for pain did not differ by the treatment group (p=0.75): use of schedule II or III narcotics was reported by 41.7%, 47.6%, and 46.1% of the C, NC, and NS patients respectively; and over-the-counter medications were taken by 15%, 14.3% and 7.8% of the C, NC and NS patients respectively. Patients in different treatment groups did not differ in baseline reported pain symptoms; however, moderately high scores were reported even by long-term survivors without disease recurrence (Figure 3a, b).
Figure 3.
a and b. Patient-reported pain intensity (a) and pain interference (b) scores (median) as measured by the Brief Pain Inventory. Among the 10 distinct patients who reported pain scores after 3 years of followup, 3 had developed metastatic rectal carcinoma and their reported pain intensity scores were 5, 3 and 2.
Prognostic factors for OS
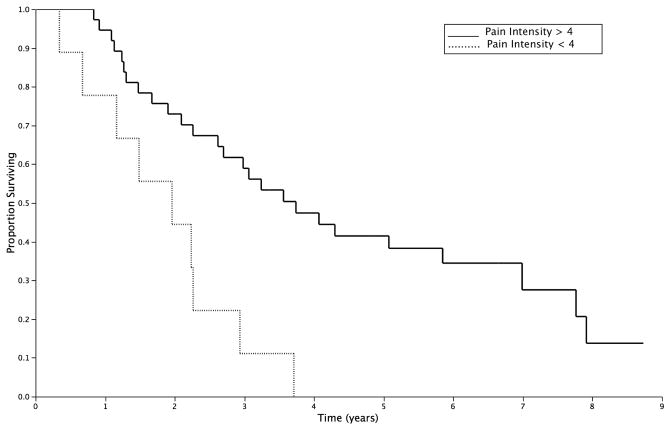
A subgroup of 54 patients were initially surveyed prior to any treatment intervention and then followed over time. In these patients, OS was not significantly associated with age, gender, baseline QOL (FACT-C score) in univariate analysis. However, both treatment group and pain intensity score significantly impacted OS. One unit higher level of pain intensity was associated with an 18% higher relative risk of dying from any cause (Table 2). Both of these variables remained independent predictors of OS in multivariate analysis (Table 2). Patients reporting a pain intensity score of 4 or less at baseline enjoyed a significantly longer median OS when compared to those reporting a high pain intensity score: 3.8 vs. 2.0years (p=0.001; Figure 4).
Table 2.
Prognostic factors for overall survival
| Estimate | Hazard ratio | Univariate p-value | Multivariate p-value | |
|---|---|---|---|---|
| Age (years) | −0.02 | 0.98 (0.96, 1.01) | 0.27 | n.a. |
| Gender | ||||
| Female | ||||
| Male | 0.12 | 1.13 (0.6, 2.11) | 0.71 | n.a. |
| Treatment | ||||
| Nonsurgical | ||||
| Noncurative surgery | −0.45 | 1.57 (0.67, 3.68) | 0.3 | 0.95 |
| Curative surgery | −1.11 | 0.33 (0.15, 0.71) | 0.005 | 0.002 |
| FACT-C score | −0.009 | 0.99 (0.97, 1.01) | 0.33 | n.a. |
| Pain intensity score | 0.17 | 1.18 (1.02, 1.37) | 0.03 | 0.009 |
Data from subgroup of 54 patients with baseline (pretreatment) FACT-C and pain scores.
Figure 4.
Overall survival of patients from diagnosis of LRRC as stratified by baseline pain intensity score.
Score ≤4: solid line; Score >4: hashed line.
Discussion
Traditionally, survival has been the primary efficacy measure of cancer treatments, and the main consideration in making treatment decisions. In the case of LRRC, however, survival gain must be considered in the context of the significant trade-offs experienced by patients during and after treatment. To our knowledge, this is the first prospective assessment of patient-reported outcomes associated with both curative and non-curative treatments for LRRC. Utilizing validated instruments and longitudinal analysis, we found curative surgery maintained QOL and PWB over the longest survival duration, while non-curative surgery and nonsurgical treatment were both associated with declines in PWB over shorter survival periods. Furthermore, pre-treatment pain score was an independent prognostic indicator of long-term OS, and pain may be common during and after treatments. Taken together, these findings identify pain as a focus for care of patients with LRRC, and help inform both the surgeon and the patient when challenged to balance survival benefit with treatment sequela.
Curative surgical resection led to a significantly longer median OS (7.1 years)from the diagnosis of LRRC when compared to other treatments(1.4 for NC surgery and 1.9 years for nonsurgical palliation). These findings are consistent with those of Hahnloser et al [19] who compared the median OS of patients undergoing curative vs. non-curative resection and reported a significant difference of 3.7 vs. 2.0 years from the date of surgery (p<0.001). Another series of 105 patients treated between 1997–1999 demonstrated median OS of 3.9 vs. 1.1 years from the date of surgery respectively (p<0.001).[20] The prospective nature of our protocol and the long length of follow-up available among our surviving patients allowed us to demonstrate the durability of the survival gain. Because previous literature has established that survival benefit requires radical resection of pelvic recurrent tumor to negative margins [3, 9, 19, 21–24], our observed median OS of 7.1 years reflects the highly selected nature of this patient cohort. Indeed, curative resection is typically feasible in only 20–30% of all patients with LRRC.[3] Unique in our study, we demonstrated that in parallel to the observed differences in OS, the QOL scores from the PWB domain diverged after patients received different treatments: PWB was durably maintained over time after curative surgery but declined after non-curative treatments. Therefore, when curative resection can be achieved, patients may expect durable gains in overall survival and maintenance of physical well-being and QOL.
However, the majority of patients with LRRC are precluded from curative resection, by extensive pelvic disease, extra-pelvic metastases, or patient choice. Indeed, a recent population-based series found that 60% of the patients with LRRC were treated by either non-curative surgery (26%) or palliative chemoradiation (34%).[8] We observed median OS of 1.4 and 1.9 years in patients undergoing non-curative surgery and nonsurgical interventions (p=0.26). These figures largely corroborate those reported in other series [3, 8, 19, 20] and reflect the reported efficacy of modern systemic therapy for patients with stage IV colorectal cancer. Although quality rather than quantity of life may be the primary concern among these patients, no comparative QOL data had been available in the literature. We detected no significant differences in OS, QOL scores, or pain symptom scores at any time point, between patients undergoing non-curative surgery or palliative treatments. These findings further support our previous observation that the median quality-adjusted survival did not differ between patients treated with non-therapeutic surgery vs. non-operatively: 0.81 vs. 1.01 quality-adjusted life years. [25] But when healthcare costs were considered, non-therapeutic surgery was far less cost-effective than non-operative treatments: $45,647 vs. $19,283(p<0.005). [25] Taken together, these suggest that non-curative surgery offers no distinct advantages over nonsurgical treatments. Thus, LRRC patients are likely best cared for by a multidisciplinary team where nonsurgical treatment options are maximally considered. In addition, the undertaking of non-curative surgery should be selective and goal-directed. While intestinal bypass or diversion may palliative some patients, abdominal exploration that leaves gross residual tumor likely benefits few. The incidence of non-therapeutic laparotomy has been reported to be as high as 23%[19], and it may be reduced by optimal preoperative tumor imaging using high-resolution pelvic MRI and PET, and by judicious assessment of what can realistically be accomplished intra-operatively based on surgical experience.
Pain is one of the most common presenting symptoms among patients with LRRC, and was specifically assessed by a validated instrument in this study. QOL and pain scores collected prior to treatment provided us a unique opportunity to investigate the prognostic value of these baseline patient-reported outcomes. Several investigators had demonstrated that baseline QOL can help predict OS in cancer patients [26], including those with pelvic tumors of prostatic and gynecologic origins. [27–32] In our study, the intensity of pain was an independent prognostic indicator of OS, after controlling for demographic and other clinical variables, with significantly shorter median OS observed for patients rating pain greater than 4 out of 10(3.8 vs. 2.0; p=0.001). One previous study had found that the median OS among patients who presented with pain symptoms was significantly shorter than others: 2.1 years, compared to 2.6 years for patients with non-pain symptoms and 3.3 years for those who were asymptomatic (p<0.001). However, the pain symptom was not formally rated and was not evaluated as an independent variable in a multivariate analysis.[19] One may speculate that greater pain reflects more advanced pelvic disease and thus worse OS. Because increasing pain is associated with worse QOL [33], greater pain may reflect poor psychosocial adaptation to their disease and worse QOL, which may in turn factor into poorer overall outcome. Besides the intensity of pain, our data also revealed the long duration of pain experienced by surviving patients. Factors that predispose to chronic pain in surgical patients had been identified to include: preexisting pain, repeat surgery, psychological vulnerability, radiation, chemotherapy, and finally depression and anxiety. [34] Taken together, we identify disease-and treatment-related pain as a significant symptom among patients with LRRC. Detailed knowledge and assessment of pain over time represents a first step toward providing optimal medical, psychosocial and multidisciplinary interventions to treat pain. The prognostic importance of pain underscores the critical importance of such interventions.
While the current study represents the largest prospective longitudinal assessment of QOL and pain in patients with LRRC, it is not without limitations. The cohort size remains relatively small after considering the different treatment groups. This precluded meaningful comparisons between treatment groups among the subgroup of patients followed from before treatment initiation. The absence of significant differences in other domains of QOL may also be partially accounted for by our cohort size. Alternatively, it may reflect biases inherent in the patient population collected in our study: patients with LRRC who are well enough to travel to a tertiary referral center likely enjoy or at least have adapted to adequate levels of emotional, social and functional well-being. Yet the significant differences that were able to be identified in PWB despite our cohort size signify the magnitude and the clinical relevance of our findings.
In conclusion, although durable survival and quality of life benefits can be achieved among patients with LRRC, careful patient selection is required. These gains were only realized in patients suitable for curative resection, as non-curative surgery offered little advantages over palliative treatments. Pain should be a targeted focus of multi-disciplinary survivor care in patients with LRRC. Finally, we have demonstrated that formal assessment of patient-reported outcomes complement standard clinical variables in predicting survival. The disease and treatment effects known only to the patients reported herein highlight the limitations of assessing clinical outcomes alone in patients with LRRC and provide significant complementary value in clinical decision-making.
Synopsis.
Surgical decisions in locally recurrent rectal cancerare challenging. We prospectively assessed patient-reported quality of life and pain after curativeornon-curative surgery and nonsurgical treatmentsover time, and report the prognostic value of these patient-reported outcomes.
Acknowledgments
Authors are grateful for the assistance in data analysis provided by Wei Qiao from Department of Biostatics, The University of Texas MD Anderson Cancer Center, Houston TX.
Footnotes
No disclosures
Accepted for oral presentation at the 63rd Annual Meeting of the Society of Surgical Oncology, St. Louis, MO, 2010.
References
- 1.Heald RJ. The ‘Holy Plane’ of rectal surgery. J R Soc Med. 1988;81:503–8. doi: 10.1177/014107688808100904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen MB, Laurberg S, Holm T. Current management of locally recurrent rectal cancer. Colorectal Dis. 2009 doi: 10.1111/j.1463-1318.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- 4.Silberfein EJ, Kattepogu KM, Hu CY, et al. Long-term survival and recurrence outcomes following surgery for distal rectal cancer. Annals of Surgical Oncology. 2010 doi: 10.1245/s10434-010-1119-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri-Brennan J, Steele RJ. The impact of recurrent rectal cancer on quality of life. Eur J Surg Oncol. 2001;27:349–53. doi: 10.1053/ejso.2001.1115. [DOI] [PubMed] [Google Scholar]
- 6.de Chaisemartin C, Penna C, Goere D, et al. Presentation and prognosis of local recurrence after total mesorectal excision. Colorectal Dis. 2009;11:60–6. doi: 10.1111/j.1463-1318.2008.01537.x. [DOI] [PubMed] [Google Scholar]
- 7.Bakx R, Visser O, Josso J, Meijer S, Slors JF, van Lanschot JJ. Management of recurrent rectal cancer: a population based study in greater Amsterdam. World J Gastroenterol. 2008;14:6018–23. doi: 10.3748/wjg.14.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2007;14:447–54. doi: 10.1245/s10434-006-9256-9. [DOI] [PubMed] [Google Scholar]
- 9.Bedrosian I, Giacco G, Pederson L, et al. Outcome after curative resection for locally recurrent rectal cancer. Dis Colon Rectum. 2006;49:175–82. doi: 10.1007/s10350-005-0276-5. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Bigas MA, Chang GJ, Skibber JM. Barriers to rehabilitation of colorectal cancer patients. J Surg Oncol. 2007;95:400–8. doi: 10.1002/jso.20778. [DOI] [PubMed] [Google Scholar]
- 11.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 12.Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res. 1999;8:181–95. doi: 10.1023/a:1008821826499. [DOI] [PubMed] [Google Scholar]
- 13.Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330:592–6. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 14.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 15.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Cleeland CS. The Brief Pain Inventory User Guide. Houston, TX: The University of Texas M. D. Anderson Cancer Center; 2009. [Google Scholar]
- 18.FACT-C scoring sheet. [cited; Available from: www.facit.org.
- 19.Hahnloser D, Nelson H, Gunderson LL, et al. Curative potential of multimodality therapy for locally recurrent rectal cancer. Ann Surg. 2003;237:502–8. doi: 10.1097/01.SLA.0000059972.90598.5F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miner TJ, Jaques DP, Paty PB, Guillem JG, Wong WD. Symptom control in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2003;10:72–9. doi: 10.1245/aso.2003.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Boyle KM, Sagar PM, Chalmers AG, Sebag-Montefiore D, Cairns A, Eardley I. Surgery for locally recurrent rectal cancer. Dis Colon Rectum. 2005;48:929–37. doi: 10.1007/s10350-004-0909-0. [DOI] [PubMed] [Google Scholar]
- 22.Caricato M, Borzomati D, Ausania F, Valeri S, Rosignoli A, Coppola R. Prognostic factors after surgery for locally recurrent rectal cancer: an overview. Eur J Surg Oncol. 2006;32:126–32. doi: 10.1016/j.ejso.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Hansen MH, Balteskard L, Dorum LM, Eriksen MT, Vonen B. Locally recurrent rectal cancer in Norway. Br J Surg. 2009;96:1176–82. doi: 10.1002/bjs.6699. [DOI] [PubMed] [Google Scholar]
- 24.Kusters M, Dresen RC, Martijn H, et al. Radicality of resection and survival after multimodality treatment is influenced by subsite of locally recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2009;75:1444–9. doi: 10.1016/j.ijrobp.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Miller AR, Cantor SB, Peoples GE, Pearlstone DB, Skibber JM. Quality of life and cost effectiveness analysis of therapy for locally recurrent rectal cancer. Dis Colon Rectum. 2000;43:1695–701. doi: 10.1007/BF02236852. discussion 701–3. [DOI] [PubMed] [Google Scholar]
- 26.Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10:865–71. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 27.Nout RA, Putter H, Jurgenliemk-Schulz IM, et al. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC-2 trial. J Clin Oncol. 2009;27:3547–56. doi: 10.1200/JCO.2008.20.2424. [DOI] [PubMed] [Google Scholar]
- 28.Halabi S, Vogelzang NJ, Kornblith AB, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol. 2008;26:2544–9. doi: 10.1200/JCO.2007.15.0367. [DOI] [PubMed] [Google Scholar]
- 29.Carey MS, Bacon M, Tu D, Butler L, Bezjak A, Stuart GC. The prognostic effects of performance status and quality of life scores on progression-free survival and overall survival in advanced ovarian cancer. Gynecol Oncol. 2008;108:100–5. doi: 10.1016/j.ygyno.2007.08.088. [DOI] [PubMed] [Google Scholar]
- 30.Maisey NR, Norman A, Watson M, Allen MJ, Hill ME, Cunningham D. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur J Cancer. 2002;38:1351–7. doi: 10.1016/s0959-8049(02)00098-9. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan PW, Nelson JB, Mulani PM, Sleep D. Quality of life as a potential predictor for morbidity and mortality in patients with metastatic hormone-refractory prostate cancer. Qual Life Res. 2006;15:1297–306. doi: 10.1007/s11136-006-0003-2. [DOI] [PubMed] [Google Scholar]
- 32.Movsas B, Moughan J, Sarna L, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. J Clin Oncol. 2009;27:5816–22. doi: 10.1200/JCO.2009.23.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esnaola NF, Cantor SB, Johnson ML, et al. Pain and quality of life after treatment in patients with locally recurrent rectal cancer. J Clin Oncol. 2002;20:4361–7. doi: 10.1200/JCO.2002.02.121. [DOI] [PubMed] [Google Scholar]
- 34.Burton AW, Fanciullo GJ, Beasley RD, Fisch MJ. Chronic pain in the cancer survivor: a new frontier. Pain Med. 2007;8:189–98. doi: 10.1111/j.1526-4637.2006.00220.x. [DOI] [PubMed] [Google Scholar]