Abstract
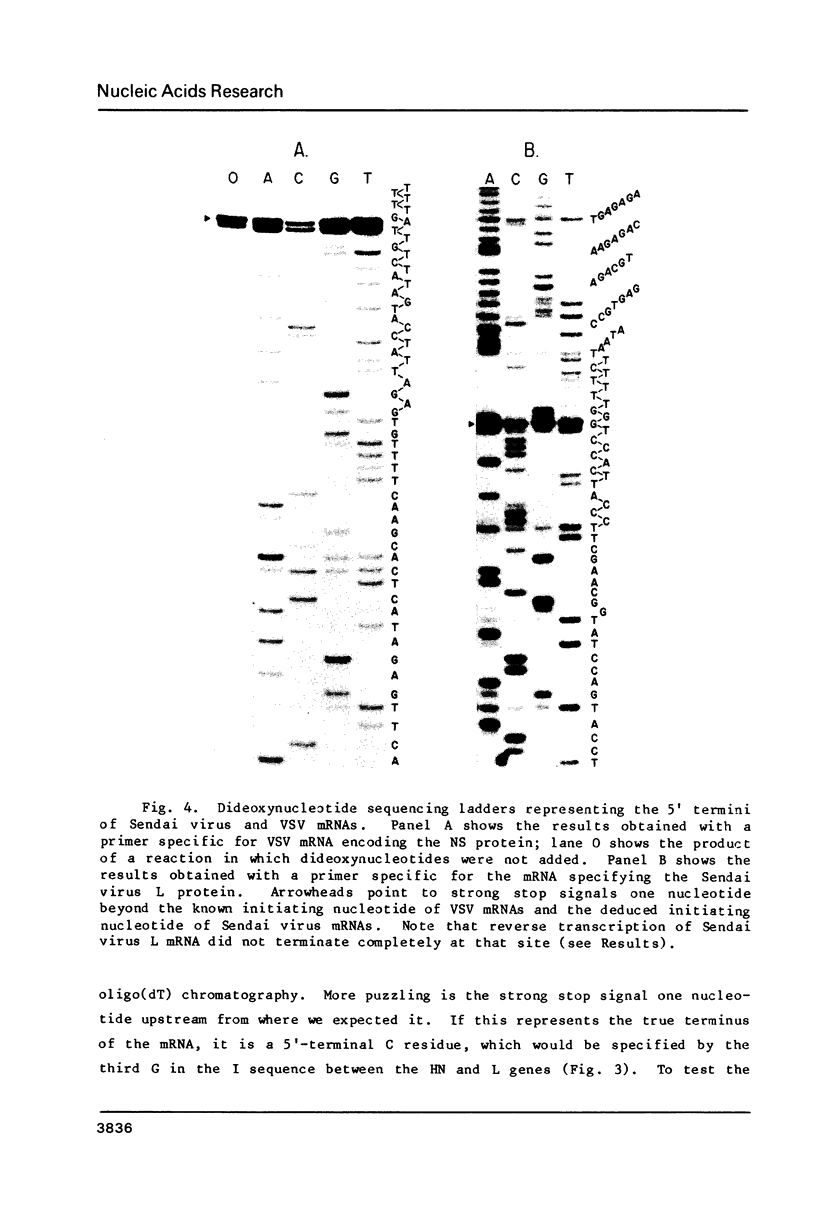
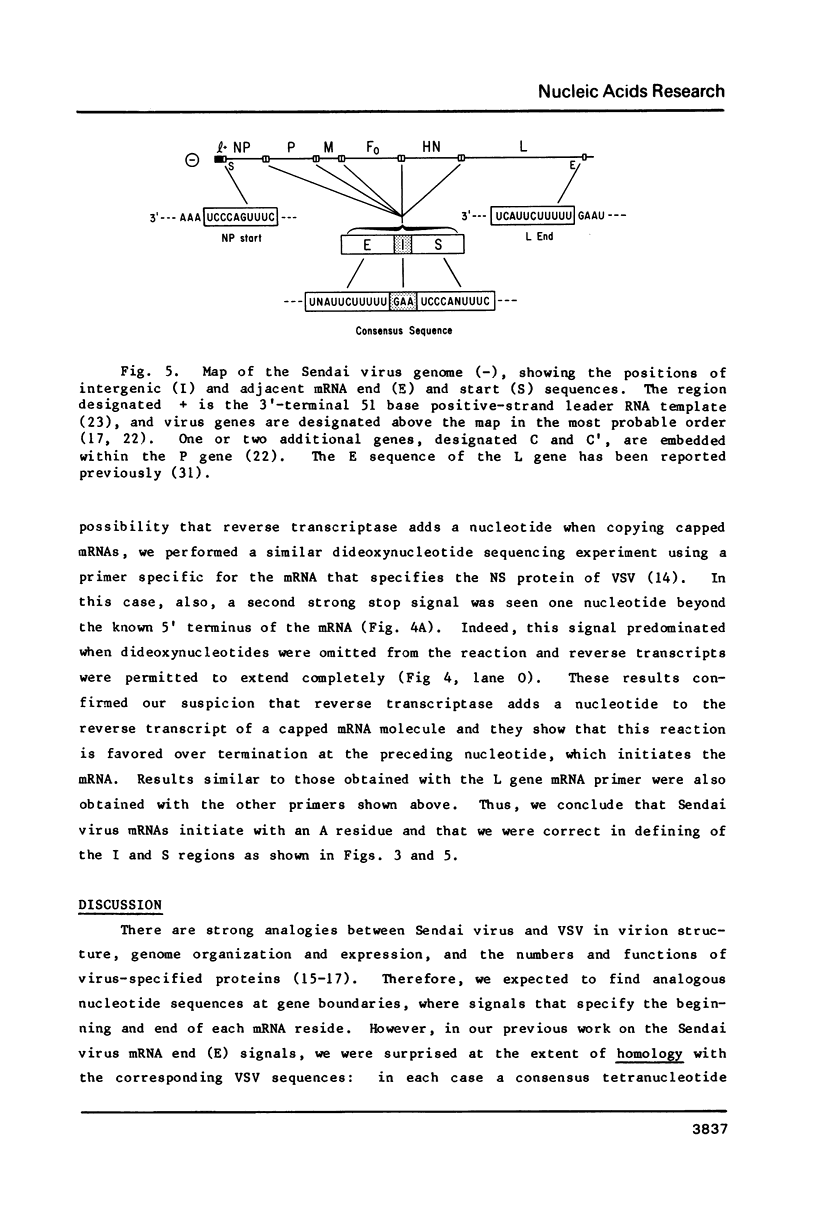
All of the consensus intergenic and transcription initiation sequences of the genome of Sendai virus, a paramyxovirus, have been determined. The boundary between the intergenic sequence, 3'-GAA, and the mRNA start signal, 3'- UCCCANUUUC , was identified by sequencing the 5' termini of specific viral mRNA molecules. One of the five intergenic trinucleotides differed from the rest, consisting of 3'- GGG , and single base substitutions were observed in two of the mRNA start signals. The Sendai virus intergenic sequence was similar to the analogous sequence (3'-GA) of vesicular stomatitis virus (VSV), a member of another family of negative-strand RNA viruses, the rhabdoviruses , but there was no sequence homology between the mRNA start signals of the two viruses. Nevertheless, these mRNA start signals were organized in the same way, being ten bases long and possessing two consensus regions, divided by one (Sendai virus) or two (VSV) variable internal nucleotides. These findings extend the evidence that both families of negative-strand RNA viruses descended from a common ancestor and that an archetypal mechanism of transcriptional regulation has been conserved in their evolution.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball L. A., Wertz G. W. VSV RNA synthesis: how can you be positive? Cell. 1981 Oct;26(2 Pt 2):143–144. doi: 10.1016/0092-8674(81)90297-x. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. W., Portner A., Kingsbury D. W. Synthesis of Sendai virus polypeptides by a cell-free extract from wheat germ. J Gen Virol. 1976 Oct;33(1):117–123. doi: 10.1099/0022-1317-33-1-117. [DOI] [PubMed] [Google Scholar]
- Dowling P. C., Giorgi C., Roux L., Dethlefsen L. A., Galantowicz M. E., Blumberg B. M., Kolakofsky D. Molecular cloning of the 3'-proximal third of Sendai virus genome. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5213–5216. doi: 10.1073/pnas.80.17.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B. M., Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983 Dec;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Glazier K., Raghow R., Kingsbury D. W. Regulation of Sendai virus transcription: evidence for a single promoter in vivo. J Virol. 1977 Mar;21(3):863–871. doi: 10.1128/jvi.21.3.863-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K. C., Bishop D. H., Roy P. 5'-terminal sequences of spring viremia of carp virus RNA synthesized in vitro. J Virol. 1979 Jun;30(3):735–745. doi: 10.1128/jvi.30.3.735-745.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Conserved polyadenylation signals in two negative-strand RNA virus families. Virology. 1982 Jul 30;120(2):518–523. doi: 10.1016/0042-6822(82)90055-1. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Morgan E. M., Kitchingman G., Kingsbury D. W. Molecular clones representing Sendai virus genes P, NP and M. J Gen Virol. 1983 Aug;64(Pt 8):1679–1688. doi: 10.1099/0022-1317-64-8-1679. [DOI] [PubMed] [Google Scholar]
- Hamlyn P. H., Browniee G. G., Cheng C. C., Gait M. J., Milstein C. Complete sequence of constant and 3' noncoding regions of an immunoglobulin mRNA using the dideoxynucleotide method of RNA sequencing. Cell. 1978 Nov;15(3):1067–1075. doi: 10.1016/0092-8674(78)90290-8. [DOI] [PubMed] [Google Scholar]
- Herman R. C. Conditional synthesis of an aberrant glycoprotein mRNA by the internal deletion mutant of vesicular stomatitis virus. J Virol. 1983 Jun;46(3):709–717. doi: 10.1128/jvi.46.3.709-717.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W. The molecular biology of paramyxoviruses. Med Microbiol Immunol. 1974;160(2-3):73–83. doi: 10.1007/BF02121714. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Boy de la Tour E., Bruschi A. Self-annealing of Sendai virus RNA. J Virol. 1974 Jul;14(1):33–39. doi: 10.1128/jvi.14.1.33-39.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D., Bruschi A. Antigenomes in Sendai virions and Sendai virus-infected cells. Virology. 1975 Jul;66(1):185–191. doi: 10.1016/0042-6822(75)90189-0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J. Structure of the gene N:gene NS intercistronic junction in the genome of vesicular stomatitis virus. Cell. 1979 Jul;17(3):673–681. doi: 10.1016/0092-8674(79)90274-5. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. The pathway of eukaryotic mRNA formation. Annu Rev Biochem. 1983;52:441–466. doi: 10.1146/annurev.bi.52.070183.002301. [DOI] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Identification of transcriptive and replicative intermediates in Sendai virus-infected cells. Virology. 1972 Mar;47(3):711–725. doi: 10.1016/0042-6822(72)90561-2. [DOI] [PubMed] [Google Scholar]
- Re G. G., Gupta K. C., Kingsbury D. W. Sequence of the 5' end of the Sendai virus genome and its variable representation in complementary form at the 3' ends of copy-back defective interfering RNA species: identification of the L gene terminus. Virology. 1983 Oct 30;130(2):390–396. doi: 10.1016/0042-6822(83)90093-4. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Synthesis, cleavage and sequence analysis of DNA complementary to the 26 S messenger RNA of Sindbis virus. J Mol Biol. 1981 Aug 15;150(3):315–340. doi: 10.1016/0022-2836(81)90550-7. [DOI] [PubMed] [Google Scholar]
- Robertson J. S., Schubert M., Lazzarini R. A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell. 1980 Feb;19(2):415–421. doi: 10.1016/0092-8674(80)90515-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda E., Maruyama T., Arrand J. R., Griffin B. E. Host-dependent evolution of three papova viruses. Nature. 1980 May 15;285(5761):165–167. doi: 10.1038/285165a0. [DOI] [PubMed] [Google Scholar]