Abstract
The nematode Caenorhabditis elegans (C. elegans) adult hermaphrodite has 302 invariant neurons and is suited for cellular and molecular studies on complex behaviors including learning and memory. Here, we have developed protocols for classical conditioning of worms with 1-propanol, as a conditioned stimulus (CS), and hydrochloride (HCl) (pH 4.0), as an unconditioned stimulus (US). Before the conditioning, worms were attracted to 1-propanol and avoided HCl in chemotaxis assay. In contrast, after massed or spaced training, worms were either not attracted at all to or repelled from 1-propanol on the assay plate. The memory after the spaced training was retained for 24 h, while the memory after the massed training was no longer observable within 3 h. Worms pretreated with transcription and translation inhibitors failed to form the memory by the spaced training, whereas the memory after the massed training was not significantly affected by the inhibitors and was sensitive to cold-shock anesthesia. Therefore, the memories after the spaced and massed trainings can be classified as long-term memory (LTM) and short-term/middle-term memory (STM/MTM), respectively. Consistently, like other organisms including Aplysia, Drosophila, and mice, C. elegans mutants defective in nmr-1 encoding an NMDA receptor subunit failed to form both LTM and STM/MTM, while mutations in crh-1 encoding the CREB transcription factor affected only the LTM.
The major advantage of invertebrate systems for the study of learning and memory is the relative simplicity of their nervous systems. Furthermore, invertebrate nervous systems consist of so-called identified neurons whose size, position, electrical properties, basic synaptic connections, and physiological and behavioral functions are more or less invariant from animal to animal of a given species (Kandel 1976). In associative learning, particularly classical conditioning, animals learn to associate a conditioned stimulus (CS) with an unconditioned stimulus (US). Memory can last in various phases from as short as seconds, as is found in short-term memory (STM), or as long as hours to a lifetime, as is found in long-term memory (LTM). Between STM and LTM in Drosophila, amnesiac-dependent anesthesia-sensitive middle-term memory (MTM) exists for several hours (Tully and Quinn 1985; Folkers et al. 1993). The cellular and molecular mechanisms behind these phases of memory seem to be distinct (DeZazzo and Tully 1995; Hammer and Menzel 1995). For example, LTM, but not STM, can be disrupted by treatments such as electroconvulsive shock or inhibitors of protein synthesis (Davis and Squire 1984). Memory processing, storage, and retrieval are each remarkably dynamic, and one of the hallmarks of memory is a progressive consolidation from initially labile STM, which is short lived and vulnerable to disruption such as anesthesia, into LTM, which is highly resistant both to experimental manipulation and to the passage of time.
Typically, STM/MTM is induced by massed training, and LTM by spaced training. Spaced training consists of repeated training sessions with an intertrial interval (ITI) (also called “a resting interval”) and generates memory dependent on mRNA and protein synthesis, and massed training comprises repeated trials without an ITI and induces memory independent of mRNA and protein synthesis (Tully et al. 1994; Beck and Rankin 1995; Crow et al. 1997; Epstein et al. 2003; Fulton et al. 2005). The augmentation in memory induced by spaced training is called the spacing effect and is a common phenomenon in the animal kingdom, including humans (Carew et al. 1972; Tully et al. 1994; Gerber et al. 1998; Beck et al. 2000; Rose et al. 2002; Cepeda et al. 2006). An interstimulus interval (ISI) is also a crucial parameter affecting the outcome of classical conditioning in intact animal studies. In general, when presentation of a CS precedes that of a US by a brief interval, optimal conditioning is observed. For this “forward conditioning,” studies of CS–US interval effects typically show an asymmetric, inverted U-shaped gradient relating the magnitude of conditioning to the ISI (Jones 1962; Schneiderman and Gormezano 1964, Hawkins et al. 1986). In contrast, successful “backward conditioning,” in which presentation of a US precedes that of a CS, has also been observed less frequently (Dostalek 1976; Spetch et al. 1981; Durkovic and Damianopoulos 1986).
C. elegans detects various environmental cues such as odorants and tastants mainly through its amphid sensilla. The amphids are the largest chemosensory organs, and each amphid consists of 12 sensory neurons with ciliated dendrites, as well as one sheath and one socket cell (Ward et al. 1975; Ware et al. 1975). These amphid neurons have roles in chemotaxis, thermotaxis, mechanosensation, osmotaxis, and dauer pheromone sensation (Bargmann and Mori 1997; Driscoll and Kaplan 1997; Riddle and Albert 1997; de Bono and Maricq 2005; Bargmann 2006). Chemotaxis of C. elegans to cations, anions, cyclic nucleotides, and amino acids was first described by Ward (1973), and since then this list has been extended further and includes many olfactory stimuli (Bargmann et al. 1993). The sensory neurons required for chemosensory responses have been identified by laser microsurgery of identified neurons (Bargmann and Horvitz 1991). In addition, the wiring diagrams of all neurons have been reconstructed from electron micrographs of serial thin sections of the entire C. elegans body (White et al. 1986).
C. elegans can learn a variety of nonassociative and associative tasks (Ishihara et al. 2002; Mohri et al. 2005; Torayama et al. 2007; Ardiel and Rankin 2010). Mechanosensory habituation as nonassociative learning is one of the most studied learning paradigms in C. elegans (Rankin et al. 1990; Rankin and Broster 1992; Rose et al. 2002; Rose and Rankin 2006). Associative learning in C. elegans has first been suggested from the finding that worms return to their temperature of cultivation if they had food at that temperature (Hedgecock and Russell 1975). Most of associative learning paradigms in C. elegans are based on pairing chemical cues or cultivation temperature with food or starvation. Conditioning worms with sodium chloride in the absence of food leads to a significant reduction in chemotaxis compared with conditioning in the presence of food (Wen et al. 1997; Saeki et al. 2001; Tomioka et al. 2006). Similar observations have been made in olfactory paradigms (Colbert and Bargmann 1997; Morrison et al. 1999; Nuttley et al. 2002). C. elegans can also learn to avoid odors associated with infection by pathogenic bacteria, a behavior analogous to mammalian conditioned taste aversion (Zhang et al. 2005). Mutant screens for worms defective in learning have resulted in the identification of lrn-1 and lrn-2, which affect both taste learning and olfactory learning (Wen et al. 1997; Morrison et al. 1999). Therefore, screens based on these complex behaviors should be useful in the identification of many new genes.
In some cases, the C. elegans learning paradigms meet strict criteria for associative learning set forth in the psychology literature (Rankin 2000). More often, however, C. elegans learning paradigms have a mixed character in which the distinction between associative learning and nonassociative sensitization, habituation, and adaptation is not clear, particularly when pairing chemical cues or cultivation temperature with food or starvation (Bargmann 2006). This is partly because C. elegans behaviors are dramatically affected by the presence and absence of food (Gray et al. 2005). Rather than pairing chemical cues with food or starvation, therefore, it would be preferable for subsequent analysis of neuronal circuits responsible for associative learning and memory that two defined chemical cues are used for conditioning of worms. Indeed, diacetyl and acetic acid were successfully used as CS and US, respectively, to induce olfactory associative memory in C. elegans, although it was not shown whether the memory formation is dependent on protein synthesis or not (Morrison et al. 1999; Morrison and van der Kooy 2001).
In the present study we have developed a protocol for the study of aversive olfactory learning and associative LTM in C. elegans. In this paradigm, we conditioned worms with 1-propanol as a CS, and hydrochloric acid (HCl) as a US. Spaced training of worms with 1-propanol and HCl induced LTM, while massed training induced STM/MTM, which was disrupted by cold-shock anesthesia. The formation of the LTM, but not the STM/MTM, is dependent on mRNA and protein syntheses. Moreover, it has also been found that several C. elegans mutants are defective in the LTM formation.
Results
Aversive classical conditioning of worms with propanol and acidic pH
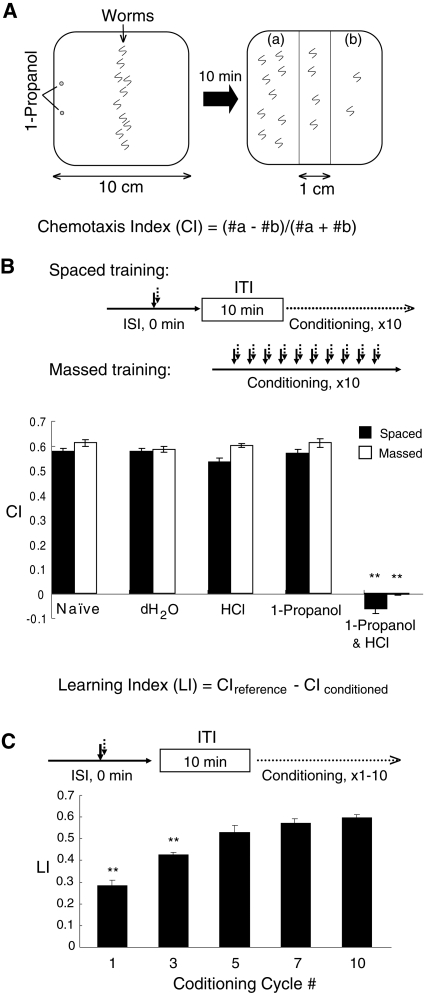
An olfactory cue, 1-propanol, is an attractant for C. elegans (Fig. 1; Bargmann et al. 1993), while worms are repelled from acidic pH lower than pH 4.0 (Sambongi et al. 2000; Supplemental Fig. S1). Utilizing 1-propanol and acidic pH as a CS and a US, respectively, we developed classical conditioning protocols for the study of associative learning in C. elegans. Worms repeatedly conditioned with deionized H2O (dH2O), HCl (pH 4.0), or 1.0% aqueous 1-propanol by spaced (with a 10-min ITI) or massed (without an ITI) training were as sensitive to 1-propanol as naive worms and were indistinguishably attracted to 1-propanol from naive worms in the chemotaxis assay (Fig. 1B; Supplemental Fig. S2). In contrast, worms conditioned with both 1-propanol and HCl by spaced or massed training avoided 1-propanol or were not attracted at all by 1-propanol, respectively (Fig. 1B). Learning index (LI) was calculated by subtracting the chemotaxis index (CI) (Fig. 1A) of conditioned worms with both 1-propanol and HCl from the CI of reference worms, which was the mean of CI values of worms conditioned with HCl alone and 1-propanol alone (Fig. 1B). When worms were conditioned repeatedly with (spaced training) or without (massed training) ITI by soaking them briefly (<1.0 sec) in a solution containing both 1-propanol and HCl, LI values of the trained worms were elevated and reached a plateau (Fig. 1C) as the cycle number of the trials was increased up to 10 times.
Figure 1.
Chemotaxis assay and classical conditioning of C. elegans. (A) Schematic representation of chemotaxis assay of worms to 1-propanol, which was carried out on square agar plates as described in the Materials and Methods. Worms were allowed to move freely on the agar for 10 min at room temperature. Chemotaxis index (CI) values were calculated from the equation shown. (B) CI values of worms to 1-propanol after spaced or massed training with chemicals indicated. Flowcharts of the spaced and massed training protocols used are shown at top. LI values were calculated by using the equation shown. The CI value of reference worms (CIreference) was the mean value of CI values of worms conditioned with HCl alone and 1-propanol alone. Bars are means ± SEM (n = 9 assays). Asterisks indicate statistically significant differences (**P <0.01) determined by one-way ANOVA with the Bonferroni/Dunn test, in comparison to the CI of naive worms. (C) LI values of worms repeatedly conditioned as indicated on the horizontal axis. At each cycle of the trials, worms were simultaneously conditioned with a solution containing 1.0% 1-propanol and 100 µM HCl (pH 4.0) with a 10-min ITI. Bars are means ± SEM (n = 9 assays). Asterisks indicate statistically significant differences (**P <0.01) determined by one-way ANOVA with Bonferroni/Dunn test, in comparison to the LI of worms trained with 10 conditioning cycles.
Optimal ISI and ITI lengths for memory acquisition and retention
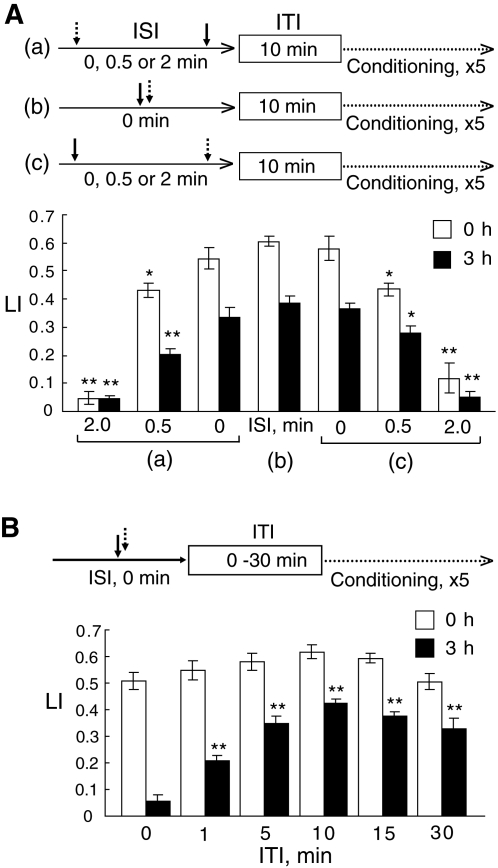
To optimize the conditioning protocols, we examined the effect of an ISI on memory acquisition and retention. The ISI is a period of time between two stimulations of worms with CS and US. Figure 2A shows conditioning protocols for “backward conditioning,” in which worms were stimulated with HCl before 1-propanol stimulation, “simultaneous conditioning,” in which worms were stimulated with a solution containing both 1-propanol and HCl, and “forward conditioning,” in which worms were stimulated with HCl after 1-propanol stimulation. The conditioning protocols with various lengths of ISI were repeated five times with a 10-min ITI.
Figure 2.
Effects of ISI and ITI lengths on memory acquisition and retention. (A) Effects of ISI lengths on memory acquisition and retention. Flowcharts of backward (a), simultaneous (b), and forward (c) conditioning protocols used are shown at top. In the backward conditioning (a), worms were first stimulated with HCl as a US (dotted arrow), and then with 1-propanol as a CS (solid arrow) for 0 min, 0.5 min, or 2 min after the US stimulation. In the simultaneous conditioning (b), worms were soaked in a solution containing both 1-propanol and HCl. In the forward conditioning (c), worms were first stimulated with 1-propanol and then with HCl for 0 min, 0.5 min, and 2 min after the CS stimulation. These procedures were repeated five times with a 10-min ITI, and then worms were examined for their LI immediately (open bars) and 3 h (closed bars) after the completion of the repetitive conditionings. Data are means ± SEM (n = 10 assays). Asterisks indicate statistically significant differences (*P <0.05; **P < 0.01) determined by one-way ANOVA with Bonferroni/Dunn test, in comparison to the LI of worms simultaneously conditioned in b. (B) Effects of ITI lengths on memory acquisition and retention. A flowchart of the conditioning used is shown at top. Worms were simultaneously stimulated by being soaked in a solution containing both 1-propanol and HCl, followed by various ITI lengths ranging from 0 min through 30 min. These conditioning procedures were repeated five times, and then the worms were tested for LI values immediately (open bars) or 3 h (closed bars) after the completion of the repetitive conditionings. Data are means ± SEM (n = 9 assays). Asterisks indicate statistically significant differences (**P <0.01) determined by one-way ANOVA with Bonferroni/Dunn test, in comparison to the LI of worms conditioned without an ITI.
Of ISI lengths tested, the simultaneous conditioning was the best for both memory acquisition and 3-h retention. LI values of worms conditioned with the CS or US immediately (a 0-min ISI) after US or CS, respectively, were statistically indistinguishable from those of worms simultaneously conditioned with a solution containing both 1-propanol and HCl. However, LI values measured immediately or 3 h after the final trial of the conditioning were decreased with longer ISI lengths in both of the forward and backward conditionings. When an ISI between the CS and US was longer than 2 min, both of the forward and backward conditionings failed to induce the memory. Thus, the simultaneous conditioning is most efficient for inducing the associative memory of two stimuli, 1-propanol and HCl.
As shown above in Figure 1C, multiple trials of the conditioning enhanced the LI. Studies of other organisms have shown that an ITI between conditioning trials is a crucial factor in the efficacy of memory formation, memory retention in particular (Yin et al. 1994; Carew 1996). Therefore, we also examined the effect of ITI lengths on the memory acquisition and retention. Worms were given five trains of the conditioning, of which ITI lengths ranged from 0 min through 30 min. The memory acquisition and retention were analyzed by measuring the LI values immediately and 3 h, respectively, after the trainings with various ITI lengths (Fig. 2B). Five trial sessions with no ITI (massed training) induced memory statistically indistinguishable from that induced by the spaced training with ITIs when assayed with the LI immediately after the trainings. However, the conditioned response was no longer observed beyond 3 h. In contrast, when assayed 3 h after the training, the LI of worms conditioned by the spaced training was elevated as the ITI length was increased up to 10 min, and then the LI was gradually decreased when ITIs were longer than 10 min. These results demonstrate that the 10-min ITI is most efficient for worms to retain the memory for 3 h after the training. For subsequent experiments, therefore, we conditioned worms by repeating the trial 10 times with or without a 10-min ITI as spaced or massed conditioning, respectively.
Memory retention and extinction
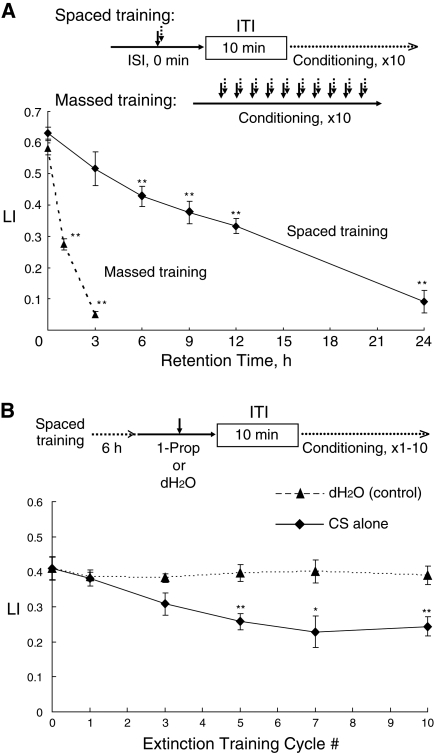
With optimized ISI and ITI lengths as well as with optimal trial numbers of the conditioning, we also measured the period of time (retention time) that the memory induced by the massed or spaced training was retained. Well-fed worms were conditioned 10 times by massed or spaced training with a solution containing both 1-propanol and HCl, and then the worms were transferred to NGM plates with a bacterial lawn, where they were allowed to move and eat at 20°C during retention intervals. Figure 3A shows various retention times of the memory induced by the massed or spaced training. Memory acquisition after the massed and spaced training was similar to each other. However, memory induced by the massed training was no longer observable within 3 h, as also shown above in Figure 2B (a 0-min ITI). In contrast, memory induced by the spaced training was retained for up to 24 h.
Figure 3.
Memory retention and extinction learning. (A) Memory retention induced by massed or spaced training. Flowcharts of the spaced and massed training protocols used are shown at top. In the spaced training, worms were simultaneously stimulated by being immersed in a solution containing 1-propanol and HCl. This procedure was repeated 10 times with a 10-min ITI, and the LI of the worms was assayed 0 h through 24 h (retention intervals) after the completion of the spaced training (solid line). In the massed training, worms were simultaneously stimulated with 1-propanol and HCl. After this conditioning was repeated 10 times without an ITI, the worms were assayed for LI 0 h, 1 h, and 3 h after the completion of the massed training (broken line). Data points are means ± SEM (n = 9–15 assays). Asterisks indicate statistically significant differences (**P <0.01) determined by one-way ANOVA with Turkey-Kramer's test, in comparison to the LI measured immediately after the trainings. (B) Extinction learning. After the spaced training 10 times simultaneously with 1-propanol and HCl described above in A, worms were transferred to NGM plates seeded with E. coli and were allowed to freely move and eat at 20°C for 6 h. The worms were then conditioned only with the CS (solid line) in the absence of the US as described in “Extinction” of Materials and Methods. This extinction training was repeated one to 10 times as indicated on the horizontal axis. Immediately after the extinction learning, worms were tested for LI. As a control (broken line), worms were also immersed in dH2O, instead of 1-propanol, at room temperature. Data points are means ± SEM (n = 9 assays). Asterisks indicate statistically significant differences (*P < 0.05; **P <0.01) determined by two-sided Student's t-test, in comparison to LI values of worms after conditioning with dH2O by the same cycle number.
Furthermore, when the worms conditioned by the spaced training were repeatedly exposed to the CS in the absence of the US, their LI values were progressively decreased (Fig. 3B), suggesting that extinction learning can also occur in the simple C. elegans nervous system. During the extinction, the conditioned worms showed a statistically significant decrease in LI values, compared with worms treated with dH2O as a negative control in the same way as that with the CS alone. This decrease is not due to habituation or adaptation, since chemotactic activity of the worms exposed repeatedly to the CS alone is similar to that of the control worms treated with dH2O (Supplemental Fig. S3). Under the experimental conditions for the extinction learning, the decrease in LI values was not complete even after 10-cycle extinction training trials as observed in other organisms such as Aplysia (Carew et al. 1981) and Drosophila (Qin and Dubnau 2010).
Propanol-specific associative learning
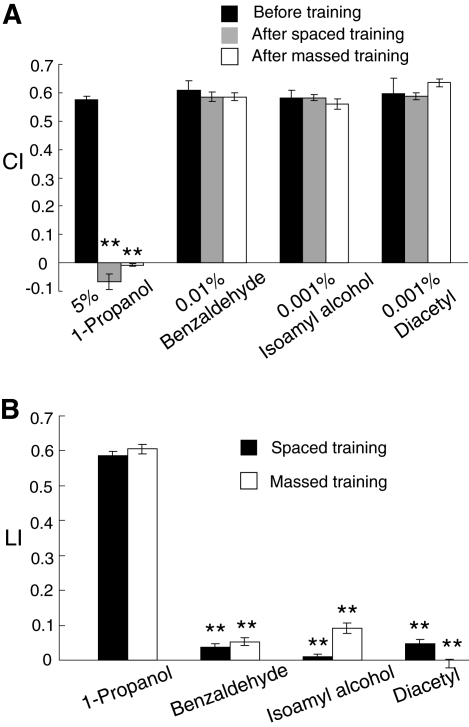
We then asked whether or not the STM/MTM and LTM formations were specific for 1-propanol. Worms conditioned simultaneously 10 times with 1-propanol and HCl by the massed or spaced training were tested for their chemotaxis to benzaldehyde, isoamyl alcohol, and diacetyl (Fig. 4A), and their LI values were calculated from their CI values. The concentrations of the stimuli in the chemotaxis assay were adjusted based on the CI values of naive worms to the stimuli. As shown in Figure 4B, the worms conditioned with 1-propanol and HCl could learn 1-propanol as a specific stimulus, since they could not associate the US with benzaldehyde, isoamyl alcohol, or diacetyl. These stimuli are sensed by AWA or AWC olfactory sensory neurons (Bargmann et al. 1993), which are responsible for the detection of most, if not all of the attractive olfactory cues. Therefore, it is likely that 1-propanol is also sensed by one of these neurons, suggesting that two different stimuli sensed by the same sensory neuron can induce memory in different ways, probably through different neural circuits, from each other.
Figure 4.
Propanol-specific associative learning. (A) Wild-type worms were conditioned 10 times simultaneously with 1-propanol and HCl by spaced (with a 10-min ITI) or massed training. Immediately after the training, worms were assayed for their ability of chemotaxis to 5% 1-propanol, 0.01% benzaldehyde, 0.001% isoamyl alcohol, or 0.001% diacetyl spotted on the edge of chemotaxis agar plates. Note that chemotactic behaviors of the trained worms to benzaldehyde, isoamyl alcohol, and diacetyl were not affected by the training. Data are means ± SEM (n = 9 assays). Asterisks indicate statistically significant differences (**P <0.01) determined by one-way ANOVA with Bonferroni/Dunn test, in comparison to the CI of naive worms (before training). (B) Associative learning of 1-propanol with HCl was specific for 1-propanol. LI values were calculated from the data shown in A. Data are means ± SEM (n = 9 assays). Asterisks indicate statistically significant differences (**P <0.01) determined by one-way ANOVA with Bonferroni/Dunn test, in comparison to the LI of worms assayed with 1-propanol as a stimulus.
Effect of translation and transcription inhibitors on memory acquisition and retention
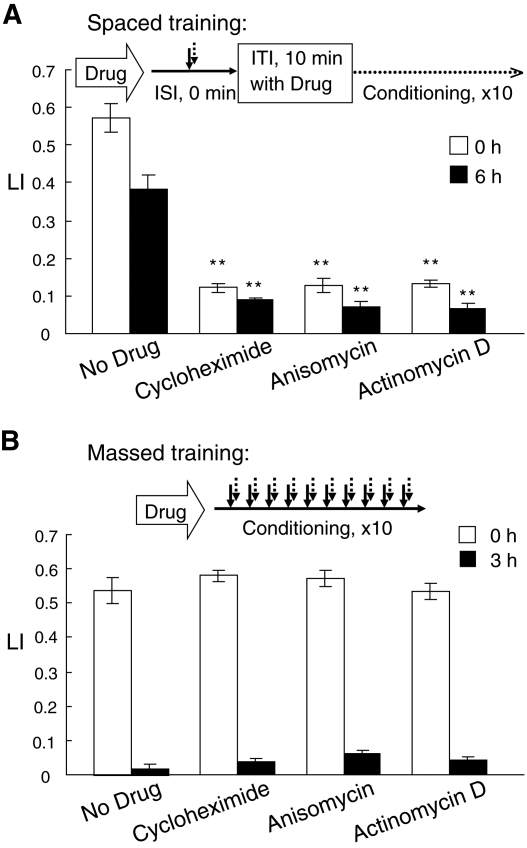
Next, we examined the effect of mRNA and protein synthesis inhibitors on memory induced by massed or spaced training since LTM, but not STM/MTM requires both protein synthesis and mRNA transcription (Flood et al. 1973; Mizumori et al. 1987; Tully et al. 1994; Crow et al. 1997). Before the spaced training, worms were cultivated on agar plates spread with bacteria in the presence of 0.3 µg/mL of cycloheximide, 0.3 µg/mL of anisomycin, or 0.1 µg/mL of actinomycin D at a final concentration for 2 h, and then during the resting intervals of the spaced training, worms were also placed on agar plates spread with bacteria that contain the drug. Therefore, worms were cultivated on agar plates containing the drug for ∼3.7 h in total. As shown in Figure 5A, the spaced training of the worms failed to induce the memory, indicating that both transcription and translation are required for memory formation. As shown in Figure 5B, in contrast, memory induced by the massed training required neither transcription nor translation, since the memory was normally induced in worms cultivated on agar plates spread with bacteria in the presence of the drug for 4 h before the conditioning started. The final concentrations of the drugs in agar plates were determined as the lowest concentrations that prevent the LTM formation (Supplemental Fig. S4), but did not affect worm's chemotaxis to 1-propanol (Supplemental Fig. S5). Under similar conditions used for the training in the presence of the drug, ∼50% of protein synthesis was indeed inhibited by the drug treatment as shown in Supplemental Figure S6. These results indicate that the memories generated by the massed and spaced trainings are STM/MTM and LTM, respectively.
Figure 5.
Effect of translation and transcription inhibitors on memory acquisition and retention. (A) A flowchart of spaced training used is shown at top. Worms were cultivated on an NGM plate spread with bacteria, which contained one of the indicated drugs for 2 h, and trained 10 times with a 10-min ITI as shown in the flowchart. During the ITI, worms were placed on an NGM plate with a bacterial lawn, which contains the indicated drug. The worms were tested for their LI by chemotaxis assay immediately (open bars) and 6 h (closed bars) after the completion of the spaced training. (B) A flowchart of massed training used is shown at top. Worms were cultivated for 4 h on an NGM plate spread with a bacterial lawn, which contained one of the indicated drugs, and trained 10 times without an ITI. The worms were tested for their LI by chemotaxis assay immediately (open bars) and 3 h (closed bars) after the completion of the massed training. Data are means ± SEM (n = 9 assays). Asterisks indicate statistically significant differences (**P <0.01) determined by one-way ANOVA with the Bonferroni/Dunn test, in comparison to the LI of worms untreated with drug.
Sensitivity of memory to disruption
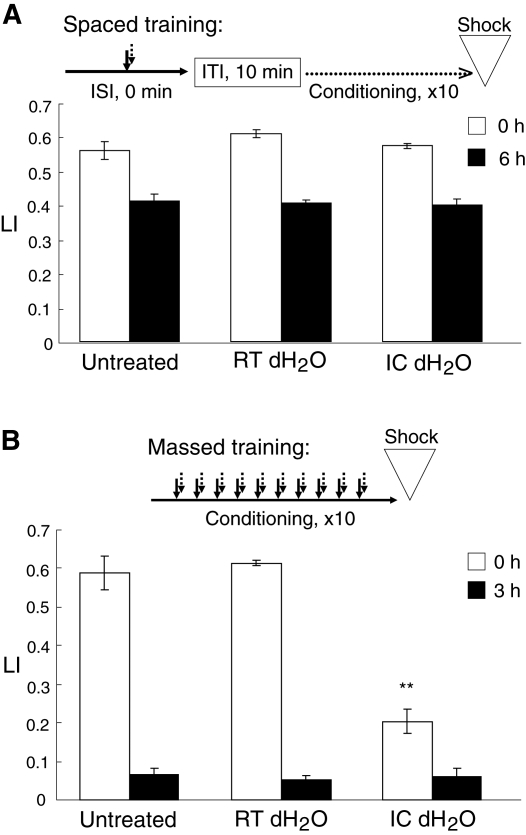
Before consolidation, memory is vulnerable to disruption and can be sensitive to anesthesia such as cold shock (Tully et al. 1994). Therefore, we examined whether the memory induced by the massed training, but not the memory induced by the spaced training, is sensitive to cold shock. Immediately after the massed or spaced training, worms were anesthetized by soaking them in ice-cold dH2O for 5.0 sec. After recovering the worms at room temperature for 5 min on an agar plate with bacteria, the worms were assayed for chemotaxis to 1-propanol. As shown in Figure 6, the cold shock did not affect the memory acquisition and retention induced by the spaced training, while the memory induced by the massed training was markedly erased by the cold shock.
Figure 6.
Sensitivity of memory to disruption. (A) A flowchart of spaced training used is shown at top. Worms were simultaneously stimulated with 1-propanol and HCl. Immediately after repeated conditioning 10 times with a 10-min ITI, the worms were soaked in either room-temperature (RT) dH2O or ice-cold (IC) dH2O, and then tested for LI values after being cultivated on NGM plates with a bacterial lawn at 20°C for 0 h (open bars) and 6 h (closed bars). (B) A flowchart of massed training used is shown at top. Worms were conditioned 10 times with a solution containing both 1-propanol and HCl, and then soaked in either room-temperature (RT) dH2O or ice-cold (IC) dH2O. Immediately (open bars) or 3 h (closed bars) after the treatment, the worms were tested for LI by chemotaxis assay. Data are means ± SEM (n = 9 assays). Asterisks indicate a statistically significant difference (**P <0.01) determined by one-way ANOVA with Bonferroni/Dunn test, in comparison to the LI of worms untreated.
These results indicate that the memory after the spaced training is resistant to cold shock, and is consolidated during the repetitive conditioning with a 10-min ITI. Since the memory induced by the spaced training was retained for ∼24 h, required transcription and translation for its formation, and was resistant to the cold-shock anesthesia, it is therefore classified as LTM by definition. In contrast, the memory after the massed training is classified as STM/MTM, since it was no longer observable within 3 h, required neither protein synthesis nor mRNA transcription for its acquisition, and was disrupted by the cold-shock anesthesia. Until amnesiac dependency of the memory is examined, however, it cannot be distinguished whether the memory is STM or MTM. Unfortunately, on the C. elegans genome, an ortholog of the amnesiac gene has not yet been found.
C. elegans mutants defective in STM/MTM and/or LTM
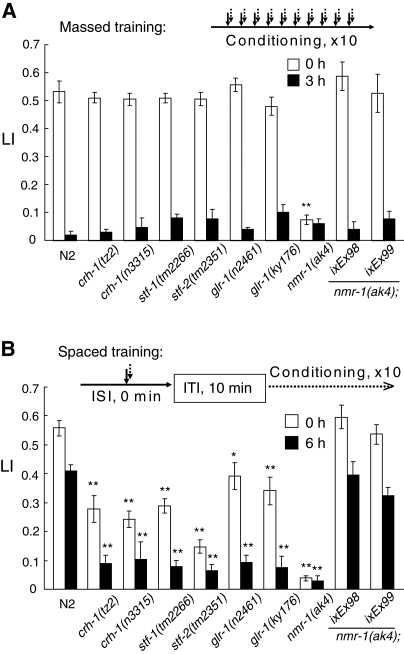
The C. elegans genome encodes “learning and memory genes,” including crh-1 encoding the ubiquitous transcription-factor CREB (cAMP responsible element binding protein), glr-1 and nmr-1 encoding α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type and N-methyl-D-aspartate (NMDA)-type glutamate receptor subunits, respectively, and stf-1 and stf-2 encoding the double-stranded RNA-binding protein Staufen isoforms. These genes have been shown to play crucial roles in classical conditioning in Aplysia, C. elegans, Drosophila, and mice (Dash et al. 1990; Morrison and van der Kooy 2001; Dubnau et al. 2003; Rose et al. 2003; Xia et al. 2005). Therefore, we also examined whether these “learning and memory genes” are involved in the generation of memory after the massed or spaced training (Fig. 7). Like the wild-type N2, all of the mutants did not show detectable defects in avoidance of HCl, pH 4.0 (Supplemental Fig. S1) or in motility after the spaced or massed training (Supplemental Table S1). However, the mutants were slightly less sensitive to 1-propanol than the wild type (Supplemental Table S2), and 1-propanol concentrations used for chemotaxis assay were therefore adjusted based on the concentrations that produce similar CI values for wild type and mutants. Nonetheless, 1.0% aqueous 1-propanol was used for the spaced and massed trainings, since higher concentrations than 1.0% affected chemotactic activity of worms to 1-propanol (Supplemental Fig. S2). As shown in Figure 7, 1.0% aqueous 1-propanol was successfully used to condition all of the wild type and mutants, except for nmr-1, to induce STM/MTM at similar levels. Mutations in crh-1, glr-1, and stf-1 and stf-2 affected only the formation of the LTM, whereas mutants defective in nmr-1 failed to form both the STM/MTM and LTM. The nmr-1(ak4) transgenic lines, nmr-1(ak4);ixEx98 and 99, which have an extrachromosomal wild-type nmr-1 gene, were successfully trained to form the STM/MTM and LTM at the wild-type levels by the massed and spaced trainings, respectively. Hence, all of the genes tested were required for the acquisition and retention of the LTM. In contrast, none of the genes examined, except for nmr-1, was essential for the STM/MTM. These results are consistent with those in Aplysia, Drosophila, and mice.
Figure 7.
C. elegans mutants defective in learning and memory. (A) A massed training protocol of wild-type and mutant worms is shown at top. The worms were trained 10 times by being soaked in a solution containing both 1-propanol and HCl, and then tested for LI values by chemotaxis assay immediately (open bars) and 3 h (closed bars) after the training. (B) A spaced training protocol used for the worms indicated is shown at top. The worms were stimulated by being soaked in a solution containing both 1-propanol and HCl. This conditioning was repeated 10 times with a 10-min ITI. The worms were tested for LI values by chemotaxis assay immediately (open bars) and 6 h (closed bars) after the training. Data are means ± SEM (n = 9 assays). Asterisks indicate statistically significant differences (**P <0.01) determined by one-way ANOVA with the Bonferroni/Dunn test, in comparison to the LI of N2 worms.
Discussion
In the present study we have developed classical conditioning protocols for the study of associative learning and memory in C. elegans. The aversive olfactory conditioning with 1-propanol and HCl as a CS and US, respectively, has been shown to share many of the defining features of associative learning in vertebrate and invertebrate species, as exemplified by classical (Pavlovian) conditioning. These include stimulus and paring specificity, contiguity learning, and both short/middle-, as well as long-term retention. Furthermore, it is also possible to extinguish the learned behavior to some extent by extinction training, in which the presentation of the reinforcing stimulus is withheld. The STM/MTM and LTM are successfully induced by the massed training and spaced training, respectively; the LTM formation is protein synthesis dependent, while STM/MTM is not. Only the difference between the two training protocols is an ITI between the trials in the spaced training. The optimal ITI length was determined to be 10 min for both acquisition and 3-h retention of the LTM (Fig. 2B). This optimal ITI length is similar to those of other organisms, including fruit flies, honeybees, and crickets (Beck et al. 2000; Menzel et al. 2001; Matsumoto and Mizunami 2002; Giurfa et al. 2009). Although the spacing effect has long been observed at the behavioral level, the underlying cellular and molecular mechanisms are poorly understood. Mitogen-activated protein kinase (MAPK) activity has been implicated in memory formation in invertebrates and vertebrates (Kandel 2001; Kelleher et al. 2004; Mayford 2007; Cammarota et al. 2008), and more recent studies suggest that MAPK activation during ITI is required for LTM (Ye et al. 2008; Pagani et al. 2009).
There are convincing examples of classical conditioning that simultaneous pairing is as effective, or more effective than forward pairing (Heth and Rescorla 1973; Mahoney and Ayres 1976; Rescorla 1980; Tully and Quinn 1985; Barnet et al. 1991, 1993; Lent and Kwon 2004). Consistent with these examples, the results described in the present study demonstrate that the most efficacious procedure for the classical conditioning inducing the LTM is to have the simultaneous onset of the CS and US, and also show that the backward pairing is as effective as the forward pairing (Fig. 2A). The closer the CS and US are together in time, the greater the LTM induced. Indeed, Lin and Glanzman (1997) have found that associative long-term synaptic changes are sensitive only to the amount of temporal contiguity between stimuli, and can mediate simultaneous, backward, and forward pairings. In contrast, there are results indicating that the most efficacious procedure for many types of classical conditioning is to have the onset of the CS precede that of the US (Maier et al. 1976; Hellstern et al. 1998; Matsumoto and Mizunami 2002). Temporal parameters that characterize different classical conditioning paradigms may result from underlying, intrinsic different mechanisms. Alternatively, all types of associative learning may be intrinsically sensitive only to the temporal correlation between stimuli, not to stimulus order. According to this view, the order specificity that characterizes some forms of classical conditioning may be due to neuronal circuits that transmit the stimuli to a critical site for associative learning.
In the present study, we have also analyzed the effects of various mutations of genes, nmr-1, glr-1, crh-1, stf-1, and stf-2, on the formation of STM/MTM and LTM (Fig. 7). All of the mutations except for nmr-1 affected only the LTM; the nmr-1 mutant was defective in the formation of both STM/MTM and LTM. In C. elegans, nmr-1, a homolog of NMDA receptor subunits, is expressed only in six pairs of neurons (AVA, AVD, ADE, RIM, AVG, and PVC) (Brockie et al. 2001a,b). In these neurons, the NMDA receptor may act as a molecular coincidence detector for 1-propanol and HCl signals in synaptic plasticity, where synaptic strengthening required for both STM/MTM and LTM can result from coincidental firing of the pre- and postsynaptic neurons (Gustafsson and Wingstrom 1988; Kauer et al. 1988; Bliss and Collingridge 1993; Bailey et al. 2000). Influx of calcium through the NMDA receptor into the postsynaptic cells can result in activation of several protein kinases including MAPK (Bailey et al. 2000; Wang et al. 2007), which may in turn phosphorylate the transcription-factor CREB encoded by crh-1 expressed in C. elegans head neurons (Kimura et al. 2002; Suo et al. 2009). CREB is a member of the basic region/leucine zipper (bZip) family of transcription factors, which is regulated by increases in the intracellular levels of cAMP and calcium (Carlezon et al. 2005), and activates a cascade of genes that leads to LTM (Dash et al. 1990; Yin et al. 1994; Kogan et al. 1996). The stf-1 and stf-2 encode highly conserved dsRNA-binding Staufen proteins and are involved in the formation of LTM cooperatively with pumilio in Drosophila (Dubnau et al. 2003). Vertebrate Staufen localizes to dendritic sites in hippocampal neurons and are implicated in translational control at distal synaptic sites. Depletion of Staufen was found to significantly reduce both β-actin mRNA containing ribonucleoproteins and β-actin mRNA at dendritic sites, suggesting Staufen regulates the dendritic cytoskeleton (Loya et al. 2010). Also, Staufen may regulate the synthesis of glutamate receptors through microRNAs (Karr et al. 2009). glr-1, which encodes one of subtypes of ionotropic glutamate receptor channels, is critical for LTM in C. elegans, and the expression and localization altered by conditioning are necessary for the formation of long-term habituation (Rose et al. 2005). It has also been found that glr-1 mutants are deficient in an olfactory associative learning task, in which diacetyl is paired with acetic acid, as well as in nonassociative learning (habituation) with the same diacetyl stimulus (Morrison and van der Kooy 2001). In this associative learning paradigm, the attractive response of naive worms to diacetyl was reduced after the conditioning, but did not completely disappear like the learned behavior seen in the present study. This may be due to the short 1.0-min ITI, and/or due to dual aversive and appetitive effects of acetic acid (Frøkjær-Jensen et al. 2008). The associative STM/MTM induced by the massed training in the present study may be different from the nonassociative learning (habituation), although it is not clear whether the nonassociative habituation is STM or not. The STM/MTM of the present study may be formed at the level of neural circuits since NMDA receptors are involved, while the nonassociative habituation may occur in the sensory neuron AWA itself.
Thus, we have found that C. elegans can learn and form associative LTM after spaced training, which is retained for >24 h after the conditioning, is sensitive to inhibitors of mRNA and protein synthesis, while associative STM/MTM induced by massed training, which is no longer observable within 3 h after the conditioning, is resistant to the inhibitors. These are major features of LTM and STM/MTM (Tully et al. 1994; Crow et al. 1997; Epstein et al. 2003; Fulton et al. 2005). Furthermore, the associative LTM is stimulus and paring specific, depends on contiguous CS–US stimulation, and can be partially extinguished by extinction learning. During the course of the present study, Kauffman et al. (2010) have reported long-term associative memory induced by spaced training with butanone and food in C. elegans, in which cold shock efficiently erased the LTM, but not STM/MTM. This is different from our results, in which cold shock erased only the STM/MTM, but not LTM as observed in other organisms (Yamada et al. 1992; Tully et al. 1994; Tamura et al. 2003). In the cold-shock protocol by Kauffman et al. (2010), worms were placed at −20°C for 15 min, in contrast to the protocol in the present study, in which worms were placed in ice-cold water for 5.0 sec. The two different cold-shock protocols may have different effects on LTM and STM/MTM.
Materials and Methods
Strains and culture media
All strains were derived from the wild-type C. elegans variety Bristol, strain N2. Mutant strains, crh-1(tz2), glr-1(n2461), and nmr-1(ak4) used in this study were provided by the Caenorhabditis Genetics Center at the University of Minnesota, Minneapolis, MN. Other mutants, stf-1(tm2266) and stf-2(tm2351), were obtained from National Bioresource Project for the Nematode (Tokyo Women's Medical University School of Medicine, Tokyo, Japan). crh-1(n3315) and glr-1(ky176) were generous gifts from Mark Alkema (University of Massachusetts School of Medicine, MA) and Andres Maricq (University of Utah, Salt Lake City, UT), respectively. The wild-type N2 and mutant strains were grown on NGM (50 mM NaCl, 20 g/L of agar, 2.5 g/L of peptone, 1.0 mM cholesterol, 1.0 mM CaCl2, 1.0 mM MgSO4, and 25 mM potassium phosphate at pH 6.0) seeded with Escherichia coli (E. coli) OP50 or NA22 to adulthood under unstarved conditions at 20°C using standard methods (Brenner 1974).
Transgenic strains
Transgenic lines were made using standard protocols (Mello et al. 1991). To generate nmr-1 rescue lines, a 13-kb nmr-1 genomic DNA fragment was amplified by PCR, using oligonucleotide primers, 5′-CACCGCGGCCGCGACAAAAGAAAACCAAATATTGTA and 5′-ATCTGCAGCATGCTGAGTTCCGAATCACTGATC, and N2 genomic DNA as a template. A resulting PCR product was purified from agarose gel by using a QIAquick Gel Extraction Kit (QIAGEN), and then the purified PCR product, 10 ng/μL, was coinjected with lin-44p::GFP (Murakami et al. 2001), 50 ng/μL, into nmr-1(ak4). Two days after DNA injection, four worms expressing GFP were allowed to self-fertilize. Two transgenic lines that express GFP at high frequencies were termed as nmr-1(ak4);ixEx98[nmr-1 gDNA; lin-44p::GFP] and nmr-1(ak4);ixEx99[nmr-1 gDNA; lin-44p::GFP], and were used as nmr-1(ak4)-rescued lines for experiments. The genotype of the transgenic lines was confirmed by PCR amplification of a portion of the gene using oligonucleotide primers, 5′-GTTCAACGTTACATTGAGGTAG and 5′-CTTCATATTCACAAGCCCAAGTCTT, and genomic DNA as a template (Supplemental Fig. S7). To prepare genomic DNA, worms suspended in lysis buffer (2.5 mM KCl, 5 mM Tris-HCl at pH 8.0, 0.23% Tween-20, and 200 µg/mL of proteinase K) were incubated at 55°C for 4 h. Genomic DNA was purified from the lysates by phenol/chloroform extraction, followed by ethanol precipitation.
Worm preparation and chemotaxis assay
Well-fed worms on day 4 after hatching were used to minimize the effects of age, locomotion, and olfactory sensitivity on assays. Naive worms, about 100, were removed from their NGM plates immediately before testing by washing them off with a 0.25% aqueous gelatin (WAKO Pure Chemical Industries) solution into 1.5-mL Eppendorf tubes (Eppendorf). After the tubes were allowed to stand still for 2 min at room temperature, worms were collected at the bottom of the tubes by removing the supernatant with a pipette or an aspirator. Likewise, the worms were washed twice with a 1.0-mL 0.25% aqueous gelatin solution. The worms were then placed along a central line of chemotaxis assay plates with a blunted pipette tip, and an excess of water was removed with a piece of Kimwipes (Kimberly-Clark).
Chemotaxis assay plates were prepared by mixing 15 g/L of Bactoagar (Becton Dickinson KK), 5 mL/L of 1.0 M potassium phosphate (pH 6.0), 1.0 mL/L of 1.0 M CaCl2, and 1.0 mL/L of 1.0 M MgSO4. These stock solutions were sterilized by autoclaving before mixing. Agar plates were made by pouring 14 mL of the mixture into square plates (10 cm × 10 cm) (Becton Dickinson), and then were left with lids at room temperature overnight. A total of 2 µL each of 5% (unless otherwise stated) aqueous 1-propanol (WAKO) was spotted at two places along the square plate edge (Fig. 1A). The worms were allowed to move freely on the plate for 10 min at room temperature. Chemotaxis assay was terminated by killing the worms by placing 1.0 mL of chloroform on the lid. A particular CI value was calculated as (number of worms in area “a” − number of worms in area “b”)/total number of worms in areas “a” and “b” (Fig. 1A). A learning index (LI) was calculated by subtracting the CI of conditioned worms (CIconditioned) from that of reference worms (CIreference) (Fig. 1B). The CIreference was the mean of CI values of worms treated with the CS alone and US alone as conditioned worms. Chemotaxis assay was also carried out by using 1-propanol or isoamyl alcohol diluted with dH2O, or benzaldehyde, or diacetyl diluted with ethyl alcohol as a stimulus, which was spotted along the edge of the chemotaxis assay plates. Unless otherwise stated, all of the chemotaxis assays were carried out at least in triplicate on three separate days (typically nine assays in total).
Simultaneous conditioning with CS and US
Before conditioning, worms were washed from their NGM plates directly into a worm collector that had been previously washed with 0.25% aqueous gelatin solution. Worm collectors were made from a transparent plastic pipe (3.5-cm length, 30-mm external diameter, 2-mm wall thickness) (Asahi Kasei) by attaching nylon mesh (30-μm mesh size) (SEFAR) to the bottom of the tube with glue (Aron Alpha/High Speed EX, Toagosei). A ∼50-mL mixture of 1.0% 1-propanol and 100 µM HCl (pH 4.0), in a glass slide staining dish with a lid (Matsunami Glass) was used for simultaneous conditioning of worms with CS and US. A 100-μM aqueous HCl (pH 4.0) was made by diluting concentrated HCl (Nacalai Tesque) with dH2O, which was prepared by using Millipore Synthesis A10, immediately before use. The concentration of HCl as US was determined as the lowest acidic pH that did not affect chemotaxis of wild-type worms to 1-propanol after conditioning five times by spaced training with a 10-min ITI (Supplemental Fig. S8). The simultaneous conditioning was carried out by briefly (<1.0 sec) dipping a worm collector with worms into a glass slide staining dish with a solution containing both 1-propanol and HCl. Then, the worm collector was gently immersed once in ∼1.0 L of dH2O in a beaker. An excess of water in the collector was removed with a piece of Kimtowels (Kimberly-Clark), and then the collector with worms was placed on an NGM plate seeded with E. coli OP50 during an ITI for the worms to rest. This cycle of conditioning was repeated up to 10 times with various ITI lengths. After the final trial, the worms were washed with ∼1.0 L of dH2O as described above, and then suspended in a ∼1.0-mL 0.25% aqueous gelatin solution. The worm suspension was transferred to a 1.5-mL Eppendorf tube with a blunted pipette tip, and the worms were collected to the bottom of the tube by gravity for ∼2 min at room temperature. Likewise, the worms were washed twice with ∼1.0 mL 0.25% aqueous gelatin solution. After the wash, the worms were placed along a central line on a chemotaxis assay plate with a blunted pipette tip, and the gelatin solution was removed with a piece of Kimwipes as much as possible.
Conditioning with various ISI lengths
Conditioning with ISI was carried out as described above in the simultaneous conditioning section, except that brief (<1.0 sec each) exposures to CS and US were separated by various lengths of time ranging from 0 sec to 2 min as an ISI. The order of stimulation with CS and US was also changed as forward (CS → US) or backward (US → CS) conditioning. After a brief (<1.0 sec) exposure to the second stimulus, worms were briefly washed by gently immersing a worm collector in ∼1.0 L of dH2O in a beaker. After removing an excess of water with a piece of Kimtowels, the worms were placed on an NGM plate seeded with E. coli OP50 during a 10-min ITI as described above. After repeating the conditioning five times, the worms were transferred to a chemotaxis assay plate for testing as described above. All other aspects of conditioning, testing, and scoring were exactly as described above.
CS-alone conditioning
Conditioning with a CS alone, as a reference for unconditioned effects of 1-propanol, was performed as described above. A glass slide staining dish containing ∼50 mL 1-propanol diluted at a ratio of 1/100 with dH2O was used for the CS-alone conditioning. After a brief (<1.0 sec) exposure to the CS, worms were immersed in dH2O instead of HCl during the conditioning. All other aspects of conditioning, testing, and scoring were exactly as described above.
US-alone conditioning
Conditioning with a US alone, as a reference for unconditioned effects of HCl (pH 4.0), was performed as described above. A glass slide staining dish containing ∼50 mL 100 µM HCl (pH 4.0) was used for the US-alone conditioning. After briefly (<1.0 sec) being immersed in dH2O instead of 1-propanol, worms were briefly (<1.0 sec) immersed in 100 µM HCl (pH4.0) in a glass slide staining dish at room temperature, and were then gently washed with dH2O as describe above. All other aspects of conditioning, testing, and scoring were exactly as described above.
Massed training
Worms were conditioned with a CS and US simultaneously, with ISI, with the CS alone, or with the US alone as described above. The trial was repeated either five or 10 times without an ITI between two consecutive trials. Immediately after washing with dH2O, worms were subjected to the next cycle of the trial. All other aspects of conditioning, testing, and scoring were exactly as described above.
Spaced training
Worms were conditioned as described above, except that the worms rested on an NGM plate seeded with E. coli OP50 for 10 min (unless otherwise indicated) between two consecutive trials at room temperature. The trial was repeated either five or 10 times, unless otherwise stated. All other aspects of conditioning, testing, and scoring were exactly as described above.
Extinction
After spaced training 10 times with a 10-min ITI described above, worms were transferred to NGM plates seeded with E. coli OP50, and were allowed to freely move and eat at 20°C for 6 h. The worms were then washed from their NGM plates directly into a worm collector that had been previously washed with 0.25% aqueous gelatin solution, and were conditioned only with a CS. This conditioning was carried out by briefly (<1.0 sec) dipping the worm collector with worms into a slide staining dish containing ∼50 mL of 1.0% aqueous 1-propanol. Then, the worm collector was gently immersed once in ∼1.0 L of dH2O in a beaker. An excess of water in the collector was removed with a piece of Kimtowels, and then the collector with worms was placed on an NGM plate seeded with E. coli OP50 during an ITI for the worms to rest. This conditioning only with the CS was repeated one to 10 times with a 10-min ITI. All other aspects of conditioning, testing, and scoring were exactly as described above.
Drug treatment
NGM culture media containing drug was prepared by mixing 15 g/L of Bactoagar, 5 mL/L of 1.0 M potassium phosphate (pH 6.0), 1.0 mL/L of 1.0 M CaCl2, and 1.0 mL/L of 1.0 M MgSO4 with 0.3 µg/mL of cycloheximide (200 mg/mL stock solution) (Sigma), 0.3 µg/mL of anisomycin (10 mg/mL stock solution) (A.G. Scientific), or 0.1 µg/mL of actinomycin D (10 mg/mL stock solution) (MP Biomedicals) at a final concentration. These stock solutions were sterilized by autoclaving or filtering before mixing. Agar plates were made by pouring 8 mL of the mixture into culture dishes (6 cm in diameter) (Kord-Valmark Labware), and then by being left with lids at room temperature overnight. A day before the experiments, the agar plates were spread with a concentrated E. coli OP50 paste and were left with lids at room temperature overnight. Worms were placed on the plates, and were allowed to freely move and eat at 20°C for 4 h before massed training or for 2 h before spaced training. During the 10-min ITI of the spaced training, the worms were also placed on plates containing the drug. All other aspects of conditioning, testing, and scoring were exactly as described above.
Cold-shock anesthesia
Immediately after massed training, worms in a collector were gently washed by immersing the collector in ∼1.0 L of dH2O in a beaker at room temperature, and then were immersed in ice-cold dH2O for 5.0 sec. An excess of water was removed from the collector with a piece of Kimtowels, and then the collector with worms was placed on an NGM plate seeded with E. coli OP50 at room temperature for 5 min or 3 h. Then, the worms were gently washed by immersing the collector in ∼1.0 L of dH2O in a beaker at room temperature, and were placed on a chemotaxis assay plate for testing as described above.
Immediately after a final ITI on an NGM plate seeded with E. coli OP50 in spaced training, a collector with worms was gently washed by immersing the collector in ∼1.0 L of dH2O in a beaker at room temperature, and then was immersed in ice-cold dH2O for 5.0 sec. The worms were subjected to a chemotaxis assay as described above. All other aspects of conditioning, testing, and scoring were exactly as described above.
Motility assay
After massed or spaced training, worms were examined for their motility. C. elegans moves on an agar plate by making a stereotypical sine wave. The movement of the head from peak to peak of the curve (frequency) was counted as one body bend. After the training, worms in a collector were washed with ∼1.0 L of dH2O in a beaker, and then were placed in a drop of dH2O on a chemotaxis assay plate, or an NGM plate seeded with and without E. coli OP50, using a blunted pipette tip. The drop of dH2O used for the transfer of worms was adsorbed with a piece of Kimwipes. Five minutes after the transfer, the number of body bends in a 10-sec interval was sequentially counted for each of 20 worms once the worms started moving in a forward direction on the assay plate.
HCl avoidance assay
An HCl avoidance assay was carried out on a quadrant plate (10 cm in diameter) (Kord-Valmark) (Wicks et al. 2000). A pair of opposite quadrants of a plate were filled with a mixture of 15 g/L of Bactoagar, 10 mL/L of 5 M NaCl, 1.0 mL/L of 1.0 M CaCl2, and 1.0 mL/L of 1.0 M MgSO4, with or without 1.0 mL/L of 1.0 N HCl. The pH values of the agar in the presence and absence of HCl were 4.0 and 6.0, respectively, when measured by using a pH meter (Model TPX-90, Toyo Chemical Laboratories). These solutions were sterilized by either autoclaving or filtrating before mixing. Well-fed worms on day 4 after hatching were washed three times with a 0.25% aqueous gelatin solution as described above to remove bacteria, and about 100 worms were placed at the center of four quadrants. The number of worms on the four quadrants was counted in 10 min at room temperature. Avoidance index (AI) values were calculated by the number of worms on the quadrants without HCl, subtracted by the number of worms on the quadrants with HCl.
Protein labeling
To radioactively label bacterial cells as a source of food for worms, a single colony of E. coli NA22 was inoculated into 100 mL of low-sulfate minimal medium, which was made by mixing 20 mL of 5 × M9 buffer (30 g of NaHPO4, 15 g of KH2PO4, and 25 g of NaCl per liter), 1.0 mL of 2 M NH4Cl, 0.5 mL of 20% glucose, 1.3 mL of 5 mM MgSO4, 500 µCi of [35S]-labeled cysteine/methionine mixture (1175 Ci/mmol, 10 mCi/mL) (American Radiolabeled Chemicals), and a 2-μg/mL (final concentration) unlabeled cysteine and methionine mixture (1:3 ratio) (WAKO) with distilled water as previously described (Lewis and Fleming 1995). After overnight growth at 37°C, the bacteria were harvested by a 10-min centrifugation at 3500g. The bacterial pellet was then resuspended in 45 mL of low-sulfate minimal medium. The resulting bacterial suspension, 1.0 mL, was spread on the surface of NGM agar plates (10 cm in diameter) containing 0.3 µg/mL of cycloheximide, 0.3 µg/mL of anisomycin, or 0.1 µg/mL of actinomycin D at a final concentration, and the plates were left overnight at room temperature.
To radioactively label worms, approximately 1000 well-fed animals on day 4 after hatching were put on an NGM agar plate with an unlabeled NA22 bacterial lawn that contained 0.3 µg/mL of cycloheximide, 0.3 µg/mL of anisomycin, or 0.1 µg/mL of actinomycin D at a final concentration at 20°C for 2 h. The worms were washed off with M9 buffer from the NGM plate directly into a worm collector that had been previously washed with 0.25% aqueous gelatin solution, and were then transferred to the NGM plate with radioactively labeled NA22 in the presence or absence of 0.3 µg/mL of cycloheximide, 0.3 µg/mL of anisomycin, or 0.1 µg/mL of actinomycin D at a final concentration at 20°C for 2 h. The worms were washed three times with 5 mL 1 × M9 buffer without MgSO4, and were then suspended in 200 µL of 1 × M9 buffer without MgSO4. After incubation at 20°C for 10 min, the worms were sonicated five times for 5 sec with a 1-min interval on ice in the presence of protease inhibitors (Complete, EDTA-free, Roche). Protein concentrations of the worm suspensions were measured by using a BCA protein assay kit (ThermoFisher Scientific). After adding an equal volume of 20% aqueous trichloroacetic acid (WAKO), the worm suspension was cooled at −20°C for 1.0 h. The resulting protein precipitate was collected on a glassfiber filter (GF/C, Whatman) by aspiration using a diaphragm dry vacuum pump (DTU-20, ULVAC Technologies). The filter was dried for 5 min by aspiration, and radioactivity of the filter was counted in a 10-mL liquid scintillation cocktail (CLEAR-SOL II, Nacalai Tesque) with a liquid scintillation counter (LSC-6000, Hitachi Aloka Medical).
Statistical analysis
All results are expressed as means with standard errors of the means (SEM) calculated from four to 15 assays, each of which had about 100 worms. Statistical analysis of data was done by two-sided Student's t-test for comparison between two groups or one-way ANOVA with Bonferroni/Dunn test or Tukey-Kramer's test for multiple comparisons between groups. P ≤ 0.05 was considered statistically significant. All analyses were carried out by using Excel 2003 (Microsoft) with the add-in software Statcel2 (OMS).
Acknowledgments
We thank M. Alkema and A. Maricq for crh-1(n3315) and glr-1(ky176) strains, respectively. We also thank our laboratory members for critically reading the manuscript. Some C. elegans strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR), and National Bioresource Project for the Nematode, Japan.
Footnotes
[Supplemental material is available for this article.]
References
- Ardiel EL, Rankin CH 2010. An elegant mind: Learning and memory in Caenorhabditis elegans. Learn Mem 17: 191–201 [DOI] [PubMed] [Google Scholar]
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER 2000. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci 1: 11–20 [DOI] [PubMed] [Google Scholar]
- Bargmann CI 2006. Chemosensation in C. elegans. WormBook 25: 1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR 1991. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7: 729–742 [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Mori I 1997. Chemotaxis and thermotaxis. In C. elegans II (ed. Riddle DL et al. ), pp. 717–737 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74: 515–527 [DOI] [PubMed] [Google Scholar]
- Barnet RC, Arnold HM, Miller RR 1991. Simultaneous conditioning demonstrated in second-order conditioning: Evidence for similar associative structure in forward and simultaneous conditioning. Learn Motiv 22: 253–268 [Google Scholar]
- Barnet RC, Grahame NJ, Miller RR 1993. Temporal encoding as a determinant of blocking. J Exp Psychol Anim Behav Process 19: 327–341 [DOI] [PubMed] [Google Scholar]
- Beck CDO, Rankin CH 1995. Heat shock disrupts long-term memory consolidation in Caenorhabditis elegans. Learn Mem 2: 161–177 [DOI] [PubMed] [Google Scholar]
- Beck CDO, Schroeder B, Davis RL 2000. Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. J Neurosci 20: 2944–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL 1993. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361: 31–39 [DOI] [PubMed] [Google Scholar]
- Brenner S 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie PJ, Madsen DM, Zheng Y, Mellem J, Maricq AV 2001a. Differential expression of glutamate receptor subunits in the nervous system of Caenorhabditis elegans and their regulation by the homeodomain protein UNC-42. J Neurosci 21: 1510–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie PJ, Mellem JE, Hills T, Madsen DM, Maricq AV 2001b. The C. elegans glutamate receptor subunit NMR-1 is required for slow NMDA-activated currents that regulate reversal frequency during locomotion. Neuron 31: 617–630 [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Medina JH, Izquierdo I 2008. ERK1/2 and CaMKII-mediated events in memory formation: Is 5HT regulation involved? Behav Brain Res 195: 120–128 [DOI] [PubMed] [Google Scholar]
- Carew TJ 1996. Molecular enhancement of memory formation. Neuron 16: 5–8 [DOI] [PubMed] [Google Scholar]
- Carew TJ, Pinsker HM, Kandel ER 1972. Long-term habituation of a defensive withdrawal reflex in Aplysia. Science 175: 451–454 [DOI] [PubMed] [Google Scholar]
- Carew TJ, Walters ET, Kandel ER 1981. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci 1: 1426–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA Jr, Duman RS, Nestler EJ 2005. The many faces of CREB. Trends Neurosci 28: 436–445 [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D 2006. Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychol Bull 132: 354–380 [DOI] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI 1997. Environmental signals modulate olfactory acuity, discrimination, and memory in Caenorhabditis elegans. Learn Mem 4: 179–191 [DOI] [PubMed] [Google Scholar]
- Crow T, Siddiqi V, Dash PK 1997. Long-term enhancement but not short-term in Hermissenda is dependent upon mRNA synthesis. Neurobiol Learn Mem 68: 343–350 [DOI] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER 1990. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature 345: 718–721 [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR 1984. Protein synthesis and memory: A review. Psychol Bull 96: 518–559 [PubMed] [Google Scholar]
- de Bono M, Maricq AV 2005. Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci 28: 451–501 [DOI] [PubMed] [Google Scholar]
- DeZazzo J, Tully T 1995. Dissection of memory formation: From behavioral pharmacology to molecular genetics. Trends Neurosci 18: 212–218 [DOI] [PubMed] [Google Scholar]
- Dostalek C 1976. Backward conditioning in man and the criteria of conditioning. Activ Nerv 18: 26–30 [PubMed] [Google Scholar]
- Driscoll M, Kaplan J 1997. Mechanotransduction. In C. elegans II (ed. Riddle DL et al. ), pp. 645–677 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, et al. 2003. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol 13: 286–296 [DOI] [PubMed] [Google Scholar]
- Durkovic RG, Damianopoulos EN 1986. Forward and backward classical conditioning of the flexion reflex in the spinal cat. J Neurosci 6: 2921–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein HT, Child FM, Kuzirian AM, Alkon DL 2003. Time windows for effects of protein synthesis inhibitors on Pavlovian conditioning in Hermissenda: Behavioral aspects. Neurobiol Learn Mem 79: 127–131 [DOI] [PubMed] [Google Scholar]
- Flood JF, Rosenzweig MR, Bennett EL, Orme AE 1973. The influence of duration of protein synthesis inhibition on memory. Physiol Behav 10: 555–562 [DOI] [PubMed] [Google Scholar]
- Folkers E, Drain P, Quinn WG 1993. radish, a Drosophila mutant deficient in consolidated memory. Proc Natl Acad Sci 90: 8123–8127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Ailion M, Lockery SR 2008. Ammonium-acetate is sensed by gustatory and olfactory neurons in Caenorhabditis elegans. PLoS ONE 3: e2467 10.1371/journal.pone.0002467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Kemenes I, Andrew RJ, Benjamin PR 2005. A single time-window for protein synthesis-dependent long-term memory formation after one-trial appetitive conditioning. Eur J Neurosci 21: 1347–1358 [DOI] [PubMed] [Google Scholar]
- Gerber B, Wustenberg D, Schutz A, Menzel R 1998. Temporal determinants of olfactory long-term retention in honeybee classical conditioning: Nonmonotonous effects of the training trial interval. Neurobiol Learn Mem 69: 71–78 [DOI] [PubMed] [Google Scholar]
- Giurfa M, Fabre E, Flaven-Pouchon J, Groll H, Oberwallner B, Vergoz V, Roussel E, Sandoz JC 2009. Olfactory conditioning of the sting extension reflex in honeybees: Memory dependence on trial number, interstimulus interval, intertrial interval, and protein synthesis. Learn Mem 16: 761–765 [DOI] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, Bargmann CI 2005. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci 102: 3184–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Wigström H 1988. Physiological mechanisms underlying long-term potentiation. Trends Neurosci 11: 156–162 [DOI] [PubMed] [Google Scholar]
- Hammer M, Menzel R 1995. Learning and memory in the honeybee. J Neurosci 15: 1617–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Carew TJ, Kandel ER 1986. Effects of interstimulus interval and contingency on classical conditioning of the Aplysia siphon withdrawal reflex. J Neurosci 6: 1695–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock EM, Russell RL 1975. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci 72: 4061–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstern F, Malaka R, Hammer M 1998. Backward inhibitory learning in honeybees: A behavioral analysis of reinforcement processing. Learn Mem 4: 429–444 [DOI] [PubMed] [Google Scholar]
- Heth CD, Rescorla RA 1973. Simultaneous and backward fear conditioning in the rat. J Comp Physiol Psychol 82: 434–443 [DOI] [PubMed] [Google Scholar]
- Ishihara T, Iino Y, Mohri A, Mori I, Gengyo-Ando K, Mitani S, Katsura I 2002. HEN-1, a secretory protein with an LDL receptor motif, regulates sensory integration and learning in Caenorhabditis elegans. Cell 109: 639–649 [DOI] [PubMed] [Google Scholar]
- Jones JE 1962. Contiguity and reinforcement in relation to CS-UCS intervals in classical aversive conditioning. Psychol Rev 69: 176–186 [DOI] [PubMed] [Google Scholar]
- Kandel ER 1976. Cellular basis of behavior. An introduction to behavioral neurobiology. W.H. Freeman and Co, San Francisco, CA [Google Scholar]
- Kandel ER 2001. The molecular biology of memory storage: A dialogue between genes and synapses. Science 294: 1030–1038 [DOI] [PubMed] [Google Scholar]
- Karr J, Vagin V, Chen K, Ganesan S, Olenkina O, Gvozdev V, Featherstone DE 2009. Regulation of glutamate receptor subunit availability by microRNAs. J Cell Biol 185: 685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC, Nicoll RA 1988. A persistent postsynaptic modification mediates long-term potentiation in hippocampus. Neuron 10: 911–917 [DOI] [PubMed] [Google Scholar]
- Kauffman AL, Ashraf JM, Corces-Zimmerman MR, Landis JN, Murphy CT 2010. Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. PLoS Biol 8: e1000372 10.1371/journal.pbio.1000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S 2004. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 116: 467–479 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Corcoran EE, Eto K, Gengyo-Ando K, Muramatsu MA, Kobayashi R, Freedman JH, Mitani S, Hagiwara M, Means AR, et al. 2002. A CaMK cascade activates CRE-mediated transcription in neurons of Caenorhabditis elegans. EMBO Rep 3: 962–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schutz G, Silva AJ 1996. Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol 7: 1–11 [DOI] [PubMed] [Google Scholar]
- Lent DD, Kwon H-W 2004. Antennal movements reveal associative learning in the American cockroach Periplaneta americana. J Exp Biol 207: 369–375 [DOI] [PubMed] [Google Scholar]
- Lewis JA, Fleming JT 1995. Basic culture methods. In Caenorhabditis elegans: Modern biological analysis of an organism (ed. Epstein HF, Shakes DC), pp. 3–29 Academic Press, San Diego, CA [Google Scholar]
- Lin XY, Glanzman DL 1997. Effect of interstimulus interval on pairing-induced LTP of Aplysia sensorimotor synapses in cell culture. J Neurophysiol 77: 667–674 [DOI] [PubMed] [Google Scholar]
- Loya CM, Van Vactor D, Fulga TA 2010. Understanding neuronal connectivity through the post-transcriptional toolkit. Genes Dev 24: 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney WJ, Ayres JJB 1976. One-trial simultaneous and backward fear conditioning as reflected in conditioned suppression of licking in rats. Anim Learn Behav 4: 357–362 [Google Scholar]
- Maier SF, Rapaport P, Wheatley KL 1976. Conditioned inhibition and the UCS-CS interval. Anim Learn Behav 4: 217–220 [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Mizunami M 2002. Temporal determinants of long-term retention of olfactory memory in the cricket Gryllus bimaculatus. J Exp Biol 205: 1429–1437 [DOI] [PubMed] [Google Scholar]
- Mayford M 2007. Protein kinase signaling in synaptic plasticity and memory. Curr Opin Neurobiol 17: 313–317 [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V 1991. Efficient gene transfer in C. elegans: Extrachromosomal maintenance and integration of transforming sequences. EMBO J 10: 3959–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R, Manz G, Menzel R, Greggers U 2001. Massed and spaced learning in honeybees: The role of CS, US, the intertrial interval, and the test interval. Learn Mem 8: 198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJ, Channon V, Rosenzweig MR, Bennett EL 1987. Anisomycin impairs long-term working memory in a delayed alternation task. Behav Neural Biol 47: 1–6 [DOI] [PubMed] [Google Scholar]
- Mohri A, Kodama E, Kimura KD, Koike M, Mizuno T, Mori I 2005. Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics 169: 1437–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison GE, van der Kooy D 2001. A mutation in the AMPA-type glutamate receptor, glr-1, blocks olfactory associative and nonassociative learning in Caenorhabditis elegans. Behav Neurosci 115: 640–649 [DOI] [PubMed] [Google Scholar]
- Morrison GE, Wen JYM, Runciman S, van der Kooy D 1999. Olfactory associative learning in Caenorhabditis elegans is impaired in lrn-1 and lrn-2 mutants. Behav Neurosci 113: 358–367 [DOI] [PubMed] [Google Scholar]
- Murakami M, Koga M, Ohshima Y 2001. DAF-7/TGF-β expression required for the normal larval development in C. elegans is controlled by a presumed guanylyl cyclase DAF-11. Mech Dev 109: 27–35 [DOI] [PubMed] [Google Scholar]
- Nuttley WM, Atkinson-Leadbeater KP, van der Kooy D 2002. Serotonin mediates food-odor associative learning in the nematode Caenorhabditis elegans. Proc Natl Acad Sci 99: 12449–12454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani MR, Oishi K, Gelb BD, Zhong Y 2009. The phosphatase SHP2 regulates the spacing effect for long-term memory induction. Cell 139: 186–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Dubnau J 2010. Genetic disruptions of Drosophila Pavlovian learning leave extinction learning intact. Genes Brain Behav 9: 203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH 2000. Context conditioning in habituation in the nematode Caenorhabditis elegans. Behav Neurosci 114: 496–505 [PubMed] [Google Scholar]
- Rankin CH, Broster BS 1992. Factors affecting habituation and recovery from habituation in the nematode Caenorhabditis elegans. Behav Neurosci 106: 239–249 [DOI] [PubMed] [Google Scholar]
- Rankin CH, Beck CD, Chiba CM 1990. Ceanorhabditis elegans: A new model system for the study of learning and memory. Behav Brain Res 37: 89–92 [DOI] [PubMed] [Google Scholar]
- Rescorla RA 1980. Simultaneous and successive associations in sensory preconditioning. J Exp Psycho Anim Behav Process 6: 207–216 [DOI] [PubMed] [Google Scholar]
- Riddle DL, Albert PS 1997. Genetic and environmental regulation of dauer larva development. In C. elegans II (ed. Riddle DL et al. ), pp. 739–768 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- Rose JK, Rankin CH 2006. Blocking memory reconsolidation reverses memory-associated changes in glutamate receptor expression. J Neurosci 26: 11582–11587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Kaun KR, Rankin CH 2002. A new group-training procedure for habituation demonstrates that presynaptic glutamate release contributes to long-term memory in Caenorhabditis elegans. Learn Mem 9: 130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Kaun KR, Chen SH, Rankin CH 2003. GLR-1, a non-NMDA glutamate receptor homolog, is critical for long-term memory in Caenorhabditis elegans. J Neurosci 23: 9595–9599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Sangha S, Rai S, Norman KR, Rankin CH 2005. Decreased sensory stimulation reduces behavioral responding, retards development, and alters neuronal connectivity in Caenorhabditis elegans. J Neurosci 25: 7159–7168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki S, Yamamoto M, Iino Y 2001. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J Exp Biol 204: 1757–1764 [DOI] [PubMed] [Google Scholar]
- Sambongi Y, Takeda K, Wakabayashi T, Ueda I, Wada Y, Futai M 2000. Caenorhabditis elegans senses protons through amphid chemosensory neurons: Proton signals elicit avoidance behavior. Neuroreport 11: 2229–2232 [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Gormezano I 1964. Conditioning of the nictitating membrane of the rabbit as a function of CS-US interval. J Comp Physiol Psychol 57: 188–195 [DOI] [PubMed] [Google Scholar]
- Spetch ML, Wilkie DM, Pinel JP 1981. Backward conditioning: A re-evaluation of the empirical evidence. Psychol Bull 89: 163–175 [PubMed] [Google Scholar]
- Suo S, Culotti JG, Van Tol HHM 2009. Dopamine counteracts octopamine signalling in a neural circuit mediating food response in C. elegans. EMBO J 28: 2437–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Chiang AS, Ito N, Liu HP, Horiuchi J, Tully T, Saitoe M 2003. Aging specifically impairs amnesiac-dependent memory in Drosophila. Neuron 40: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Tomioka M, Adachi T, Suzuki H, Kunitomo H, Schafer WR, Iino Y 2006. The insulin/PI 3-kinase pathway regulates salt chemotaxis learning in Caenorhabditis elegans. Neuron 51: 613–625 [DOI] [PubMed] [Google Scholar]
- Torayama I, Ishihara T, Katsura I 2007. Caenorhabditis elegans integrates the signals of butanone and food to enhance chemotaxis to butanone. J Neurosci 27: 741–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T, Quinn WG 1985. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A 157: 263–277 [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M 1994. Genetic dissection of consolidated memory in Drosophila. Cell 79: 35–47 [DOI] [PubMed] [Google Scholar]
- Wang JQ, Fibuch EE, Mao L 2007. Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem 100: 1–11 [DOI] [PubMed] [Google Scholar]
- Ward S 1973. Chemotaxis by the nematode Caenorhabditis elegans: Identification of attractants and analysis of the response by use of mutants. Proc Natl Acad Sci 70: 817–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S, Thomson N, White JG, Brenner S 1975. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol 160: 313–337 [DOI] [PubMed] [Google Scholar]
- Ware RW, Clark D, Crossland K, Russell RL 1975. The nerve ring of the nematode Caenorhabditis elegans: Sensory input and motor output. J Comp Neurol 162: 71–110 [Google Scholar]
- Wen JYM, Kumar N, Morrison G, Rambaldini G, Runciman S, Rousseau J, van der Kooy D 1997. Mutations that prevent associative learning in C. elegans. Behav Neurosci 111: 354–368 [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Trans R Soc Lond B 314: 1–340 [DOI] [PubMed] [Google Scholar]
- Wicks SR, de Vries CJ, van Luenen HGAM, Plasterk RHA 2000. CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans. Dev Biol 221: 295–307 [DOI] [PubMed] [Google Scholar]
- Xia S, Miyashita T, Fu TF, Lin WY, Wu CL, Pyzocha L, Lin IR, Saitoe M, Tully T, Chiang AS 2005. NMDA receptors mediate olfactory learning and memory in Drosophila. Curr Biol 15: 603–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Sekiguchi T, Suzuki H, Mizukami A 1992. Behavioral analysis of internal memory states using cooling-induced retrograde amnesia in Limax flavus. J Neurosci 12: 729–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Shobe JL, Sharma SK, Marina A, Carew TJ 2008. Small G proteins exhibit pattern sensitivity in MAPK activation during the induction of memory and synaptic facilitation in Aplysia. Proc Natl Acad Sci 105: 20511–20516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JCP, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T 1994. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79: 49–58 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu H, Bargmann CI 2005. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 438: 179–184 [DOI] [PubMed] [Google Scholar]