Summary
A major kerosene explosion disaster occurred in oil-producing Nigeria in October 2001. One hundred and twenty-five burn patients were treated at the Lagos State University Teaching Hospital in a 25-day period of 12/10/01 to 6/11/01. All but two of the patients sustained fire/flame burns resulting from hurricane lantern and cooking stove explosions in home or enclosed environments. In a scene reminiscent of petrol bomb explosions, most burns were extensive, covering the face, chest, and abdomen. Burns were relatively deep because the clothing was usually perfused with the splashed fuel. Severity was greater in females than males, as they were more in contact with lamps and cooking stoves in the household. Almost 50% of the patients required hospitalization upwards of 3 weeks.
Keywords: adulterated, kerosene, burn, disaster, nigeria
Abstract
En octobre 2001 il s’est vérifié un grave désastre causé par une explosion de kérosène en Nigeria, pays producteur de pétrole. Cent vingt-cinq patients ont été traités auprès du Lagos State Teachíng Hospital pendant une période de 25 jours (12/10/01 - 6/11/01). Tous les patients sauf deux ont subi des brûlures par feu ou par flammes provoquées par l’explosion d’une lampe-tempête ou d’un poêle de cuisine dans un environnement domestique ou clos. Dans un scénario qui rappelait les explosions de cocktails Molotov, la plupart des brûlures étaient étendues et couvraient le visage, la cage thoracique et l’abdomen. Les brûlures étaient relativement profondes parce que les vêtements, dans la plupart des cas, étaient trempés du kérosène versé. La sévérité des brûlures était majeure chez les patients du sexe féminin parce que les femmes étaient en contact plus immédiat avec les lampes et les instruments de cuisine. Presque 50% des patients ont nécessité une hospitalisation d’au moins trois semaines.
Introduction
Production of petrol or gasoline goes through a series of fractionating processes, from an initial crude form to several products, one of which is kerosene, a highly hazardous product. Nigeria, an OPEC member, is one of the world’s largest producers of petrol, with a poor population of 120 million that depends largely on kerosene as a substitute for an erratic electric power supply for hurricane lamp illumination and stoves for cooking. Consequently, burns and fires are common in the country. This latest event led to an urgent creation of a Burns and Emergency Response Unit to handle the disaster and establish a state of preparedness for other future disasters.*
Materials and methods
All the patients involved in the kerosene burn disaster treated at the Lagos State University Teaching Hospital were studied. Parameters considered were gender, age, localization, extent and depth of the burn, the need for hospitalization, and average healing time and mortality.
All patients had a standard therapeutic regime based on the Muir & Barclay fluid resuscitation formula, which was modified according to the clinical response of each patient; all had prophylactic antibiotics. A topical antimicrobial agent, silver sulphadiazine, was applied daily after a Hibitane bath. Nutritional support was by the enteral route and was based on the patient’s caloric requirements supplemented by milk shakes and Casilan feed. Burn wound sepsis was minimal and was managed by change to the prophylactic antibiotic needed.
Results
A total number of 123 patients, 71 females and 52 males, were attended to at the Lagos State University Teaching Hospital over a 25-day period (12/10/01-6/11/01). The average age of the patients was 25 years, and the percentage mean total body surface area (TBSA) of burn 47%, with a third-degree component of 37%.
Twelve of the 123 patients died, nine of whom were female and three male. Five of these patients had burns above 60% TBSA, while five others were referred in a septic state and succumbed to multiple organ failure; one patient had 80% burns in association with CRF; another patient, 70 years old with CVA, died of 20% burns. The last patient sustained 40% burns in a state of pregnancy.
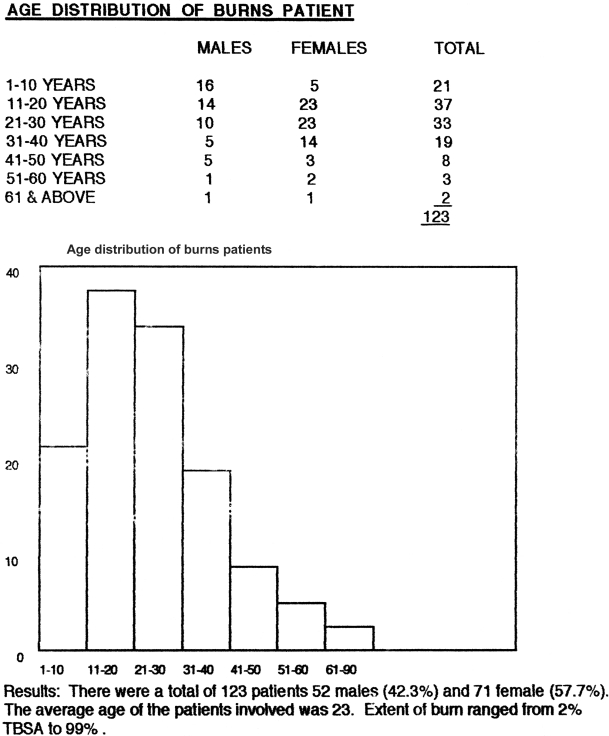
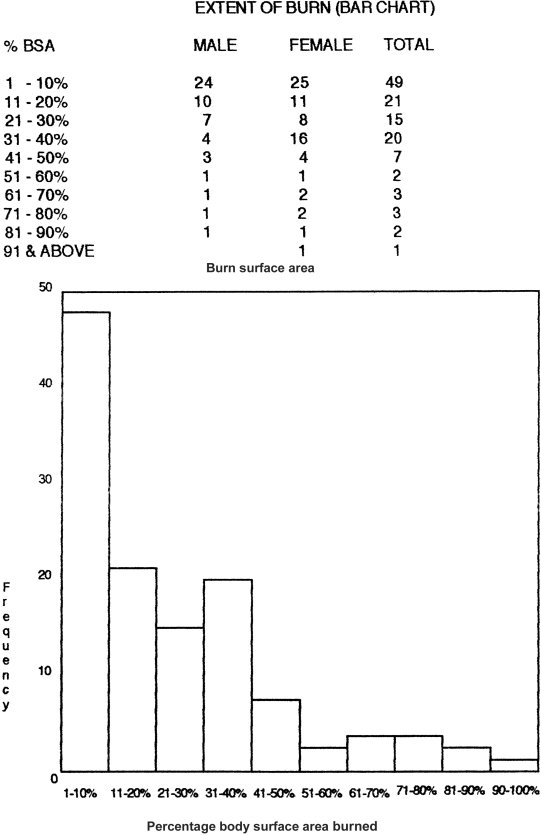
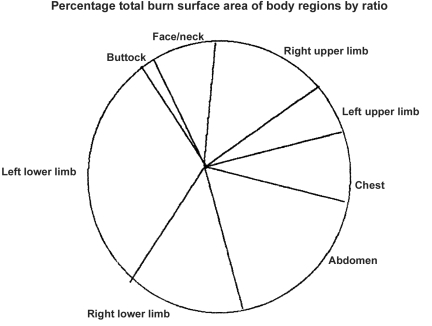
There were more females than males in a ratio of 1.3 to 1. The accidents were all domestic; the commonest sites were the left upper limb (usually used by right-handed people to carry a lamp) and the right upper limb (used to ignite the lamp); the abdomen suffered the greatest amount of deep burns and was next in percentage to the left upper limb, as the patient usual squatted on the floor to light the hurricane lamp, explaining the chest and facial burns (Figs. 1 , 2 ).
Fig. 1. Age distribution of burn patients.
Fig. 2. Extent of burn (bar chart).
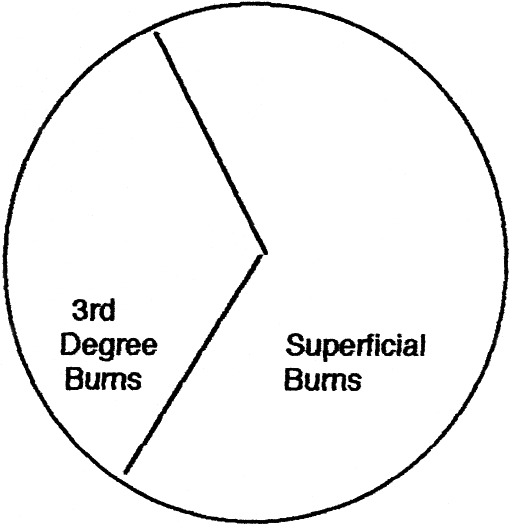
Based on the data of the patients admitted, third-degree burns constituted 37% of the total burn surface areas affected ( Fig. 3 ).
Fig. 3. Depth of burns.
Sixty-nine patients, i.e 56%, required hospitalization; 54 (44%) were treated and discharged the same day or referred to private hospitals. Of the admitted patients five developed Staphylococcus infection sensitive to erythro-mycin in spite of being under the prophylactic cover of cephalosporins. No Pseudomonas infection was cultured nor was any gross burn wound sepsis noticed in any of the patients on admission. One patient developed within 14 days a lesion simulating keloid on the back and buttock, and the rapid exuberant growth of the mass around the buttock a week after with necrosis on the surface presently indicated a fungal infection.
The male-to-female ratio was close in minor and very extensive burns but a wide disparity was noted in the 31-40% TBSA category. Mostly women were affected, in the child-rearing age and handling most of the domestic chores in this country (Figs. 4 , 5 ).
Fig. 4. Ratio of burn patient admissions.
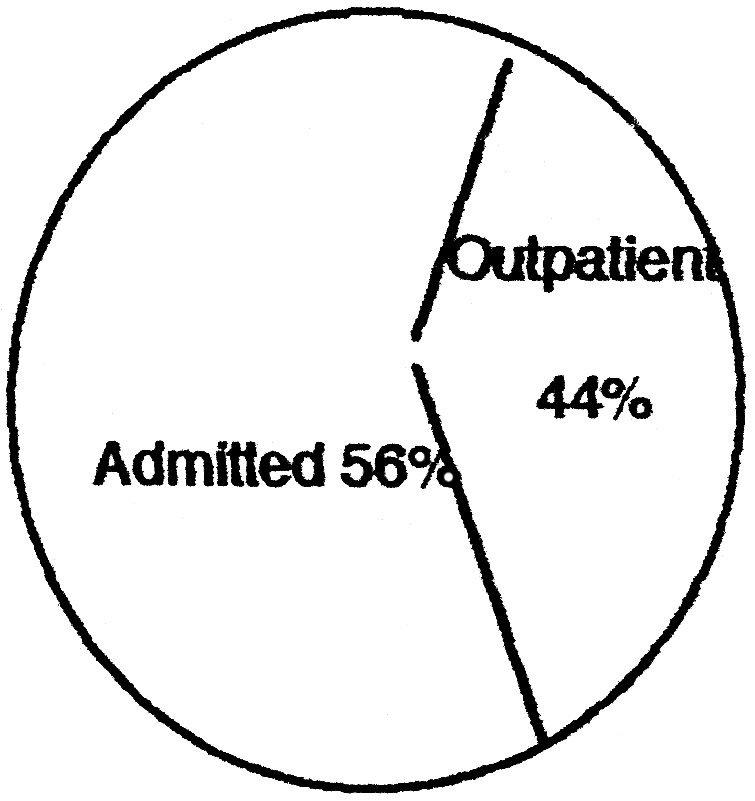
Fig. 5. Distribution of BSA within the body sites.
A total number of 69 out of the 123 required admission, i.e 56%; 44% were treated on an out-patient basis. Of the 69 that required admission 12 died and seven were treated and discharged within three weeks, leaving 50 patients on admission with various degrees of burns. There were 37 admissions to the burns unit out of the total number of 69 that required admission, i.e. 32%, corresponding to the more severely burned patients.
Discussion
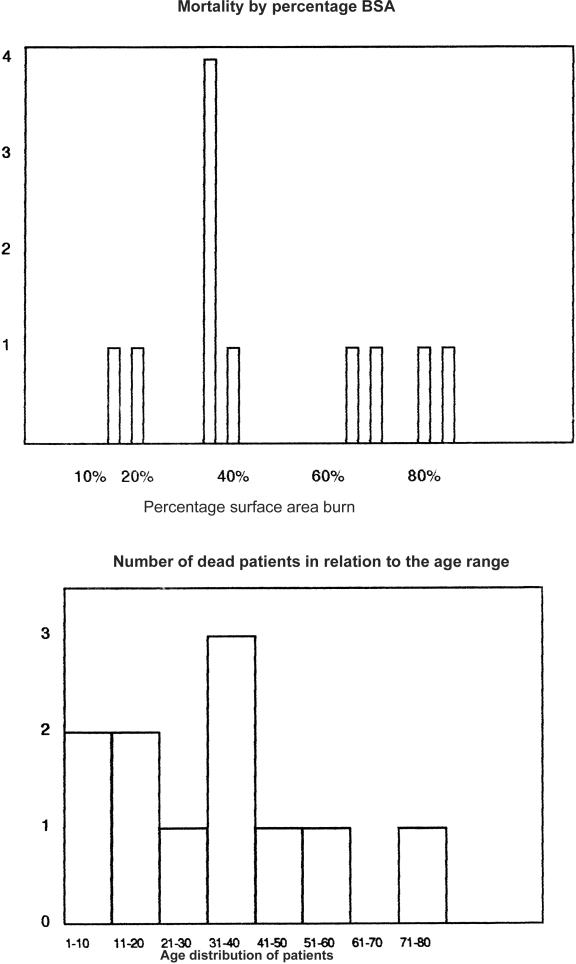
The burn patient is at a serious risk of invasion of tissue by micro-organisms due to loss of the local skin barrier in combination with defects in systemic host defences. Four of our patients succumbed within the first few days of admission owing to sepsis from referral hospitals. A defect in cell mediated immunity occurs following thermal injury due to circulating humoral suppressor factors, 1, 2decrease in the number of T helper cells, 3and an increase in the number of suppressor T cells. 4, 5The death of patients with burns of 60% and above that were admitted primarily into our unit could be explained as mortality increasing with the size of the burn; and for a given size, prognosis worsened with advancing years after early adulthood. 8, 9Since 1949, predictions of mortality have been made from the percentage BSA and patient age. 10In our results, more patients in their teens survived extensive burn injury than patients above 30 years of age having the same extent of burn. This confirms findings in other studies in which the overall mortality of children under age 15 is less than that of adults having the same BSA. 11, 12
Fig. 6. Mortality by percentage BSA.
Comparing patients within the same age range and BSA, survival was not affected by the sex of the patient as in other previous studies. 13, 14, 15, 16
Our mortality figure of approximately 10% compares favourably with reported mortality figures in other burn units 17in disaster situations.
Transfer from the emergency unit to the burn unit was resisted in many cases by patients because of the anxiety of being separated from their relations and the foreknowledge that only severe patients some of whom died were managed in the unit.
This limited movement of patients into the burn unit to only very severe injuries (usually of 40% BSA and above).
This is a cultural element that needs to be taken into consideration in international emergency missions.
References
- 1.Constantian M.B. Associated sepsis with an immunosuppressive polypeptide in the serum of burn patients. Ann. Surg. 1978;188:200–9. doi: 10.1097/00000658-197808000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ninneman J.L., Condie T., Davies S.E., Crockett R.A. Isolation of immunosupressive serum component following thermal injury. J. Trauma. 1982;22:837–44. doi: 10.1097/00005373-198210000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Ninneman J.L., Stockland A.E. Participation of prostaglandin in immunosuppression following thermal injury. J. Trauma. 1984;24:201–7. doi: 10.1097/00005373-198403000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Antonacci A.G., Reaves L.E., Calvaho S.E, et al. Flow cytometric analysis of lymphocyte subpopulations after thermal injury in human beings. Surg. Gynaecol. Obstet. 1984;159:1–8. [PubMed] [Google Scholar]
- 5.Miller C.L., Baker C.C. Changes in lymphocytic activity after thermal injury: The role of suppressor cells. J. Clin. Invest. 1979;63:202–10. doi: 10.1172/JCI109290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munster A.M. Post-traumatic immunosuppression is due to activation of suppressor cells. Lancet. 1976:1329. doi: 10.1016/s0140-6736(76)92658-1. [DOI] [PubMed] [Google Scholar]
- 7.McIrvine A.J., O’Mahony J.B., Saporoschetz I., Mannic J.A. Use of monoclonal antibodies and functional assays to define the role of suppressor cells. Ann. Surg. 1982:196–304. doi: 10.1097/00000658-198209000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moyer C.A. An assessment of the therapy of burns: a clinical study. Ann. Surg. 1953;137:628–38. doi: 10.1097/00000658-195305000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes B.A. Mortality of burns at the Massachusetts General Hospital, 1939-1954. Ann. Surg. 1957;145:210–22. doi: 10.1097/00000658-195702000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bull J.P., Squire J.R. A study of mortality in a burn unit. Standard for the evaluation of alternative methods of treatment. Ann. Surg. 1953;137:628–38. doi: 10.1097/00000658-194908000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bull J.P. Revised analysis of mortality due to burns. Lancet. 1971;2:1133–34. doi: 10.1016/s0140-6736(71)91286-4. [DOI] [PubMed] [Google Scholar]
- 12.Currer P.W., Luterman A., Braum D.W., Shires G.T. Burn injury analysis of survival and hospitalization time for 937 patients. Ann. Surg. 1980;192:472–8. doi: 10.1097/00000658-198010000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritternburg M.S., Maddox R.N., Schmidt F.H., et al. Probit analysis of burn mortality in 1831 consecutive burns treated at a burns unit. Ann. Surg. 1966;164:123–38. doi: 10.1097/00000658-196607000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiths R.W., Cross N.L., Laing J.E. A low-volume burn resuscitation regimen: Assessment of performance by probit analysis. Br. J. Surg. 1981;68:225–8. doi: 10.1002/bjs.1800680403. [DOI] [PubMed] [Google Scholar]
- 15.Zwack B.E., Azen S.P., Imbus S.H., Chang Y.C. Multifactorial probit analysis of mortality in burned patients. Ann. Surg. 1979;189:1–5. doi: 10.1097/00000658-197901000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry C.C., Wachtel T.L., Frank H.A. An analysis of factors which predict mortality in hospitalized burn patients. Burns. 1982;9:38–45. doi: 10.1016/0305-4179(82)90134-6. [DOI] [PubMed] [Google Scholar]
- 17.Barnes B.A., Constable J.D., Burke J.F. Mortality of burns at the Massachusetts General Hospital 1955-1969. Transactions of the 3rd International Congress on Research in Burns. In: Matter P., Barclay J.L., editors. Research in Burns. Burns Hans Huber; 1971. pp. 430–3. [Google Scholar]
- 18.Gunn S.W.A., Masellis M. The World Health Organization Collaborating Centre for prevention and treatment of burns and fire disasters: The Mediterranean Council for Burns and Fire Disasters. Annals Burns and Fire Disasters. 1998;11:3–6. [Google Scholar]