Summary
Objective. Burns represent a major health problem worldwide, with high mortality and morbidity and economic loss even with small burns. Changes in medical treatment protocols depending on a new mechanism involved in the pathogenicity of burns, i.e. oxidative stress (such as the use of povidone-iodine alone or in combination with vitamin E and vitamin C) may improve the outcome and reduce the economic loss. Patients and methods. Thirty-eight thermally injured patients of different age groups, sex, and occupation with different burn size, admitted to the burn unit in Baquba General Hospital, Iraq, were involved in this clinical trial. The patients were allocated to three groups: group A (8 patients), treated according to hospital policy; group B (17 patients), treated with topical povidone-iodine ointment; and group C (13 patients), treated with topical povidone-iodine ointment with systemic once daily 400 mg vitamin E and 500 mg vitamin C in addition to the classical antibiotic used by our hospital. In each group of oxidative stress parameters, the thyroid, liver, and kidney function test, microbiological studies, the mortality rate and healing time measurements, and economic studies were performed using standard methods. Results. Treatment with topical povidone-iodine ointment or in combination with systemic vitamin E and vitamin C was found to be of significant benefit in improving oxidative stress parameters, the mortality rate, healing time, and cost, and was free of any adverse thyroid, hepatic, or renal effects. Conclusion. Treatment of thermally injured patients with topical povidone-iodine ointment significantly improved oxidative stress parameters, indicating its antioxidant effect. Further investigation is needed to explain the exact mechanism by which povidone-iodine exerts this antioxidant effect. Treatment with topical povidone-iodine ointment alone or in combination with systemic vitamin E and vitamin C significantly improves the outcome of thermally injured patients in a safe way, thanks to the newly emerged mechanism - oxidative stress - involved in burns pathogenesis.
Keywords: antioxidant, vitamin, vitamin c, povidone-iodine, ointment, burns
Abstract
But. Les brûlures constituent un important problème dans le monde entier qui est accompagné d’une mortalité et morbidité élevée et des pertes économiques, même en cas de brûlures mineures. Il est possible que certains progrès dans les protocoles des soins médicaux qui dépendent d’un nouveau mécanisme intéressé à la pathogénicité des brûlures, c’est-à-dire le stress oxidatif (comme par exemple l’emploi de la polyvidone iodée seule ou en combinaison avec la vitamine E et la vitamine C), améliorent les résultats et réduisent les coûts économiques. Patients et méthodes. Trente-huit patients atteints de lésions thermiques provenant d’une variété de groupes d’âge, de sexe et de métier et d’extension de brûlure, hospitalisés dans l’Unité des Brûlures de l’Hôpital Général de Baquba, Iraq, ont été suivis dans cet essai clinique. Les Auteurs de l’étude ont divisé les patients en trois groupes: groupe A (8 patients), traité selon la pratique de l’hôpital; groupe B (17 patients), traité avec l’onguent de polyvidone iodée topique; et groupe C (13 patients), traité avec l’onguent de polyvidone iodée topique associé à une dose systémique une fois par jour de 400 mg de vitamine E et de 500 mg de vitamine C, en plus de l’antibiotique classique employé dans notre hôpital. Dans chaque groupe de paramètres de stress oxidatif, les Auteurs ont utilisé des méthodes standard pour effectuer le test de la fonction thyroïdienne, hépatique et rénale, des études microbiologiques, des évaluations du taux de mortalité et du temps de guérison et enfin des études de nature économique. Résultats. Les Auteurs ont trouvé que le traitement avec l’onguent de polyvidone iodée topique ou en combinaison avec la vitamine E et la vitamine C systémique avait un effet bénéficial significatif sur l’amélioration des paramètres de stress oxidatif, le taux de mortalité, le temps de guérison, et le coût; en outre, cette modalité de traitement n’avait aucun effet négatif thyroïdien, hépatique ou rénal. Conclusion. Le traitement des patients atteints de lésions thermiques moyennant l’onguent de polyvidone iodée topique a amélioré en manière significative les paramètres de stress oxidatif, ce qui indiquait son effet antioxidant. Il faudra effectuer d’autres recherches pour expliquer l’exacte mécanisme par lequel la polyvidone iodée exerce cet effet antioxidant. Le traitement avec l’onguent de polyvidone iodée topique seul ou en combinaison avec la vitamine E et C systémique améliore en manière significative les résultats que l’on peut obtenir dans le traitement des patients atteints de lésions thermiques, sans exposer les patients à aucun risque, grâce au mécanisme récemment découvert - le stress oxidatif - intéressé à la pathogenèse des brûlures.
Introduction
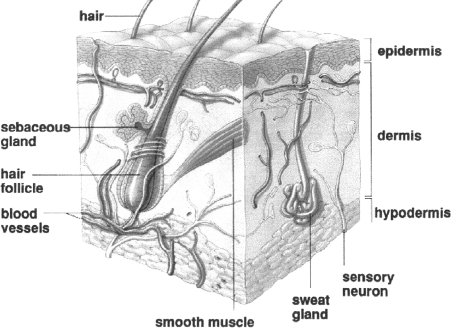
The skin, one of the body’s largest organs, functions as a protective and regulatory barrier between the body and the external environment. Human skin comprises two major tissue layers: the epidermis and the dermis ( Fig. 1 ). The epidermis is the outer layer of the skin and is made up of four layers:
Fig. 1. Structure of human skin.
Stratum corneum: the layer that retains water. This layer consists of dead, dried out (keratinized) cells that are constantly being shed.
Stratum granulosum: characterized by further accumulation of keratin in the cytoplasm.
Stratum spinosum: composed of 3-5 layers of cells with an increasing number of keratin filaments.
Stratum basale: the layer in which new skin cells are produced.
The true skin, dermis, or corium is the inner layer of the skin. It is composed of connective tissues and pressure sensors, nerves, pain sensors, hair follicles, and sweat glands; this layer also controls heat balance. 1, 2
Burns
The skin separates us from our environment, provides the bulk of cooling for heat stress, regulates the egress of body fluids, and prevents outside agents and bacteria from entering the body. When the skin is exposed to excessive heat, such as from fire, electricity, or corrosive chemicals, the resulting tissue damage is known as a burn, and we lose some or all of the skin’s protective to a degree that depends on the extent of the burn. 3
Classification of burns
Burn injuries can be classified either on the basis of the severity of tissue damage or on the basis of the extent or depth of the injury. Severity is expressed as first-, second- and third-degree burns, extent is expressed as a percentage of the total surface area, and depth is classified as partial or full thickness, 4as follows:
According to severity:
First-degree burns: these affect only the outer layer of the skin (epidermis), causing pain and redness
Second-degree burns: these extend to the layer below the epidermis (dermis), causing pain, redness, and blisters that may ooze
Third-degree burns: these involve all layers of the skin and may also damage the underlying bones, muscles, and tendons. The burn site appears pale, charred, or leathery and there is generally no sensation in the area because the nerve endings have been destroyed 5
According to extent: using the Rule of Nines, which is a quick way of estimating the surface area affected by a burn, as follows:
- face and scalp 9%
- back 18%
- perineum 1%
- arm (each) 9%
- front 18%
- upper arm (each) 9%
- lower leg (each) 9%
In children, the head is more than 9%, and a good way of estimating the burn area is to say that the child’s palm is 1% of the surface area. 6
According to depth:
Partial thickness: there is damage to the epidermis but the dermis is intact, and the skin can therefore regenerate. There is also what is called deep partial, which is the term used when much of the dermis has been lost but there are epithelial pockets. With infection or inappropriate care, this can become full thickness
Full thickness: both the epidermis and dermis are destroyed and the skin will not regenerate 7
Pathophysiological alterations in burn injury
Vascular changes in burned skin: almost immediately after the burn, the vessels in the adjacent area are altered. At first, an intense vasoconstriction is caused by the release of numerous vasoactive substances from the injured cells. After a few hours, the vessels dilate as kinins are released from the damaged mast cells. During vasodilation, the capillaries become more permeable. This results in abnormal osmotic and hydrostatic pressure gradients, which force intravascular fluid into the interstitial spaces. Cellular injury triggers the release of inflammation mediators, further contributing to local or systemic increase in capillary permeability. 8
Water and heat losses: in addition to the direct reactions to a thermal burn, burns which destroy the epidermis will allow increased imperceptible water losses of up to 15 times normal. As the water evaporates, body heat is lost, which can lead to the development of hypothermia; 9these losses must be considered when preparing treatment plans.
Infection potential: following a severe skin burn from any source, the skin undergoes coagulative necrosis and becomes an excellent medium for bacteria. Because the local blood supply is also compromised, the local defence mechanisms may be inadequate. The degree and consequences of the resultant bacterial invasion will vary directly with the severity of the wound and can be modified by subsequent therapy. 10This bacterial invasion is one of the most frequent and fatal complications of a serious burn and should be treated aggressively from the beginning. 11
Treatment approach
Appropriate treatment for burns depends on the extent of tissue damage, the cause of the burn, and whether or not infection is present. All burns (with the exception of mild, first-degree burns) require immediate medical attention because of the risk of infection, dehydration, and other potentially serious complications.
Treatment objectives are the prevention and treatment of shock, control of bacterial proliferation, and conversion of an open wound to a closed one. Other important considerations are the maintenance and preservation of body function and appearance, healing within a minimum period, and the patient’s mental and emotional stability. 12
Treatment starts with intravenous (IV) fluid and the maintenance of the airways in unconscious patients. The volume of IV fluid given is calculated by the following formula:
volume = weight (kg) x percentage burn x 4 ml
This volume is given at different rates: in the first 8 h, half of the total; in the next 16 h, half the remainder; and in next 24 h, the remainder. 13
Other medication includes topical 14and systemic antibiotics, analgesia, 15and routine medication such as tetanus toxoid and cimetidine. 16
Burns and oxidative stress
Oxidative stress is caused by the presence of free radicals or radical-generating agents in concentrations that overwhelm natural radical-blocking or -scavenging mechanisms. 17
A skin burn is a common traumatic injury that results in both local tissue damage and a systemic mediator-induced response; there is evidence of both local and systemic oxidant changes manifested by increased oxygen free radical activity 18and lipid peroxidation in animal burn models and also in burned humans. 19Results obtained by Huo et al. showed that both oxygen free radicals and lipid peroxidation play a major role in the injuries caused by skin burns. 20In addition, it has been shown that burn injury causes a remarkable decrease in superoxide dismutase and total antioxidant status 21and a reduction in antioxidant scavenging capacity when compared with control. 22Nagane et al. suggested that therapeutic use of antioxidants could be beneficial in the clinical management of burn patients. 23
Source of free radicals in burns
It has been shown that after burn trauma, tissue adenosine triphosphate (ATP) levels gradually fall, and increased adenosine monophosphate (AMP) is converted to hypoxanthine, providing a substrate for xanthine oxidase. These complicated reactions produce hydrogen peroxide and superoxide, clearly recognized free radicals. 24In addition to xanthine oxidase related free radical generation in burn trauma, adherent-activated neutrophils produce additional free radicals. 19, 24
Patients and methods
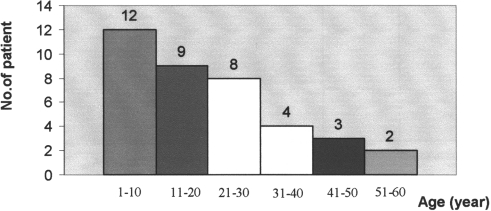
Thirty-eight burn patients (18 males and 20 females) of varying age from 1 to 60 yr (mean ± SD, 21.26 ± 16.7) ( Fig. 2 ) and varying burn percentage ( Fig. 3 ) (10-80% estimated according to the Rule of Nines) were admitted to the burn unit in the Department of Surgery in Baquba General Hospital, Diyala, Iraq, over a period of 6 months.
Fig. 2. Distribution of age groups in burned patients.
Fig. 3. Distribution of burn percentage in burned patients.
The causes of the patients’ burns ( Fig. 4 ) were direct flame, boiling water, chemical burns, and accidental explosion (this study was conducted in time of war - March 2003).
Fig. 4. Distribution of causes of burn.
The patients’ past medical history was negative except for one patient who had diabetes mellitus and another with asthma and hypertension; the social history revealed nothing significant except for one patient only who was a smoker.
The drug treatment given to the patients in the burn unit included intravenous fluid such as Ringer’s solution and glucose water given according to the Parkland method; 13the patients also received systemic antibiotics according to the classical method approved by hospital policy. The antibiotics included ampicillin, gentamicin, ampiclox, cephalosporin, refampicin, cephalothin, and cefotaxime. Local antibiotic ointments were also given to the patients according to availability in the hospital, such as Flamazine, Tetracyclin, and Fucidin ointment. Other drugs given were analgesics like pethidine, morphine, and tramadol, antipyretics like paracetamol, and others like diazepam and Tagamet, according to our hospital drug policy.
The patients were divided into three groups:
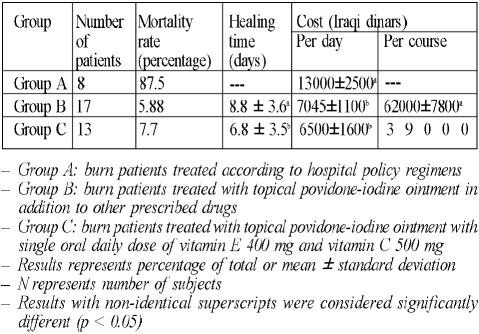
group A: 8 patients (4 males and 4 females) - this group was already present in the burn unit, managed by other surgeons according to hospital policy regimens
group B: 17 patients (8 males and 9 females) - treated with topical povidone-iodine ointment (Povicenter, Arab Center for Pharmaceuticals and Chemicals, Jordan), in addition to other prescribed drugs determined by the hospital drug policy
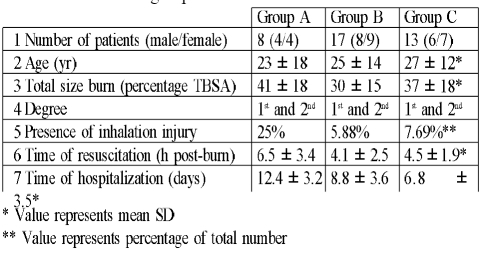
group C: 13 patients (6 males and 7 females) - treated with topical povidone-iodine ointment and oral vitamin E 400 mg once daily (Cipla, India) and oral vitamin C 500 mg once daily (MPI, Syria) until discharged, in addition to other drugs prescribed according to the hospital drug policy ( Table I presents the details of each group)
Table I. Details of groups.
In addition, 12 healthy subjects (5 males and 7 females), with the same age range as that of patients, were selected to serve as control for comparison.
Sample collection and preparation
Blood samples were collected from all subjects by venipuncture.
Ten millilitres were taken on admission (zero time) to the burns unit and then every two days of treatment and on discharge day to check changes in the parameters studied. All blood samples were collected in a heparinized plain tube. Erythrocytes were separated by centrifugation at 3000 rpm for 10 min at 4 C; the plasma obtained was used for biochemical analysis, which included:
Measurement of oxidative stress parameters
measurement of plasma malondialdehyde (MDA) level: MDA is a by-product of lipid peroxidation and its measurement is based on the reaction of thiobarbituric acid (TBA) with MDA forming TBA2-MDA adducts, according to the standard method of Stocks and Dormandy, 25modified by Gilbert et al. 26
measurement of plasma glutathione (GSH) level: glutathione contents (measured as total sulphydryl groups) were measured according to the method of Godin et al. 27
Thyroid function test: in each subject T 3(triiodothyronine) and T 4(thyroxin) plasma levels were measured by enzyme immunoassay according to the methods of Utiger 28and Wistom 29respectively; a ready-made kit was used for this purpose (BioCheck, Burlingame)
Liver function test: alkaline phosphatase activity was measured colorimetrically according to the method of Kind et al., 30utilizing a ready-made kit for this purpose (BioMerieux, France). Also, the activities of glutamate-pyruvate transaminase (SGPT) and glutamate-oxaloacetate transaminase (SGOT) enzymes were evaluated colorimetrically according to the method of Reitman, 31utilizing a ready-made kit for this purpose (RANDOX, UK)
Renal function test:
blood urea: determination of serum urea level was performed using the urease-modified Berthelot reaction, 32utilizing a ready-made kit for this purpose
serum creatinine: serum creatinine was evaluated utilizing a ready-made kit for this purpose (Biomaghreb, Tunisia), according to the method of Henry 33
Wound swabs for microbiological examination and characterization of the invading micro-organism, if present, were taken on admission and at 2-day intervals during follow-up until discharge 34
Mortality rate: burn mortality rate was evaluated, as also the relative effect of burn size, presence of inhalation injury, 35timing of resuscitation, and the plasma level of MDA and GSH 36and their effects on the mortality rate
Healing time: healing time was measured, reported as the time required for complete healing of burn wound without any sign of infection 37
Cost: cost of treatment with povidone-iodine ointment, vitamin E, and vitamin C was evaluated and compared with the classical method followed in the burn unit according to the hospital drug policy. The comparison was based on the retail prices of the drugs, including the topical agents used, intravenous fluid, antibiotics, analgesics, and other accessories like gloves, catheters, and dressing material and also including the cost of hospitalization, all laboratory tests performed, and dressing changes. The evaluation of the total cost of treatment, as in a similar previous study by Atiyeh et al. 38was calculated on a per day basis in contrast to others calculated on a per course of treatment basis 39
Statistical analysis:
the results were expressed as mean ± SD
the Student t-test was used to examine the degree of significance, and a pvalue of less than 0.05 was considered significant
Results
Treatment with topical povidone-iodine ointment showed a significant reduction (p < 0.05) in the MDA level after two days (38%) and on discharge day (71%), as well as a significant increase (p < 0.05) in the GSH level after two days (50%) and on discharge day (80%), compared to pre-treatment values ( Table II ). These data clearly prove the possible antioxidant effect of povidone-iodine ointment when used topically in burn patients.
Table II. Results of the various treatments.
Treatment with topical povidone-iodine ointment together with oral vitamin E and vitamin C significantly (p < 0.05) reduced the MDA level after two and four days (57% and 74% respectively), while the effects on plasma GSH levels were significantly increased (p < 0.05) after four days and on discharge day (96% and 142% respectively), compared to pre-treatment values ( Table II ).
Table II also shows that treatment with topical povidone-iodine ointment alone and treatment with topical povidone-iodine ointment together with oral vitamin E and vitamin C had no significant effect on serum levels of thyroid hormones T 3and T 4compared to control and pre-treatment values.
In addition, Table II clearly shows that there were no significant differences between the groups studied as regards the serum values of liver enzyme activities, compared to control. There was no significant difference in serum values of liver enzyme activities between group B, treated with topical povidone-iodine ointment, and group C, treated with topical povidone-iodine ointment together with oral vitamin E and vitamin C, compared to pre-treatment.
The results shown in Table II show that there was a significant elevation in blood urea and serum creatinine levels in all treated groups (A, B, C) at zero time compared to control. In contrast, after initiation of treatment, blood urea and serum creatinine levels decreased significantly (p < 0.05) in all groups after two and four days, returning to normal level at discharge day, compared to pre-treatment.
Table III and Fig. 5 show the incidence and distribution of the invading micro-organisms isolated from the burn patients. The percentage of positive swabs increased from 25% at zero time to 75% after two and four days in group A, while treatment with topical povidone-iodine ointment (group B) altered the incidence of wound infection from 23.5% at zero time and two days later to 17.6% after four days and at discharge time.
Table III. Effects of treatment with topical povidone-iodine ointment or topical povidone-iodine ointment with oral vitamin E 400 mg and vitamin C 500 mg on the incidence and distribution of invading micro-organisms isolated from burn patients.
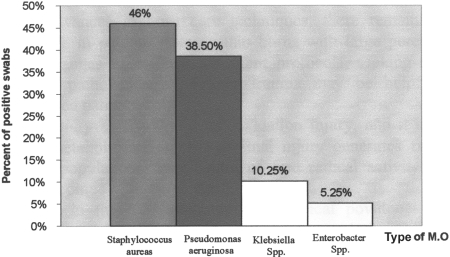
Fig. 5. Distribution of micro-organism types in positive swabs isolated from burn patients.
Treatment with topical povidone-iodine ointment together with oral vitamin E and vitamin C (group C) decreased the incidence of infection in burn patients by 7.7% after two days and by 15.3% after four days and at discharge time when compared with zero time. Table III also shows that 27.1% of swab cultures were bacteriologically positive; the micro-organisms isolated, as shown in Fig. 5 , were Staphylococcus aureus (46.%), Pseudomonas aeruginosa (38.5%), Klebsiella spp. (10.25%), and Entero-bacter spp. (5.25%).
Table IV shows the reduction in mortality rate in the groups treated. Treatment with topical povidone-iodine ointment reduced the mortality rate from 87.5% to 5.88%, and treatment with topical povidone-iodine ointment and oral vitamin E with vitamin C reduced the mortality rate from 87.5% to 7.7%, compared to the classically treated group.
Table IV. Effects of treatment with topical povidone-iodine ointment or topical povidone-iodine ointment with oral vitamin E 400 mg and vitamin C 500 mg on on mortality, healing time, and cost in burn patients.
Since seven out of the eight patients (87.5%) died during the course of treatment in group A, and one patient was discharged from the burns unit by the patient’s own decision, no healing time was calculated for group A.
Treatment with topical povidone-iodine ointment and oral vitamin E with vitamin C significantly (p < 0.05) reduced the healing time by two days (22.7%), compared to group B, which was treated with topical povidone-iodine ointment only.
Table IV does not present any values for group A in relation to cost calculated per course of treatment since the mortality rate in this group was very high, with seven patients out of eight dying and one patient being discharged from the burn unit on the patient’s own decision; in this group cost was calculated on a per day basis.
Treatment with topical povidone-iodine ointment significantly (p < 0.05) decreased the cost calculated on a per day basis, compared to group A; the reduction was 45.8% (5955 Iraqi dinars [ID]). Treatment with topical povidone-iodine ointment and oral vitamin E with vitamin C also significantly (p < 0.05) reduced the cost by 50% (6500 ID) compared to group A, while there was no significant change between groups B and C.
When cost was calculated per course of treatment, treatment with topical povidone-iodine ointment and oral vitamin E with vitamin C significantly (p < 0.05) reduced the cost by 37% (23,000 ID), compared to group B.
Discussion
Despite advances in burn care techniques, there is still a tendency for therapeutic failure in patients who sustain burns in a large percentage of the total body surface area. Changes in medical treatment protocols, seeking new mechanisms involved in the pathogenesis of burn trauma, may be helpful in the successful treatment of burn patients.
Thermal injury of the skin is an oxidation injury, associated with biological and metabolic alterations; thermal injury generates free radicals from various cellular populations, and the modulation of free radical activity with scavengers may improve outcome. 40
As shown in Table II , treatment with topical povidone-iodine ointment significantly decreased the end-product of lipid peroxidation (MDA) two days after start of treatment and at discharge, and also significantly increased the level of natural antioxidant (GSH) two days after start of treatment and at discharge, indicating the antioxidant effect of povidone-iodine. This was the first time the antioxidant effect of povidone-iodine was shown when used topically as an ointment for burn patients.
The mechanism by which povidone-iodine exerts its antioxidant effect may be due to its iodine content. It has been shown that in iodide-concentrating cells, such as stomach and thyroid cells as well as epidermis cells, iodide may act as an electron donor in the presence of H 2O 2and peroxidase, and the remaining iodine readily iodinates tyrosine. 41Also, Katamine et al. 42have shown that dietary iodides are able to defend brain cells from lipid peroxidation in rats. The antioxidant action of iodide has also been described in isolated rabbit eye. 43Muranov et al. 44have shown the protective role of iodide against cataract development induced by selinite, and he supposed that the anticataract effect of iodide could be based on a direct or indirect antioxidant mechanism.
Schmut et al. 45showed that iodide protected hyaluro-nate, a component of tear fluid and tissues of the anterior part of the eye, against UVB light-induced degradation, and he suggested that the administration of an antioxidant such as iodide to artificial tears might help prevent eye damage provoked by oxidative stress.
Additionally, Winkler et al. 46showed that the administration of NaI led to a significantly increased antioxidant capacity (using what is known as the Total Antioxidant Status determined by a calorimetric method) compared to NaC, while Tatzber et al. 47gave an explanation for the increased total antioxidant status in the presence of iodide, which may be due to an indirect protective effect via enhanced activity of enzymatic antioxidants, thereby reducing endogenous peroxides. These data may provide an explanation for results presented in Table II .
Treatment with topical povidone-iodine ointment and oral vitamin E with vitamin C also significantly reduced the MDA level after two and four days and at the same time significantly raised the level of reduced glutathione (GSH) after four days, reaching control levels at discharge, as shown in Table II .
Our results are compatible with those obtained by Fang et al.40 and Matsuda et al., 48in which the administration of vitamin E, a well-known antioxidant, improved burn outcome and the addition of vitamin C diminished early post-burn lipid peroxidation and cellular injury and dysfunction, by virtue of their ability to scavenge free radicals and inhibit the activation of the nuclear factor kappa B (NFKB), a mediator responsible for inflammation. 49
To investigate the changes that may occur in thyroid hormone levels following burns treated topically with povidone-iodine, the serum levels of thyroxin (T 4) and triiodothyronine (T 3) were measured. Table II shows that there were no significant changes in these values in burn patients treated with povidone-iodine, compared to control; also, clinical signs and symptoms considered to be attributable to thyroid dysfunction were not seen in any of the subjects, and no cases of goitre were observed.
Our results are compatible with those obtained by Balogh et al., 50who found no significant changes in thyroid function compared to control, especially when renal function was unimpaired, since the absorbed iodine responsible for these changes was quickly excreted. In contrast, data reported by Aiba et al. 51showed that long-term use of topical povidone-iodine in burn patients caused iodine toxicosis manifested as hypotension, bradycardia, metabolic acidosis, and renal failure, while in 1983 Preissler 52showed that T 4and T 3concentrations decreased significantly during the first two weeks in extensive burns treated with topical application of povidone-iodine.
Increased liver enzyme levels (SGPT, SGOT, and alkaline phosphatase) are usually regarded as expressions of cellular necrosis, especially in hepatocytes. They reflect cellular damage due to burn which according to many researchers becomes normal before the patients’ discharge. 53 54
Temporary nonsignificant elevations in the activity of GPT, GOT, and alkaline phosphatase were observed in all groups ( Table II ), indicating that treatment with topical povidone-iodine ointment alone or in combination with oral vitamin E and vitamin C had no effect on liver enzymes.
The same observation was made when checking blood urea and serum creatinine as a renal function test. The results presented in Table II show that there was a significant increase (p < 0.05) in the level of blood urea and serum creatinine at zero time in all groups compared to control; they also show that during the course of treatment in all groups there was a gradual normalization in renal function test data compared to normal control.
These data are consistent with those obtained by others 55 56and give a clear indication of the renal safety of treatment with topical povidone-iodine ointment alone or in combination with oral vitamin E and vitamin C in burn patients. Table III shows the reduction in percentage of incidence of infection in groups B and C compared to group A. Fig. 5 shows that the main micro-organism responsible for infection in burn patients was Staphylococcus aureus, which represents 46% of positive swabs isolated from burn patients, a result consistent with that obtained by Ugburo et al. 57
Pseudomonas aeruginosa, however, remains a serious cause of infection and septic mortality in burns, particularly when acquired nosocomially; 58it continues to be the predominant species isolated from burn patients even after the use of topical povidone-iodine ointment. Povidone-iodine is the combination of molecular iodine and the polymer polyvinylpyrrolidone; 59it has bactericidal, 60sporicidal, 61viricidal, 62and fungicidal 63effects. Since local infection and burn wound sepsis are among the severest problems in the treatment of thermally injured patients, early treatment with topical antiseptics decreases the infection rate and improves the survival rate of burn patients; 58in addition, with the proliferation of antibiotic- and antiseptic-resistant strains of bacteria around the world, attention is increasingly being focused on more traditional and untraditional methods for combating and preventing wound infection. 64Clinical studies have demonstrated that treatment with povidone-iodine is the most effective method against bacterial and fungal infection in burn patients. 65In contrast, Cetinkale et al. 66demonstrated that large burns were profoundly immunosuppressive and that the early use of antioxidant therapy was able to significantly restore cell-mediated immunity, which may explain the results presented in Table III in cases where treatment involved a topical antioxidant, such as povidone-iodine ointment, alone or in combination with systemic antioxidants like vitamin E and vitamin C.
Many recent data have demonstrated the cause-and-effect relationship between plasma oxidative parameters and mortality in patients with burn injury. 35Post-burn multiple organ failure (MOF) occurs mainly in patients with a high burn percentage, and MOF mortality was found to be directly proportional to the number of organs involved 67- the incidence of pulmonary failure was the highest, 68and the highest mortality was attributed to renal failure. 69MOF occurring in the early stage was more often related to burn shock, while MOF occurring in the late stage was due mainly to infection. 34
It has been shown that oxygen free radicals play an important role in the genesis and development of post-burn MOF. It has been found that antiperoxidation ability declines, reactive oxygen species increase, and lipid peroxidation becomes excessive after burn injury. 67Results obtained by Horton in 1996 suggested that xanthine-oxidase-mediated free radical production contributed, in part, to post-burn alterations in cardiac function, 70while Maass et al. showed that burn trauma activated myocardial NF-kappa B and promoted cardiomyocyte secretion of TNF-alpha, and that this inflammatory cascade preceded the appearance of cardiac dysfunction, indicating that cardiac myocyte-derived TNF-alpha contributed, in part, to post-burn cardiac contractile deficits. 71Horton et al. 72made the interesting finding that in cases of burn trauma antioxidant vitamin therapy provided cardioprotection, at least in part, by inhibiting translocation of the transcription factor NF-kappa B and by interrupting cardiac inflammatory cytokine secretion of TNF-alpha. 72La Londe et al. 73showed that antioxidants, administered post-burn, restored antioxidant defences, attenuated the altered cell energetics, and prevented mortality, indicating that oxidants were the cause of mortality; these data also suggest that there is a critical value in the decrease of antioxidant defences that results in mortality. Our data ( Table IV ), in agreement with all these findings, indicate the beneficial effect of antioxidants in reducing the mortality rate when administered to burn patients. Table IV shows a significant difference in healing time between groups B and C - there are no data available for group A since all the patients died after two to three weeks in the burn unit. The use of systemic antioxidant vitamins in addition to topical antioxidant in group C significantly (p < 0.05) reduced healing time compared to group B, in which only topical povidone-iodine ointment was used.
Recent data have shown that povidone-iodine is widely used in the local treatment of burn wounds; 74 75Juhasz 76showed that povidone-iodine was beneficial to burn wounds owing to its effect in reducing bacterial colony counts, and he suggested that the use of povidone-iodine was safe in a clinical setting. Our results are compatible with these, as regards the effectiveness and safety of the topical use of povidone-iodine in burn patients. Also, the formation of eschars after use of povidone-iodine ointment in burn patients can be easily treated with 10% salicylic acid without any problems and avoiding surgical escharotomy. Many researchers have proved the protective effect of povidone-iodine ointment against skin lesions induced by chemical and thermal stimuli; 77and clinical experience with patients after accidental heat burns has shown that the topical application of povidone-iodine ointment immediately after the stimulus significantly reduces, and often prevents, skin lesions. Furthermore, apart from being a safe and widely used disinfectant, povidone-iodine ointment can be considered an efficient protective agent against skin toxicity caused by hazardous chemicals and heat stimuli. 78 79
Wormser et al. 80showed that reduced collagenolytic activity might be one of the mechanisms by which iodine protects the skin against chemical insults, which may explain the considerable difference in healing time in the group treated with povidone-iodine ointment compared to group A, in which where there was no healing at all. Khodr et al. 81showed that treatment with an antioxidant, namely vitamin E, reduced the time required for complete wound closure of full-thickness burn injury, while another study 82showed that use of antioxidants, namely vitamin E and vitamin C, hastened the epithelialization process, results with which our data are compatible.
Table IV shows that treatment with topical povidone-iodine ointment significantly (p < 0.05) reduced the cost calculated on a per day basis, while results obtained by Wormser et al. 80indicated that use of povidone-iodine reduced the collagenase activity found in the skin, which might explain the reduction in oozing and fluid loss in burn patients. This in turn led to decreased fluid requirement in the patients, which might effectively reduce the cost. In addition, the decrease in oozing reduced the change of bedclothes from once daily to every other day, which reduced costs.
The systemic administration of vitamins E and C in addition to topical use of povidone-iodine ointment significantly reduced costs (p < 0.05) compared to group B, which was treated with topical povidone-iodine ointment only.
Huang et al. 83found that endogenous circulating factors played a role in post-burn microvascular leakage; recent studies suggest that reactive oxygen species generated by thermal injury are involved in the increased microvascular permeability, oedema formation, and tissue damage associated with burns. 84
In view of these findings, the administration of antioxidants may be of great benefit. Tanaka et al. 85 86showed that the administration of an elevated dose of ascorbic acid during the first 24 h after thermal injury significantly reduced resuscitation fluid volume requirements and wound oedema, while Matsuda et al. 87found that high-dose vitamin C infusion maintained haemodynamic stability in the presence of a reduced resuscitation fluid volume, provided that vitamin C was administered for a minimum of 8 h post-burn.
In contrast, Tanaka et al. 88showed that after delayed initiation of high-dose ascorbic acid therapy, the 24-h fluid resuscitation volume dropped to 32.5% of the Parkland formula, while Matsuda et al. 89found that the administration of high-dose vitamin C to burn patients reduced the total 24-h resuscitation volume from 4 ml/kg/% burn to 1 ml/kg/% burn; all these results could explain vitamin C’s role in the reduction in fluid resuscitation volume required for burn patients, effectively reducing the cost of treatment. This may explain our results presented in Table IV .
To sum up, the reduction in healing time shown in Table IV played an important role in reducing costs, since the duration of hospitalization was reduced by two days, leading to a significant reduction in the cost of treatment calculated on a per course basis.
Conclusion
This study showed the involvement of oxidative stress parameters in the pathogenicity of thermally injured patients manifested as elevation in the plasma level of MDA; it also showed the end product of lipid peroxidation and a reduction in the plasma level of GSH, the natural antioxidant, compared to healthy subjects. Another finding was that treatment with topical povidone-iodine ointment significantly improved these parameters, indicating the antioxidant effect of topical povidone-iodine ointment when used to treat burns. The exact mechanism by which povidone-iodine ointment exerts its antioxidant effect requires further deep investigation.
Treatment with topical povidone-iodine ointment alone or in combination with systemic vitamin E and vitamin C not only improved the oxidative stress condition in thermally injured patients but also effectively interfered with the incidence of infection and significantly reduced it; it also significantly reduced the mortality rate and healing time and effectively reduced the cost of treatment, compared to classical treatment.
It is important to bear in mind that the treatment of thermally injured patients with topical povidone-iodine ointment alone in combination with systemic vitamin E and vitamin C was safe and had no effect on thyroid gland function or on liver and renal function tests during the course of treatment.
References
- 1.Holbrook K.A., Sybert V. “Dermatology in General Medicine”. 1988. The structure and development of skin. pp. 93–131. [Google Scholar]
- 2.Odland G.F. “Structure of the Skin Biochemistry and Physiology of the Skin”. 1983;63 [Google Scholar]
- 3.Demling R.H. Burns. N. Engl. J. Med. 1985;313:1389–98. doi: 10.1056/NEJM198511283132205. [DOI] [PubMed] [Google Scholar]
- 4.Tobiasen J., Hieber J.M., Edlich R.F. The abbreviated burn severity index. Ann. Emerg. Med. 1981;11:260–2. doi: 10.1016/s0196-0644(82)80096-6. [DOI] [PubMed] [Google Scholar]
- 5.Roi L.D., Flora J.D., Davis T.M., et al. A severity grading chart for the burned patient. Ann. Emerg. Med. 1981;10:161–3. doi: 10.1016/s0196-0644(81)80386-1. [DOI] [PubMed] [Google Scholar]
- 6.Lund C.C., Browder N.C. The estimation of areas of burns. Surg. Gynecol. Obstet. 1944;79:352–8. [Google Scholar]
- 7.Heimbach D.M., Afromowitz M.A., Engrav L.H., Marvin J.A., Perry B. Burn depth estimation - man or machine. J. Trauma. 1984;24:373–8. [PubMed] [Google Scholar]
- 8.Pitt R.M., Paeker J.C. Analysis of altered capillary pressure and permeability after thermal injury. J. Surg. Res. 1987;42:693–702. doi: 10.1016/0022-4804(87)90013-8. [DOI] [PubMed] [Google Scholar]
- 9.Arturson M.G. The pathophysiology of severe thermal injury. J. Burn Care Rehabil. 1985;6:129–46. doi: 10.1097/00004630-198503000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Revathi G., Puri J., Jain B.K. Bacteriology of burns. Burns. 1998;24:347–9. doi: 10.1016/s0305-4179(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 11.Bowler P.G., Duerden B.I., Armstrong D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001;14:2–244. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caravajal H.F., Stewart C.E. Emergency management of burn patients The first few hours. Emerg. Med. Reports. 1987;8:129–136. [Google Scholar]
- 13.Rubin W.D., Mani M.M., Hiebert J.M. Fluid resuscitation of the thermally injured patient. Clin. Plas. Surg. 1986;152:664–9. [PubMed] [Google Scholar]
- 14.Palmieri T.L., Greenhalgh D.G. Topical treatment of pediatric patients with burns: A practical guide. Am. J. Clin. Dermatol. 2002;3:529–34. doi: 10.2165/00128071-200203080-00003. [DOI] [PubMed] [Google Scholar]
- 15.Ulmer J.F. Burn pain management: A guideline-based approach. J. Burn Care Rehabil. 1998;19:151–9. doi: 10.1097/00004630-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin C.W. Current burn treatment. Advances in Surgery. 1984;18:145–76. [PubMed] [Google Scholar]
- 17.Halliwell B., Gutteridge J.M. “Free radicals in biology and medicine”. 1990. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen T.T., Cox C.S., Traber D.L., Gasser H., Redl H., Schlag G., Herndon D.N. Free radical activity and loss of plasma antioxidant vitamins and sulphydryl groups in patients with burns: The 1993 Moyed Award. J. Burn Care Rehabil. 1993;14:602–9. doi: 10.1097/00004630-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Youn Y.K., La Londe C., Demling R. Oxidants and the pathophysiology of burn and smoke inhalation injury. Free Radic. Biol. Med. 1992;12:409–15. doi: 10.1016/0891-5849(92)90090-4. [DOI] [PubMed] [Google Scholar]
- 20.Huo T.N., Fang R.H., Wei Y.H. Changes in lipid peroxide levels in the plasma of patients with thermal skin injuries. J. Formos. Med. Assoc. 1993;92:1034–9. [PubMed] [Google Scholar]
- 21.Cetinkale O., Belce A., Konukoglu D., Senyuva C., Gumustas M.K. Evaluation of lipid peroxidation and total antioxidant status in plasma of rats following thermal injury. Burns. 1997;23:114–6. doi: 10.1016/s0305-4179(96)00084-8. [DOI] [PubMed] [Google Scholar]
- 22.Haycock J.W., Ralston D.R., Morris B., Freedlander E., MacNeil S. Oxidative damage to protein and alterations to antioxidant level in human cutaneous thermal injury. Burns. 1997;23:533–40. doi: 10.1016/s0305-4179(97)00072-7. [DOI] [PubMed] [Google Scholar]
- 23.Nagane N.S., Bhagwat V.R., Subramanium M. Increased free radical activity in burns. Indian J. Med. Sci. 2003;57:7–11. [PubMed] [Google Scholar]
- 24.Horton J.W. Free radicals and lipid peroxidation mediated injury in burn trauma: The role of antioxidant therapy. Toxicology. 2003;189:75–88. doi: 10.1016/s0300-483x(03)00154-9. [DOI] [PubMed] [Google Scholar]
- 25.Stocks J., Dormandy T.L. The auto-oxidation of human red cell lipids induced by hydrogen peroxide. Brit. J. Haematol. 1971;20:95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert H.S., Stemp D.D., Roth E.F. A method to correct errors caused generation of interfering compounds during lipid peroxidation. Anal. Biochem. 1984;173:282–6. doi: 10.1016/0003-2697(84)90086-1. [DOI] [PubMed] [Google Scholar]
- 27.Godin D.V., Wahaieb S.A., Garnett M.E. Antioxidant enzyme alteration in experimental and clinical diabetes. Mol. and Cell. Bioch. 1988;84:223–31. doi: 10.1007/BF00421057. [DOI] [PubMed] [Google Scholar]
- 28.Utiger R.D. Serum triiodothyronine in man. Ann. Rev. Med. 1974;25:289–302. doi: 10.1146/annurev.me.25.020174.001445. [DOI] [PubMed] [Google Scholar]
- 29.Wistom G.B. Enzyme-immunoassay. Clin. Chem. 1976;22:1243. [PubMed] [Google Scholar]
- 30.Kind R.R.N., King E.J. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J. Clin. Path. 1954;7:322–6. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reitman S., Frankel S. GOT/GPT procedures. Am. J. Clin. Path. 1957;28:56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 32.Fawcett J.K., Scott J.E. Determination of urea in blood or serum. J. Clin. Path. 1960;13:156–9. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry J.B. “Clinical Diagnosis and Management” (17th edition) p. 1984.. [Google Scholar]
- 34.Revathi G., Puri J., Jain B.K. Bacteriology of burns. Burns. 1998;24:347–9. doi: 10.1016/s0305-4179(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 35.O’Mara M.S., Canshaj P., Goldfarb I.W., Slater H. Treatment and mortality trends among massively burned patients. Ann. Burns and Fire Disasters. 2000;13:73–6. [Google Scholar]
- 36.Ritter C., Andrades M., Guerreiro M., Zavaschi L., Gelain D.P., Souza L.F., Riberio C.A., Clausell N., Menna-Barreto S., Moreira J.C., Dal-Pizzol F. Plasma oxidative parameters and mortality in patients with severe burn injury. Intensive Care Med. 2003;29:1380–3. doi: 10.1007/s00134-003-1833-9. [DOI] [PubMed] [Google Scholar]
- 37.Williams R.L., Armstrong D.G. Wound healing: New modalities for a new millennium. Clin. Pediatr. Med. Surg. 1998;15:151–4. [PubMed] [Google Scholar]
- 38.Atiyeh B.S., Dham R., Kadry M., Abdallah A.F., Al Oteify M., Fathi O., et al. Benefit-cost analysis of moist exposed burn ointment. Burns. 2002;28:659. doi: 10.1016/s0305-4179(02)00075-x. [DOI] [PubMed] [Google Scholar]
- 39.Lofts J.A. Cost analysis of a major burn. N.Z. Med. J. 1991;16:488. [PubMed] [Google Scholar]
- 40.Fang C.H., Peck M.D., Alexander J.W., Babcock G.F., Warden G.D. The effect of free radical scavengers on outcome after infection of burned mice. J. Trauma. 1990;30:453–6. [PubMed] [Google Scholar]
- 41.Venturi S., Venturi M. Iodide,thyroid and stomach carcinogenesis: Evolutionary story of a primitive antioxidant. Europ. J. Endocrinol. 1999;140:371–2. doi: 10.1530/eje.0.1400371. [DOI] [PubMed] [Google Scholar]
- 42.Katamine S., Hoshino N., Totsuka K., Suzuki M. Effects of the long-term feeding of high-iodine eggs on lipid metabolism and thyroid function in rats. J. Nutr. Sci. Vitaminol. 1985;31:339–53. doi: 10.3177/jnsv.31.339. [DOI] [PubMed] [Google Scholar]
- 43.Elstner E.F., Adamczyk R., Kromer R., Furch A. The uptake of potassium iodide and its effect as an antioxidant in isolated rabbit eyes. Ophthalmologica. 1985;191:122–6. doi: 10.1159/000309572. [DOI] [PubMed] [Google Scholar]
- 44.Muranov K., Poliansky N., Winkler R., Rieger G., Schmut O., Horwath-Winter J. Protection by iodide of lens from selenite-induced cataract. Graefes. Arch. Clin. Exp. Ophthalmol. 2004;242:146–51. doi: 10.1007/s00417-003-0790-x. [DOI] [PubMed] [Google Scholar]
- 45.Schmut O., Horwath-Winter J., Rieger G., Winkler R., Trummer G., Spitzenberger H., Wachswender C. Iodide protection from UVB irradiation-induced degradation of hyaluronate and against UVB-damage of human conjunctival fibroblasts. Graefes Arch. Clin. Exp. Ophthalmol. 2004;242:279–83. doi: 10.1007/s00417-003-0829-z. [DOI] [PubMed] [Google Scholar]
- 46.Winkler R., Griebenow S., Wonisch W. Effect of iodide on total antioxidant status of human serum. Cell. Biochem. Funct. 2000;18:143–6. doi: 10.1002/(SICI)1099-0844(200006)18:2<143::AID-CBF857>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 47.Tatzber F., Griebenow S., Wonisch W., Winkler R. Dual method for the determination of peroxidase activity and peroxide-iodide leads to a significant increase of peroxidase activity in human sera. Anal. Biochem. 2003;316:147–53. doi: 10.1016/s0003-2697(02)00652-8. [DOI] [PubMed] [Google Scholar]
- 48.Matsuda T., Tanaka H., Yuasa H., Forrest R., Matsuda H., Reyes H. The effect of high-dose vitamin C therapy on post-burn lipid peroxidation. J. Burn Care Rehabil. 1993;14:624–9. doi: 10.1097/00004630-199311000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Conner E.M., Grisham M.B. Inflammation, free radicals and antioxidants. Nutrition. 1996;12:274–7. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 50.Balogh D., Bauer M., Riccabona G. The influence of povidone-iodine treatment on thyroid hormones in severe burns. J. Hosp. Infec. 1985;6:147–53. doi: 10.1016/s0195-6701(85)80061-x. [DOI] [PubMed] [Google Scholar]
- 51.Aiba M., Ninomiya J., Furuya K., Arai H., Asaumi S., Takagi A., Ohwada S., Morishita Y. Induction of a critical elevation of povidone-iodine absorption in the treatment of burn patient: Report of a case. Surg. Today. 1999;29:157–9. doi: 10.1007/BF02482241. [DOI] [PubMed] [Google Scholar]
- 52.Preissler P. Changes in thyroid hormone levels following severe burns treated topically with povidone-iodine. Langenbecks Arch. Chir. 1983;360:9–15. doi: 10.1007/BF01255579. [DOI] [PubMed] [Google Scholar]
- 53.Chiarelli A., Casadei A., Pornaro E., Siliprandi L., Mazzoleni F. Alanine and aspartate aminotransferase serum levels in burned patients: A long-term study. J. Trauma. 1987;27:790–4. doi: 10.1097/00005373-198707000-00017. [DOI] [PubMed] [Google Scholar]
- 54.Chiarelli A., Siliprandi L., Casadei A., Schiavon M., Mazzoleni F. Aminotransferase changes in burned patients. Intensive Care Med. 1987;13:199–202. doi: 10.1007/BF00254704. [DOI] [PubMed] [Google Scholar]
- 55.Vanholder R., Van den Bogaerde J., Vogelaers D., Colardyn F. Renal function in burns. Acta Anaesthesiol. Belg. 1987;38:367–71. [PubMed] [Google Scholar]
- 56.Zhu L., Yang Z., Li A. The protective effects on post-burn renal function by early enteral feeding in burned rats. Zhonghua Shao Shang Za Zhi. 2000;16:224–7. [PubMed] [Google Scholar]
- 57.Ugburo A.O., Atouebi O.A., Oyeneyin J.O., Sowemimo G.O. An evaluation of the role of systemic antibiotic prophylaxis in the control of burn wound infection at the Lagos University Teaching Hospital. Burns. 2004;30:43–8. doi: 10.1016/j.burns.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 58.Tredget E.E., Shankowsky H.A., Rennie R., Burrell R.E., Logsetty S. Pseudomonas infections in the thermally injured patients. Burns. 2004;30:3–26. doi: 10.1016/j.burns.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Ogrin R. Current thoughts on the use of povidone-iodine (Betadine) in wounds: Acute and chronic. Australian J. Paed. Med. 2002;36:13–19. [Google Scholar]
- 60.Goldenheim P.D. In vitro efficacy of povidone-iodine solution and cream against methicillin-resistant Staphylococcus aureus. Postgrad. Med. J. 1993;69 (suppl. 3):S62–5. [PubMed] [Google Scholar]
- 61.Tennen R, Setlow B., Davis K., Loshon C., Setlow P. Mechanisms of killing spores of Bacillus subtilis by iodine, glutaraldehyde and nitrous acid. J. Applied Microbiology. 2000;89:330–8. doi: 10.1046/j.1365-2672.2000.01114.x. [DOI] [PubMed] [Google Scholar]
- 62.Kawana R., Kitamura T., Nakagomi O., Matsumoto I., Arita M., Yoshihara N., et al. Inactivation of human viruses by povidone-iodine in comparison with other antiseptics. Dermatology. 1997;195 (suppl. 2):29–35. doi: 10.1159/000246027. [DOI] [PubMed] [Google Scholar]
- 63.Pierard G., Pierard-Franchimont C., Arrese J. Povidone-iodine wash solutions in the prevention of superficial fungal infections: Predictive evaluation using the corneofungimetry bioassay. European J. Clin. Pharmacol. 1997;53:101–4. doi: 10.1007/s002280050345. [DOI] [PubMed] [Google Scholar]
- 64.Flynn J. Povidone-iodine as a topical antiseptic for treating and preventing wound infection: A literature review. Br. J. Community Nurs. 2003;8 (suppl. 6):S36–42. doi: 10.12968/bjcn.2003.8.Sup2.11555. [DOI] [PubMed] [Google Scholar]
- 65.Steen M. Review of the use of povidone-iodine (PVP-I) in the treatment of burns. Postgrad. Med. J. 1993;69 (suppl. 3):S84–92. [PubMed] [Google Scholar]
- 66.Cetinkale O., Senel O., Bulan R. The effect of antioxidant therapy on cell-mediated immunity following burn injury in an animal model. Burns. 1999;25:113–8. doi: 10.1016/s0305-4179(98)00124-7. [DOI] [PubMed] [Google Scholar]
- 67.Yang Z.C. Clinical study of the pathogenesis of multiple organ failure after burns. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi. 1992;8:8–12. [PubMed] [Google Scholar]
- 68.Chen L.W., Hwang Y.C., Chen C.J., Wang J.S., Chen S.J., Hsu C.M. Burn-induced lung damage in rats is mediated by a nitric oxide/cGMP system. Shock. 2003;20:369–74. doi: 10.1097/01.shk.0000086520.18735.df. [DOI] [PubMed] [Google Scholar]
- 69.Holm C., Horbrand F., Von Donnersmarck G.H., Muhlbauer W. Acute renal failure in severely burned patients. Burns. 1999;25:171–8. doi: 10.1016/s0305-4179(98)00144-2. [DOI] [PubMed] [Google Scholar]
- 70.Horton J.W. Oxygen free radicals contribute to post-burn cardiac cell membrane dysfunction. J. Surg. Res. 1996;61:97–102. doi: 10.1006/jsre.1996.0087. [DOI] [PubMed] [Google Scholar]
- 71.Maass D.L., Hybki D.P., White J., Horton J.W. The time course of cardiac NF-kappa B activation and TNF-alpha secretion by cardiac monocytes after burn injury: Contribution to burn-related cardiac contractile dysfunction. Shock. 2002;17:293–9. doi: 10.1097/00024382-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 72.Horton J.W., White D.J., Maass D.L., Hybki D.P., Haudek S., Giroir B. Antioxidant vitamin therapy alters burn trauma-mediated cardiac NF-kappa B activation and cardiomyocyte cytokine secretion. J. Trauma. 2001;50:397–406. doi: 10.1097/00005373-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 73.La Londe C., Nayak U., Hennigan J., Demling R.H. Excessive liver oxidant stress causes mortality in response to burn injury combined with endotoxin and is prevented with antioxidants. J. Burn Care Rehabil. 1997;18:187–92. doi: 10.1097/00004630-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 74.Norman D. The use of povidone-iodine in superficial partial-thickness burns. Br. J. Nurs. 2003;12 (suppl. 6):S30–6. doi: 10.12968/bjon.2003.12.Sup1.11250. [DOI] [PubMed] [Google Scholar]
- 75.Hadjiiski O.G., Lesseva M.I. Comparison of four drugs for local treatment of burn wounds. Eur. J. Emerg. Med. 1999;6:41–7. [PubMed] [Google Scholar]
- 76.Juhasz I. Experiences with the use of povidone-iodine containing local therapeutics in dermatological surgery and in the treatment of burns: Testing for allergic sensitization in post-surgery patients. Dermatology. 2002;204 (suppl. 1):52–8. doi: 10.1159/000057726. [DOI] [PubMed] [Google Scholar]
- 77.Wormser U., Brodsky B., Green B.S., Arad-yellin R., Nyska A. Protective effect of povidone-iodine ointment against skin lesions induced by sulphur and nitrogen mustards and by non-mustard vesicants. Arch. Toxicol. 1997;71:165–70. doi: 10.1007/s002040050371. [DOI] [PubMed] [Google Scholar]
- 78.Wormser U., Brodsky B., Green B.S., Arad-yellin R., Nyska A. Protective effect of povidone-iodine ointment against skin lesions induced by chemical and thermal stimuli. J. Appl. Toxicol. 2000;20:S183–5. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat677>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 79.Wormser U., Sintortov A., Brodsky B., Amitai Y., Nyska A. Protective effect of topical iodine preparations upon heat-induced and hydrofluoric acid-induced skin lesions. Toxicol. Pathol. 2002;30:552–8. doi: 10.1080/01926230290105767. [DOI] [PubMed] [Google Scholar]
- 80.Wormser U., Brodsky B., Reich R. Topical treatment with povidone-iodine reduces nitrogen mustard-induced skin collagenolytic activity. Arch. Toxicol. 2002;76:119–21. doi: 10.1007/s00204-001-0307-5. [DOI] [PubMed] [Google Scholar]
- 81.Khodr B., Howard J., Watson K., Khalil Z. Effect of short-term and long-term antioxidant therapy on primary and secondary aging neurovascular processes. J. Gerontol. Biol. Sci. Med. Sci. 2003;58:698–708. doi: 10.1093/gerona/58.8.b698. [DOI] [PubMed] [Google Scholar]
- 82.Mallikarjuna R.C., Ghosh A., Raghothama C., Bairy K.L. Does metronidazole reduce lipid peroxidation in burn injuries to promote healing? Burns. 2002;28:427–9. doi: 10.1016/s0305-4179(01)00126-7. [DOI] [PubMed] [Google Scholar]
- 83.Huang Q., Xu W., Ustinova E., Wu M., Childs E., Hunter F., Yuan S. Myosin light chain kinase-dependent microvascular hyperpermeability in thermal injury. Shock. 2003;20:363–8. doi: 10.1097/01.shk.0000079425.0000.db. [DOI] [PubMed] [Google Scholar]
- 84.Youn Y.K., La Londe C., Demling R. Oxidants and the pathophysiology of burn and smoke inhalation injury. Free Rad. Biol. Med. 12:409. doi: 10.1016/0891-5849(92)90090-4. [DOI] [PubMed] [Google Scholar]
- 85.Tanaka H., Matsuda T., Miyagantani Y., Yukioka T., Matsuda H., Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: A randomized, prospective study. Arch. Surg. 2000;135:326–31. doi: 10.1001/archsurg.135.3.326. [DOI] [PubMed] [Google Scholar]
- 86.Tanaka H., Lund T., Wiig H., Reed R.K., Yukioka T., Matsuda H., Shimazaki S. High-dose vitamin C counteracts the negative interstitial fluid hydrostatic pressure and early edema generation in thermally injured rats. Burns. 1999;25:569–74. doi: 10.1016/s0305-4179(99)00073-x. [DOI] [PubMed] [Google Scholar]
- 87.Matsuda T., Tanaka H., Reyes H.M., Richter H.M., III, Hanumadass M.M., Shimazaki S., Matsuda H., Nyhus L.M. Antioxidant therapy using high-dose vitamin C: Reduction of post-burn resuscitation fluid volume requirements. World J. Surg. 1995;19:287–91. doi: 10.1007/BF00308640. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka H., Matsuda H., Shimazaki S., Hanumadass M., Matsuda T. Reduced resuscitation fluid volume for second-degree burns with delayed initiation of ascorbic acid therapy. Arch. Surg. 1997;132:158–61. doi: 10.1001/archsurg.1997.01430260056011. [DOI] [PubMed] [Google Scholar]
- 89.Matsuda T., Tanaka H., Williams S., Hanumadass M., Abcarian H., Reyes H. Reduced fluid volume requirement for resuscitation of third-degree burns with high-dose vitamin C. J. Burn Care Rehabil. 1991;12:525–32. doi: 10.1097/00004630-199111000-00007. [DOI] [PubMed] [Google Scholar]