Summary
Nitric oxide (NO) is an important signal molecule in many types of cells and tissues. Efficiently balanced NO production was noted to play an important role in the healing of burns. Inducible nitric oxygen synthase (iNOS) is responsible for the discontinuous synthesis of high amounts of NO. Dysregulation of nitric oxygen synthase (NOS) activity has been associated with multiple organ failure in burn patients and may therefore represent a novel therapeutic target in such circumstances. Heparin and low molecular weight heparin (LMWH) derivatives may offer therapeutic benefit for inflammatory diseases, whereas NO plays a protagonist role. Burn injury in humans has been associated with a significant increase in NO2/NO3 (nitrite/nitrate) plasma levels. In this prospective study burn patients were treated with and without LMWH to provide evidence that LMWH has NOS-reducing activity. This was proved by colorimetric and immunohistological studies. There was a significantly different NOS activity between the treated and the control group and our results suggest that LMWH was more effective in the treatment of burn patients through iNOS inhibition. Treatment with LMWH was initiated within 6 h post-burn.
Keywords: NITRIC OXIDE, BURNS, HEPARIN, INFLAMMATION
Abstract
L’oxyde nitrique (ON) est une importante molécule signal dans de nombreux types de cellules et de tissus. Il a été observé que la production efficacement équilibrée de l’ON joue un rôle important dans la cicatrisation des brûlures. La synthase de l’oxyde nitrique inductible (SONi) est responsable de la synthèse discontinue de grandes quantités de l’ON. Le dérèglement de l’activité de la synthase de l’oxyde nitrique (SON) a été associé à une défaillance multiviscérale chez les humains brûlés et peut donc représenter une nouvelle cible thérapeutique dans ces circonstances. L’héparine et les dérivés de l’héparine à bas poids moléculaire (HBPM) peuvent offrir des avantages thérapeutiques aux patients atteints de maladies inflammatoires, alors que l’ON joue un rôle de protagoniste. Les brûlures chez l’homme ont été associées à une augmentation significative des concentrations plasmatiques de NO2/NO3 (nitrites/nitrates). Dans cette étude prospective les patients brûlés ont été traités avec et sans l’emploi de l’HBPM pour avoir des preuves que c’est l’HPBM qui possède une action qui réduit la SON. Cela a été démontré par des études colorimétriques et immunohistologiques. Il a été aussi démontré que l’activité de la SON dans le groupe traité et l’activité dans le groupe témoin étaient significativament différentes. Les résultats obtenus dans cette recherche font penser que l’HBPM s’est démontrée plus efficace dans le traitement des patients brûlés à cause de l’inhibition de la SONi. Le traitement avec l’HBPM a commencé dans les six premières heures après la brûlure.
Introduction
Nitric oxide (NO) is an important signal molecule in many types of cells and tissues. Efficiently balanced NO production was noted to play an important role in the healing of burns.1 Nitric oxide is a diatomic mediator liberated on oxidation of L-arginine by the nitric oxide synthase (NOS) family of enzymes.2 Up to this point in time three NOS isoforms have been identified: two distinct NOS isoforms, constitutively expressed in cells, plus a third isoform, inducible NOS (iNOS), transcribed in response to specific stimuli. In particular, iNOS is responsible for the discontinuous synthesis of high amounts of NO.3 The enzyme synthesizes NO during the conversion of L-arginine to L-citrulline in the presence of oxygen and the co-factors reduced nicotinamide adenine ninucleotide phosphate, flavin adenine dinucleotide, flavin mononucleotide, and tetrahydrobiopterin (BH4). The amount of NO produced in cells or tissues can be determined by analysing the activity of NOS. The best index of total NO production is the sum of both NO2 and NO3 content.
Nitric oxide has complex and wide-ranging functions in vivo and has been implicated in the development of the profound inflammatory response that occurs as a result of cutaneous burn injury. In addition, dysregulation of NOS activity has been associated with multiple organ failure in burn patients and may therefore represent a novel therapeutic target in such circumstances. It is known that traditional non-steroidal anti-inflammatory drugs (NSAIDs), selective cyclooxygenase-2 inhibitors, and inhibitors of NOS impair healing.4,5 It is now well established that low molecular weight heparin (LMWH) has many superior advantages, such as a lower risk of haemorrhage and a positive effect on the serum lipid profile. However, the higher cost limits their broad usage.
Heparin and LMWH derivatives may offer therapeutic benefits in inflammatory diseases where NO plays a protagonist role.6 It has been reported that induction of iNOS was demonstrated after lung injury.7 Although iNOS does not exist under physiological conditions, it is induced by inflammatory cytokines and/or LPS in immune cells, such as macrophages, and 14 other cells, such as vascular smooth muscle cells and myocardium;8,9 iNOS is an important enzyme regulating physiological and pathophysiological processes in mammalian cells, and its expression is associated with several inflammatory diseases. Post-transcriptional mechanisms have been shown to play an important role in the regulation of iNOS expression in response to inflammatory stimuli.
Burn injury in humans has been associated with a significant increase in NO2/NO3 (nitrite/nitrate) plasma levels. 10 A study in burned rats showed that the nitric oxide content of the skin was higher than that of the plasma and visceral organs, suggesting that burned tissue may be an important site for the production of nitric oxide.11 However, the relationship between cutaneous and systemic NO has been little studied. This prospective study considers burn patients treated with and without LMWH with the aim of providing evidence that LMWH possesses NOSreducing activity, as proved by colorimetric and immunohistological studies.
Materials and methods
Study design
A total of 24 burn patients followed for 15 days were enrolled over a period of 12 months (2007-2008). Of these, 12 were treated with LMWH-FRAGMIN and 12 served as controls without heparin therapy. In both groups partial-thickness deep second-degree burns covered 25-30% of the total body surface area (TBSA). Age, sex, and aetiology were matched in both groups. The mean age of the subjects was 27.3 ± 9.08 yr. Subjects with infection were excluded. The causes of burns were noted from medical records. The study protocol was approved by the Institutional Ethics Committee and all the subjects gave their written informed consent prior to participation in the study. Tissue and blood samples were collected on days 3, 6, 9, and 15 day of healing. Heparin is an approved drug recognized by the drug Controller General in India, used in coronary artery disease. In our study the dosages of heparin were determined by the clinicians involved on the basis of TBSA. Care was taken that the dosage did not exceed that given in myocardial infarction. LMWH was given as 400IU/kg/15% TBSA every 12 h for 7 days.
Estimation of iNOS and IL-6 expressions
Monoclonal antibodies for iNOS and IL-6 were procured from Santa Cruz, USA. Secondary antibody against anti-mouse was also purchased from Santa Cruz. Fast red substrate was purchased from DAKO chemicals, Denmark, and all other chemicals were analytical grade purchased from Sigma chemicals.
Immunohistochemical analysis
Wound biopsy samples collected at three-day time intervals were subjected to analysis. Immunohistochemical staining identified the sites of cellular expression and temporal sequence of iNOS synthase protein within partialthickness burns excised from the patients at different days of healing. The sections were deparaffinized using xylene, air-dried, serially hydrated using aqueous ethanol (gradient of 70%), and finally hydrated completely. The hydrated sections were then incubated for 1 h with 2N HCl at 37 °C (for antigen exposure) and kept immersed in PBS. Sections from different post-burn days were incubated for 30 min in 2% BSA for blocking. After fine washes with PBST (PBS containing 0.05% Tween20) three or four times, the sections were again incubated for 1-2 h at 37 °C individually with primary antibodies iNOS and IL-6 to assess the inflammatory status. Sections of control and treated subjects taken on days 3, 6, 9, and 15 were probed with antibody at a ratio of 1:100. After extensive washing with PBST the sections were incubated for 2 h at 37 °C followed by washing with PBST four or five times. Then secondary antibody diluted to 1:200 was added and incubated for 1 h at 37 °C. Sections were then detected with fast red substrate (prepared with buffer provided in the kit by the manufacturers), washed in running water for 30 min, and counterstained with Mayer’s haematoxylin for 15 min. The excessive stain was washed again with water and finally mounted using crystal mountant, and images were taken with a Leica Phase contrast microscope and analysed using Leica Application Suite software.
Nitrate/nitrite estimation in plasma
A nitrate/nitrite colorimetric estimation kit was purchased from Cayman chemicals USA and assay was performed according to the manufacturer’s instructions. Patient blood samples from the control and treated groups were collected at various time intervals (days 3, 6, 9, and 15) in EDTA tubes. Using the FICOLL density gradient method, plasma was separated and stored at -20 °C until use. Briefly, 40 µl of filtered plasma was taken, and 10 µl of enzyme co-factor mixture and nitrate reductase mixture was added and incubated for 3 h. After incubation Griess reagents 1 and 2 were added and 10 min were allowed for colour development at room temperature. Absorbance was measured at 540 or 550 nm on a BIORAD ELISA reader, USA. The assay was performed in triplicates and the results were statistically analysed. In principle NO is produced in variable amounts in vitro and in vivo. The final NO products are in the form of NO2 nitrite and NO3 nitrate. The proportion of nitrite and nitrate is unpredictable. Hence the best index of measurement of NO is the sum of nitrite and nitrate. The kit provides a simple method for measuring the total NO amount.
Statistical analysis
The analysis was performed using SPSS 10.0 Version software. Mean and standard deviation and proportions are reported as relevant. The non-parametric Mann Whitney U test was used to compare the difference between the two study groups at different time points. The chi-square test was performed to compare the categorical variables between the two groups. A significance test was performed with a confidence limit of 95%, i.e. p < 0.05 was considered statistically significant.
Results
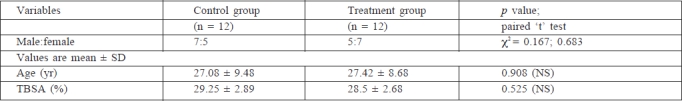
As stated above, all the subjects were matched by age, sex, and aetiology in both the groups (Table I). The causes of the burns were prominently flame and scalds. Scalds were caused by hot water and other liquids such as curry and milk. Flame burns were due to accidents, in females mainly caused by kerosene.
Table I. Patients enrolled in treated and control groups.
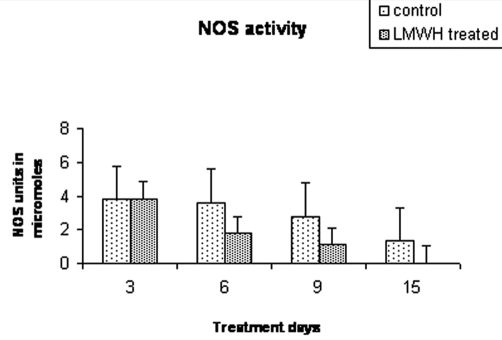
Colorimetric estimation of NOS activity
Nitrate/nitrite quantification was performed in the plasma of burn patients on different days of healing. There was no statistical difference between the two groups on day 3 (mean ± SD, group 1 vs group 2: 3.79 ± 1.34 vs 4.16 ± 1.14; p = 0.754). However, a difference in NOS activity was observed from day 6 onwards that was found to be significantly lower in the LMWH-FRAGMIN-treated group than in the control group (3.61 ± 1.00 vs 1.79 ± 0.73; p = 0.043). This difference was maintained until days 9 (2.761 ± 0.55 vs 1.1 ± 0.80; p = 0.021) and 15 (1.324 ± 0.78 vs 0.035 ± 0.026; p = 0.021) in the control and treated groups. There was a maximum content of NOS activity (4.7 µM) on day 3. In the LMWH-treated group NOS activity was not up to a detectable limit after day 9 while it was within measurable limits in the control groups even on day 15 (Fig. 1). It is evident from this figure that LMWH heparin treatment reduced the activity of NOS under the stress conditions of severe burns.
Fig. 1. Colorimetric estimation of nitrite/nitrate concentration showing downregulation of nitric oxide synthase activity in burn patient’s serum sample.
Expression of iNOS by immunohistochemistry
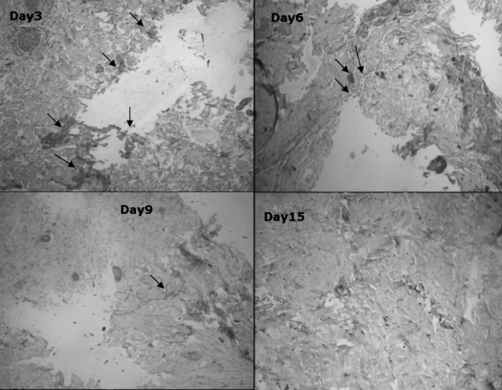
The expression of iNOS was analysed both in the control and the treated groups. On day 3 the expression was greater in both groups and it was almost similar in the initial days of healing. However, in the LMWH-treated groups (Fig. 2), the expression was reduced on day 6 compared with the control group. On day 9 the expression was negligible in the treated group, while a low expression was seen in the control group (Fig. 3). On day 15 there was no iNOS expression in the treated group and negligible expression in the control group.
Fig. 2. Light microscopy images of burn tissue samples expressing iNOS on different days of healing in LMWH-treated group; iNOS expression reduced from day 6.
Fig. 3. Light microscopy images of burn tissue samples expressing iNOS on different days of healing in control group; iNOS expressed from day 3 to day 15.
Expression of IL-6 by immunohistochemistry
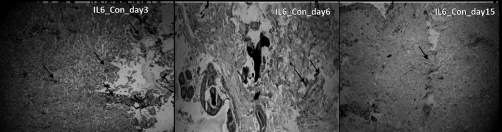
IL-6, which is one of the prominent pro-inflammatory markers, was also analysed in the burn patients’ granulation tissue. In an earlier report12 we found a reduction of IL-6 after treatment with standard heparin. Hence we looked at the action of LMWH on IL-6 and it was noted that on day 3 there was an equal expression of IL-6 in the control and treated groups. After 6 days IL 6 expression was reduced in the treated group, compared with the control group, and drastically reduced to negligible levels after 15 days in the treated group.
Fig. 4. Light microscopy images of burn tissue samples expressing IL-6 on different days of healing in control group.
Fig. 5. Light microscopy images of burn tissue samples expressing IL-6 on different days of healing in treated group.
Discussion
Nitric oxide, a highly reactive radical, is a pleiotropic mediator of numerous biological processes and is involved in the pathogenesis and control of inflammation, tumours, autoimmunity, and infectious and chronic degenerative diseases.13,14 Various reports available on the effects of iNOS in animal models have suggested that inhibition of iNOS effectively mitigates endotoxaemic changes and lung pathology.15,16 The production of NO exerts its prophylactic effect from the first 24 h post-burn. The cellular and temporal distributions of immunoreactive iNOS suggest that nitric oxide may play a role in the regulation of wound repair processes beyond the acute burn injury.
There is evidence in the literature that heparin has long been used to treat burn injuries. However, there is no strong evidence to indicate that standard heparin can improve clinical outcome. Some of the evidence from clinical and animal studies supports the anti-inflammatory role of heparin and heparin-related derivatives.17,18 Application of heparin in the local treatment of burns has also been reported.19
The present study reports that in the early days of wound healing, i.e. the inflammatory stage, NOS could be measured only in plasma or serum, with significant differences observed in day 6 samples. The efficacy of healing by LMWH was well reflected in the tissue samples in the subsequent days of healing (days 9 and 15). Bleeding time, clotting time, prothrombin time, and platelet count were measured in all patients, and the dosage was fixed accordingly. Bleeding time was not found to be altered with LMWH. Skin samples were taken by punch biopsy and wound depth was confirmed by haematoxylin and eosin staining. Biopsies were taken in nearby areas on different days of healing at the healing edge.
Dysregulation of NOS activity has been associated with multiple organ failure in burn patients and may therefore represent a novel therapeutic target in such circumstances.20 LMWH derivatives may offer therapeutic benefits in inflammatory diseases where NO plays a protagonist role in which the effects of tinzaparin on pharmacodynamic profiles of the plasma tissue factor pathway inhibitor and NO in obese subjects have been found to be effective in normal endothelial responsiveness.21
The clinical use of heparin as an anti-inflammatory agent has been held back by the fear of bleeding. The development of non-anticoagulant heparins or heparin derivatives mitigates this concern. LMWH generates less fear of bleeding owing to its defragmented nature. This study demonstrates that it exerts anti-inflammatory action through downregulation of iNOS in serum and that it can be used effectively in the treatment of burns.
Previously it was postulated that keratinocytes in a burn wound would express increased levels of iNOS, and the cellular and temporal distributions of immunoreactive iNOS suggest that nitric oxide may play a role in the regulation of wound repair processes beyond the acute burn injury.22 The close relationship between the resting glomerular filtration rate23 and basal NOS activity24 was also mirrored in the renal microcirculation. Hence regulation of NO is essential because it may complicate the metabolic activity of the burn patients. In in vitro culture of human macrophages, iNOS expression has been studied and found to be elevated under stress conditions.25
Conclusion
It is well established that LMWH offers many great advantages, such as a lower risk of haemorrhage risk, a positive effect on serum iNOS level, and ease of use. However, its higher cost limits its wide employment. To conclude, our results suggest that LMWH heparin therapy is more effective in the treatment of burn patients owing to iNOS inhibition.
Acknowledgments
Acknowledgements. The authors thank Dr Michael Saliba, Saliba Istitute of Burns, La Jolla, California, USA, for partial funding of this work.
Conflict of interest: none declared.
References
- 1.Wallace J.L. Nitric oxide as a regulator of inflammatory processes. Mem Inst Oswaldo Cruz, Rio de Janeiro. 2005;100:5–9. doi: 10.1590/s0074-02762005000900002. [DOI] [PubMed] [Google Scholar]
- 2.Wallis J.P. Nitric oxide and blood: A review. Transfusion Medicine. 2005;15:1–11. doi: 10.1111/j.1365-3148.2005.00542.x. [DOI] [PubMed] [Google Scholar]
- 3.Filippou D., Papadopoulos V.P., Triga A. et al. Nitric oxide, antioxidant capacity, nitric oxide synthase and xanthine oxidase plasma levels in a cohort of burn patients. Burns. 2007;33:1001–1007. doi: 10.1016/j.burns.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Shin S.G., Kang J.K., Lee K.R. et al. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 expression in RAW 264.7 macrophages by sesquiterpene lactones. J Toxicol Environ Health Part A. 2005;68:2119–2131. doi: 10.1080/15287390591009506. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi K., Yokota A., Tanaka A. et al. Factors involved in upregulation of inducible nitric oxide synthase in rat small intestine following administration of nonsteroidal anti-inflammatory drugs. Dig Dis Sci. 2006;51:1250–1259. doi: 10.1007/s10620-006-8045-4. [DOI] [PubMed] [Google Scholar]
- 6.Vallance P. Nitric oxide: Therapeutic opportunities. Fund & Clin Pharmacol. 2003;17:1–10. doi: 10.1046/j.1472-8206.2003.00124.x. [DOI] [PubMed] [Google Scholar]
- 7.Traber D.L., Hawkins H.K., Enkhbaatar P. et al. The role of the bronchial circulation in the acute lung injury resulting from burn and smoke inhalation. Pulm Pharmacol Ther. 2007;20:163–166. doi: 10.1016/j.pupt.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Coleman J.W. Nitric oxide: A regulator of mast cell activation and mast cell-mediated inflammation. Clin Exp Immunol. 2002;129:4–10. doi: 10.1046/j.1365-2249.2002.01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beltrán A.E., Concepción F., Manzanares D. et al. Heparin and low molecular weight heparin decrease nitric oxide production by human polymorphonuclear cells. Arch Med Res. 1999;30:116–119. doi: 10.1016/s0188-0128(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 10.Rawlingson A. Nitric oxide, inflammation and acute burn injury. Burns. 2003;29:631–640. doi: 10.1016/s0305-4179(03)00079-2. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira G.V., Shimoda K., Enkhbaatar P. et al. Skin nitric oxide and its metabolites are increased in nonburned skin after thermal injuries. Shock. 2004;22:278–282. doi: 10.1097/01.shk.0000135259.90311.33. [DOI] [PubMed] [Google Scholar]
- 12.Ravikumar T., Shanmugasundaram N., Mathangi Ramakrishnan V.K., Babu M. Low molecular weight heparin-induced pharmacological modulation of burn wound healing. Ann Burns and Fire Disasters. 2006;19:123–129. [PMC free article] [PubMed] [Google Scholar]
- 13.Mookerjee R.P., Dalton R.N., Davies N.A. et al. Inflammation is an important determinant of levels of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine (ADMA) in acute liver failure. Liver Transpl. 2007;22: 13:400–405. doi: 10.1002/lt.21053. [DOI] [PubMed] [Google Scholar]
- 14.Isenberg J.S., Ridnour L.A., Espey M.G. et al. Nitric oxide in woundhealing. Microsurgery. 2005;25:442–451. doi: 10.1002/micr.20168. [DOI] [PubMed] [Google Scholar]
- 15.Robinson E.K., Seaworth C.M., Suliburk J.W. et al. Effect of NOS inhibition on rat gastric matrix metalloproteinase production during endotoxemia. Shock. 2006;25:507–514. doi: 10.1097/01.shk.0000209543.83929.bd. [DOI] [PubMed] [Google Scholar]
- 16.Su C.F., Yang F.L., Chen H.I. Inhibition of inducible nitric oxide synthase attenuates acute endotoxin-induced lung injury in rats. Clin Exp Pharmacol Physiol. 2007;34:339–346. doi: 10.1111/j.1440-1681.2007.04553.x. [DOI] [PubMed] [Google Scholar]
- 17.Oremus M., Hanson M., Whitlock R. et al. The uses of heparin to treat burn injury. Evid Rep Technol Assess. 2006;148:1–58. [PMC free article] [PubMed] [Google Scholar]
- 18.Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122:743–752. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Troshev K., Dimitrov R. Application of heparin in the local treatment of burns. Acta Chir Plast. 2001;43:143–144. [PubMed] [Google Scholar]
- 20.Paulsen S.M., Wurster S.H., Nanney L.B. Expression of inducible nitric oxide synthase in human burn wounds. Wound Repair and Regeneration. 1998;6:142–148. doi: 10.1046/j.1524-475x.1998.60208.x. [DOI] [PubMed] [Google Scholar]
- 21.Mousa S.A., Johansen K. Pharmacodynamic effects of low molecular weight heparin in obese subjects following subcutaneous administration of 75 IU/kg on plasma tissue factor pathway inhibitor and nitric oxide. Int Angiol. 2005;24:40–42. [PubMed] [Google Scholar]
- 22.Enkhbaatar P., Traber D.L. Pathophysiology of acute lung injury in combined burn and smoke inhalation injury. Clinical Science. 2004;107:137–143. doi: 10.1042/CS20040135. [DOI] [PubMed] [Google Scholar]
- 23.Schlaich M.P., Schmitt D., Ott C. et al. Basal nitric oxide synthase activity is a major determinant of glomerular haemodynamics in humans. J Hypertens. 2008;26:110–116. doi: 10.1097/HJH.0b013e3282f1a93e. [DOI] [PubMed] [Google Scholar]
- 24.Fareed D., Iqbal O., Tobu M. et al. Blood levels of nitric oxide, C-reactive protein, and tumor necrosis factor-a are upregulated in patients with malignancy-associated hypercoagulable state: Pathophysiologic implications. Clinical and Applied Thrombosis/Hemostasis. 2004;10:357–364. doi: 10.1177/107602960401000408. [DOI] [PubMed] [Google Scholar]
- 25.Panaro M.A., Brandonisio O., Acquafredda A. et al. Evidences for iNOS expression and nitric oxide production in the human macrophages. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:210–211. doi: 10.2174/1568008033340216. [DOI] [PubMed] [Google Scholar]