Summary
Pseudomonas aeruginosa and Staphylococcus aureus remain the most important pathogens and are frequently the cause of burn wound infections in our centre. This is a particular problem in high-risk and long-stay patients and can lead to epidemics of infection in hospital settings. This study analysed P. aeruginosa and S. aureus infections in Tohid Burn Centre, Iran, in order to estimate their frequency and antibiotic susceptibilities. Out of 6704 strains examined, 4904 and 688 were found respectively to be P. aeruginosa and S. aureus in the period March 1995-September 1999, in burn patients hospitalized in this burn centre. Antimicrobial susceptibility was determined by the disk diffusion method outlined by the National Committee for Clinical Laboratory Standards. The overall frequencies of P. aeruginosa and S. aureus were respectively 73.1% and 10.3%; the remaining 16.6% consisted of other organisms. The frequency of P. aeruginosa resistance to cipro?oxacin, amikacin, and gentamicin was over 85%. The rate of S. aureus resistance to cloxacillin and cephalexin was 90%. P. aeruginosa and S. aureus were thus the commonest organisms in this centre. High frequency rates of resistance to these micro-organisms were found in this study. It is necessary to limit the use of antimicrobial agents in our epidemiological setting. In 2000 the Burn Centre was closed.
Keywords: BURN, WOUND, INFECTIONS, ANTIMICROBIAL, RESISTANCE, TEHRAN, IRAN, INCREASING, PROBLEM
Abstract
Dans le centre des brûlés des Auteurs, Pseudomonas aeruginosa et Staphylococcus aureus continuent à être les pathogènes les plus importants et fréquemment ils causent des infections chez les patients brûlés hospitalisés, ce qui constitue un problème particulier pour les patients à haut risque destinés à une hospitalisation prolongée, pouvant mener à des épidémies d'infection dans un cadre hospitalier. Les Auteurs ont analysé les infections de P. aeruginosa et de S. aureus dans le Centre des Brûlés de Tohid, Iran, dans le but d'évaluer leur fréquence et leurs susceptibilités antibiotiques. Pendant la période mars 1995-septembre 1999, sur les 6704 souches examinées, 4904 et 688 se sont démontrées être respectivement P. aeruginosa et S. aureus chez les patients brûlés hospitalisés dans ce centre. La susceptibilité antimicrobienne a été déterminée utilisant la méthode de la diffusion de disque décrite par le National Committee for Clinical Laboratory Standard. Les fréquences globales de P. aeruginosa et de S. aureus étaient respectivement 73,1% et 10,3%; les autres organismes (16.6%) étaient d'autres types. La fréquence de la résistance de P. aeruginosa à la ciprofloxacine, à l'amikacine et à la gentamicin était supérieure à 85%. Le taux de résistance de S. aureus à la cloxacilline et à la céphalexine était 90%. P. aeruginosa et S. aureus étaient donc les organismes les plus communs dans le centre. Les Auteurs ont trouvé dans cette étude des taux élevés de résistance à ces microorganismes, et il faudra limiter l'emploi des agents antimicrobiens dans ce cadre épidémiologique.
Introduction
Burn injuries by fire and hot liquids and contact with hot surfaces have been recognized as a significant and major public health problem in economically developing countries like Iran. Unfortunately, most of the burn victims in Iran are children and women.1-7
Large open wound areas containing necrotic tissue make burn patients more susceptible to infection. In addition, a general state of immunosuppression is caused by the impaired functioning of neutrophils and the cellular and humoral immune system.8-11 In these conditions, micro-organisms can easily multiply and colonize wounds to high densities. Immunologically compromised patients are also obliged to stay in high-risk intensive care units for prolonged periods of time, during which they may be submitted to endotracheal intubation and/or catheterization of the blood vessels and bladder; also, in these units, both the air and environmental surfaces are heavily contaminated. That is why burn patients are high-risk groups for infection.12,13
Pseudomonas aeruginosa - particularly in economically developing countries - is one of the most important and most common causes of serious infection in burn patients. 13-21 When infection caused by P. aeruginosa occurs in burn patients, treatment becomes very difficult and the mortality rate among infected patients is likely to reach up to 40-50%.6,7,14-16 Treatment of these infections is frequently complicated by antibiotic resistance, a problem that has been increasing over time.14-16 The emergence of multi-drug resistant strains in burns units, particularly in economically underdeveloped and developing countries like Iran, is an increasing infection control problem.14-16
The gains of established infection control measures are now being felt in the economically developed countries with purpose-built burns units.17,22 However, in economically developing countries, the establishment of such units is hindered by poverty, ignorance, poor management, and lack of personnel.17,23
This study of the bacteriological profile and antibiotic sensitivity patterns of isolates from a main referral burn centre in Tehran was carried out in order to highlight some of the problems faced by clinicians and to document burn wound infection patterns in this environment.
Materials and methods
This study was carried out during the period March 1995-September 1999 in Tohid Burn Centre, which is affiliated to the Iran University of Medical Sciences in Tehran, Iran. Tohid Burn Centre is one of two main referral burn centres in Tehran (the other is Motahari Burn Centre). In the present study, a total of 7132 samples of swabs and blood were processed from about 3864 admitted patients. Tohid Burn Center is located in the western part of Tehran and serves a population of 5.5 million people, most of them from the low and middle socio-economic classes. The inclusion criteria (admission policy) in our centre are:
children below 2 years
children with total body surface area (TBSA) ≥ 10%
adults with TBSA ≥ 15%
localized deep burns of 2% TBSA or greater
facial burns
burns of hands, feet, or perineum
suspected inhalation injury
chemical or electrical burns
associated fractures or chronic illness
suspected criminal or suicidal burns
The culture swabs from the burn wounds were taken on admission in all patients. Some patients were swabbed on day 3 and 7, depending on the clinical judgement of infection. Infection and septicaemia were suspected when a patient showed signs of disorientation, hyperpyrexia or hypothermia, circulatory embarrassment, petechial haemorrhages, black and dark discoloration in a previously clean appearing burn wound, early and rapid eschar separation, bleeding into the subcutaneous tissues, increasing oedema in surrounding areas, or leucocytosis in WBC counts.
For the isolation of P. aeruginosa, sheep blood agar and eosin methylene blue were used. Subsequently, growth at 42 ∞C in brain heart infusion, the oxidative test, and the oxidative-fermentation test for carbohydrate utilization were used for identification of P. aeruginosa.24 Also, antimicrobial susceptibility was determined by the disk diffusion method of Bauer-Kirby recommended by the American Society of Microbiology.25
All the burn patients received first aid and dressing with silver sulphadiazine 1% in the reception room of the centre. Those who needed admission had an intravenous line established and secured respiration. Early excision and grafting is the rule in the centre. Cephalothin and amikacin were administered as prophylactic antibiotics from the first day of admission in patients with ≥ 20% TBSA burns (second and third degree). The wound was inspected daily during dressing changes.
Data were compared using analysis of variance and the c2 test as appropriate. Two-tailed p values < 0.05 were considered significant. All statistical analyses were performed using Epi Info version 6.04 (CDC, USA, and WHO Geneva, Switzerland).26
Results
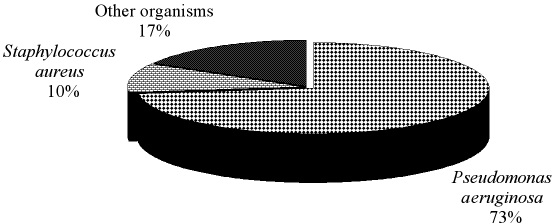
During the 4.5-yr period March 1995-September 1999, 3864 patients were admitted to our burn centre. A total number of 7132 samples were processed. From these, 6704 bacterial strains were isolated. Of the 6704 isolates, P. aeruginosa (4904 strains) accounted for 73.2% (95% CI: 72.1-74.2%) of total isolates (Fig. 1). This was followed by S. aureus (688 strains; 10.3%) (95% CI: 9.5-11%) and other organisms (1112 strains, 16.5%), such as Acinetobacter spp. and Enterobacter spp.).
Fig. 1. Frequency of the isolated bacterial strains.
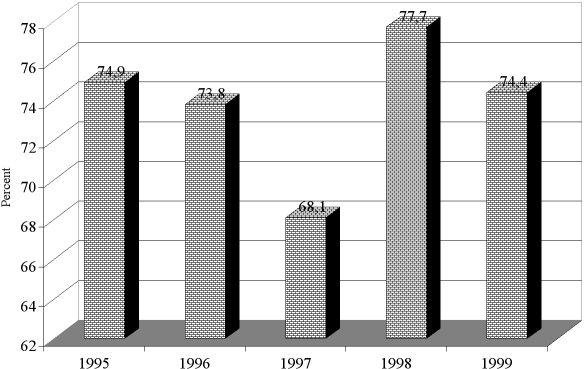
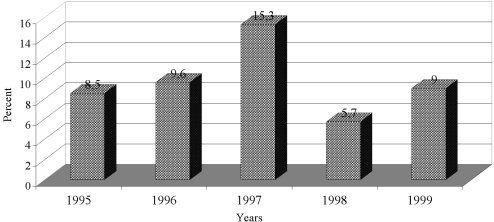
The frequency rate of P. aeruginosa is shown in the different years of study in Fig. 2. The highest and lowest frequency rates of P. aeruginosa were found in 1997 (15.3%) and 1998 (5.7%). There were statistical significant differences between the frequency rates of P. aeruginosa during the period under review (p < 0.000001). Fig. 3 shows the S. aureus frequency rate in the different years of study. The highest and lowest frequency rates of S. aureus were found in 1998 (77.7%) and 1997 (68.1%). There were statistically significant differences between the frequency rates of S. aureus during the period of study (p = 0.000001). There were also significant statistical differences between the frequency rates of P. aeruginosa and S. aureus over the period of study (p < 0.000001).
Fig. 2. Frequency rate of positive Pseudomonas aeruginosa cultures during the period of study.
Fig. 3. Frequency rate of positive Staphylococcus aureus cultures during the period of study.
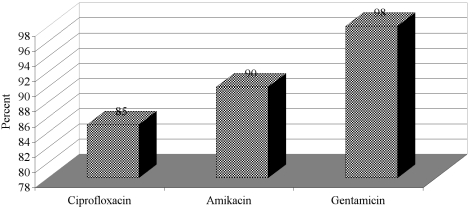
The resistance of the organisms to different antibiotics varied depending on the isolate. The frequency of P. aeruginosa resistant to carbenicillin, co-trimoxazole, ceftizoxime, and tetracycline was over 95% during the period of study. Eighty-five per cent of P. aeruginosa were resistant to ciprofloxacin. Also, P. aeruginosa was resistant to amikacin in 90% of the isolates tested and to gentamicin in 98% (Fig. 4).
Fig. 4. Frequency rate of Pseudomonas aeruginosa resistant to antimicrobial agents.
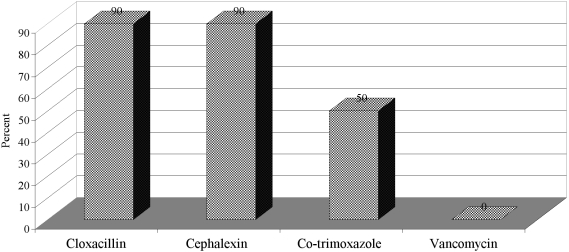
All S. aureus isolates were sensitive to vancomycin. S. aureus was resistant to cloxacillin and cephalexin in 57% and of the isolates and to co-trimoxazole in 50% (Fig. 5).
Fig. 5. Frequency rate of antimicrobials resistant to Staphylococcus aureus.
Discussion
Infection is one of the most serious complications in burn patients, and Pseudomonas, especially P. aeruginosa, is the most important, resistant, and dangerous organism in burn patient infections.14-16 P. aeruginosa thrives on the moist burn wound surface8,27 and is highly pathogenic in thermally injured immunosuppressed patients.8-11 These bacteria usually gain access to burn patients through crosscontamination of burn wounds. Pseudomonas infection is a common complication in burn patients and contributes to their morbidity and mortality.8,28 Despite advances in medical and surgical care, the prognosis remains poor, with a mortality rate of about 80% in such patients.8,29
In this study, P. aeruginosa was found to be the commonest pathogen causing wound infection and bacteraemia in Tohid Burn Centre, which is comparable to earlier studies conducted in the same burn centre.14,15 Over the past 20 years, P. aeruginosa has reportedly been found to be the most prevalent bacteria in burn centres in Tehran.14-16 In a recent study undertaken in Motahari Burn Centre (the other referral burn centre in Tehran), P. aeruginosa was reported to be the most prevalent micro-organism.16 This suggests that P. aeruginosa is the "classic pathogen" in burn wound infections in the referral burn centres in the capital city of Tehran. A similar observation was reported in the referral burn centre in the province of Fars, in southwest Iran.4,5 In many economically developing countries such as Zimbabwe,20 South Korea,19 Jordan,30 Libya,31 Nigeria, 32 India,18,33-35 and Turkey13,36 P. aeruginosa was reported to be commonest bacteria among burn patients. Although P. aeruginosa is not a classic pathogen of burn wound infections in the economically developed countries, a few burn centres in Canada and the USA,37 France,38 and Italy39 have reported P. aeruginosa as an important microorganism in burn units.
The second most common isolate in this study was S. aureus, as in other studies from Iran and other economically developing countries.13-15,17-19,31-35 This contrasts however with some other studies, especially from economically developed countries, which report S. aureus as the most predominant organism in burn infection.22,40-46
The time-related changes in the predominant flora of the burn wound from gram-positive to gram-negative recapitulate the history of burn wound infection.13,47 The predominance of gram-positive bacteria in the early phase switches to gram-negative species 4-10 days after injury. In this study, an inverse relationship between the frequencies of P. aeruginosa and S. aureus was found during the period of study (p < 0.000001). Also, previous studies in Tohid Burn Centre showed that the incidence of S. aureus decreased while that of P. aeruginosa increased during the first week of admission, compared to the day of admission. Similarly, Estahbanati et al.16 reported that in Motahari Burn Centre most burn wounds became infected with P. aeruginosa during the first week post-burn. We therefore strongly recommend that P. aeruginosa be seriously considered as the main nosocomial source of infection in Tehran referral burn centres. In addition, a study from Syria showed that in the first week of hospital stay the main organisms causing infection were S. aureus, E. coli, and other Enterobacters; in the second week Pseudomonas and Candida were the dominant organisms.48
Several reports have documented environmental sources of burn wound infections from P. aeruginosa, and this organism thus continues to be a major cause of burn injury colonization and serious wound infections.13 Moisture is a critical factor in hospital reservoirs of P. aeruginosa, as for example in respiratory equipment, medicines, disinfectants, sinks, mops, food, and vegetables. We therefore believe that the following factors could be considered as possible reasons for the high percentage of P. aeruginosa infection in our centre:
- the centre was not built as a burn centre. However, two decades ago, officials declared that this substandard building should be used as a referral burn centre, because of the high number of burn patients and the limited burn care facilities;
- as several reports have shown that burn injury is a common and increasing problem in Iran, this referral burn centre, with its approximately 80 beds, cannot respond to the high demand. All the wards are usually overcrowded, and infection control measures and isolation polices are inadequate most of the time;
- as the number of patients admitted is higher than the centre's capacity, the isolation of infected patients cannot be guaranteed - this is the key to the transmission of P. aeruginosa (the centre's classic pathogen) from person to person or from environment to person (a patient or a health care worker).
The multi-drug resistance of P. aeruginosa is another problem, along with Pseudomonas infections.15 The pattern of antibiotic sensitivity is a source of serious concern as many of the isolates are resistant to antibiotics available in Iran.15,16 This emerging pattern, highlighted in previous reports from Iran,15,16 appears to be worsening. Pseudomonas, which in our earlier study15 was only 45.2% resistant to ciprofloxacin, 48.9% to amikacin, and 88.5% to gentamicin, showed 85% resistance to ciprofloxacin, 90% to amikacin, and 98% to gentamicin in the present report. In a microbiological study of burn wound infection in the other referral burn centre in Tehran, Motahari Burn Centre, P. aeruginosa resistance to ciprofloxacin, amikacin, and gentamicin was respectively 87.7%, 53.3%, and 90.7%, which shows high antibiotic resistance.16 In this study, the rate of P. aeruginosa resistant to carbenicillin, co-trimoxazole, ceftizoxime, and tetracycline was over 95%. Estahbanati et al.16 reported that more than 90% of P. aeruginosa were resistant to ceftizoxime, carbenicillin, and ceftazidime in Motahari Burn Centre. The resistance rate of P. aeruginosa to the above-mentioned antibiotics in burn centres in Iran has increased dramatically in recent years and seems to be one of the highest in the literature. This problem could have the following explanations:
- the present prophylactic antibiotics policy in Iranian Burn Centres has caused an increasing rate of P. aeruginosa resistance to commonly used antibiotics. In addition, the rate of development of resistance of P. aeruginosa to new antibiotics is much faster than the rate of invention and development of new antibiotics;16,49,50
- there is overuse and abuse of available antibiotics such as gentamicin, amikacin, and ciprofloxacin, which in this centre are used as prophylactic or therapeutic measures;
- given that the Department of Health is in charge of importing and introducing new antibiotics (and new medications in general) into Iran, and given also that it has not yet considered or introduced broadspectrum antimicrobial agents such as imipenem and aztreonam for use in burn centres, these antibiotics are not available. Consequently, in addition to the lack of preventive measurements, broad-spectrum antibiotics that can be helpful in the treatment of burn patients with infections are not available either. It is also necessary to mention that as most burn victims come from the poorer economic classes they are unable to afford such expensive antibiotics on the black market.
The main infection control measures in burn centres in Iran are early referral, early excision and grafting, improved barrier nursing, and regular microbiological analysis of the hospital's environment and staff. However, our data and other study results show that the infection control programmes and antibacterial therapy policies have not yet reduced either the high mortality and burn wound infection rates or resistance to antibiotics.
Conclusions
We would recommend that officials take into account the following guidelines in order to decrease the rate of infection and antibiotic resistance in burn centres in Iran:
- consider absolute isolation seriously in contaminated patients, especially those with over 40% TBSA burns;
- improve barrier nursing, personnel hygiene, including hand washing before and after attending to a patient, and restriction of staff traffic;
- increase the number of intensive care unit beds so to be able to transfer patients at the most appropriate time;
- consider appropriate sterilization for all equipment;
- establish a new and effective antibiotic policy in order to revise and introduce prophylactic and therapeutic antibiotics, including the implementation of broad-spectrum antimicrobial agent choices in burn centres;
- avoid unnecessary use of antibiotics.
Acknowledgments
This work was completed at the Iran University of Medical Sciences, Tehran, Iran, and presented at the Tenth International Congress of Bacteriology and Applied Microbiology, held in Paris, France, in July 2002.
References
- 1.Rastegar Lari A., Alaghehbandan R. Epidemiological study of self-inflicted burns in Tehran, Iran. J. Burn Care Rehabil. 2003;24:15–20. doi: 10.1097/00004630-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Mehdizadeh A., Akbarian A.R., Pahlavan P.S., Tavajjohi S., Rossignol A.M., Alaghehbandan R., Groohi B. Epidemiology of burn injuries during pregnancy in Tehran, Iran. Ann. Burns and Fire Disasters. 2002;15:163–169. [Google Scholar]
- 3.Groohi B., Alaghehbandan R., Rastegar Lari A. Analysis of 1089 burn patients in province of Kurdistan, Iran. Burns. 2002;28:569–574. doi: 10.1016/s0305-4179(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 4.Rastegar Lari A., Panjeshahin M.R., Talei A.R., Rossignol A.M., Alaghehbandan R. Epidemiology of childhood burn injuries in Fars province, Iran. Epidemiology of childhood burn injuries in Fars province, Iran. J. Burn Care Rehabil. 2002;23:39–45. doi: 10.1097/00004630-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Panjeshahin M.R., Rastegar Lari A., Talei A.R., Shamsnia J., Alaghehbandan R. Epidemiology and mortality of burns in the southwest of Iran. Burns. 2001;27:219–226. doi: 10.1016/s0305-4179(00)00106-6. [DOI] [PubMed] [Google Scholar]
- 6.Alaghehbandan R., Rossignol A.M., Rastegar Lari A. Paediatric burn injuries in Tehran, Iran. Burns. 2001;27:115–118. doi: 10.1016/s0305-4179(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 7.Rastegar Lari A., Alaghehbandan R., Nikui R. Epidemiological study of 3341 burns patients during three years in Tehran, Iran. Burns. 2000;26:49–53. doi: 10.1016/s0305-4179(99)00102-3. [DOI] [PubMed] [Google Scholar]
- 8.Gang R.K., Bang R.L., Sanyal S.C., Mokaddas E., Lari A.R. Pseudomonas aeruginosa septicaemia in burns. Burns. 1999;25:611–616. doi: 10.1016/s0305-4179(99)00042-x. [DOI] [PubMed] [Google Scholar]
- 9.Ninneman J.L. Immunological defences against infection: Alterations following thermal injuries. J. Burn Care Rehabil. 1987;3:355–366. [Google Scholar]
- 10.Winkelstein A.U. What are the immunological alterations induced by burn injury? J. Trauma. 1984;24:S72–S83. doi: 10.1097/00005373-198409001-00005. [DOI] [PubMed] [Google Scholar]
- 11.Pruitt B.A. jr, MacManus A.T. The changing epidemiology of infection in burn patients. World J. Surg. 1992;16:57–67. doi: 10.1007/BF02067116. [DOI] [PubMed] [Google Scholar]
- 12.Kluytmans J. Surgical infections including burns. In: Wenzel R.P., editor. Prevention and Control of Nosocomial Infections. 3. Williams & Wilkins; Pennsylvania: 1997. pp. 841–865. [Google Scholar]
- 13.Oncul O., Yksel F., Altunay H., Açikel C., Çeliköz B., Çavuslu S. The evaluation of nosocomial infection during 1-year period in the burn unit of a training hospital in Istanbul, Turkey. Burns. 2002;28:738–744. doi: 10.1016/s0305-4179(02)00106-7. [DOI] [PubMed] [Google Scholar]
- 14.Rastegar Lari A., Alaghehbandan R. Nosocomial infections in an Iranian burn care centre. Burns. 2000;26:737–740. doi: 10.1016/s0305-4179(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 15.Rastegar Lari A., Bahrami Honar H., Alaghehbandan R. Pseudomonas infections in Tohid Burn Centre, Iran. Burns. 1998;24:637–641. doi: 10.1016/s0305-4179(98)00090-4. [DOI] [PubMed] [Google Scholar]
- 16.Karimi Estahbanati H., Pour Kashani P., Ghanaatpisheh F. Frequency of Pseudomonas aeruginosa serotypes in burn wound infections and their resistance to antibiotics. Burns. 2002;28:340–348. doi: 10.1016/s0305-4179(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 17.Ozumba U.C., Jiburum B.C. Bacteriology of burn wounds in Enugu, Nigeria. Burns. 2000;26:178–180. doi: 10.1016/s0305-4179(99)00075-3. [DOI] [PubMed] [Google Scholar]
- 18.Kaushik R., Kumar S., Sharma R., Lal P. Bacteriology of burn wounds - the first three years in a new burn unit at the Medical College Chandigarh. Burns. 2001;27:595–597. doi: 10.1016/s0305-4179(01)00023-7. [DOI] [PubMed] [Google Scholar]
- 19.Song W., Lee K.M., Kang H.J., Shin D.H., Kim D.K. Microbiologic aspects of predominant bacteria isolated from burn patients in Korea. Burns. 2001;27:136–139. doi: 10.1016/s0305-4179(00)00086-3. [DOI] [PubMed] [Google Scholar]
- 20.Igumbor E., Gwanzura L., Chirara M., Obi C., Muza D., Chihara M. Antibiotic sensitivity and plasmid profiles of Pseudomonas aeruginosa. Cent. Afr. J. Med. 2001;47:84–84. [PubMed] [Google Scholar]
- 21.Xue B., Liu X., Tang M. The change in bacterial flora and antibiotic resistance of bacteria of burn patients in our hospital during 1986-1996. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi. 1999;15:309–312. [PubMed] [Google Scholar]
- 22.Nakhla L.S., Sanden R. Microbiological aspects of burns at Mount Vernon Hospital, UK. Burns. 1991;17:309–312. doi: 10.1016/0305-4179(91)90046-j. [DOI] [PubMed] [Google Scholar]
- 23.Sowemimo G.O. Burn care in Africa: Reducing the misery index. The 1993 Everett Idris Evans Memorial Lecture. J. Burn Care Rehabil. 1993;14:589–594. doi: 10.1097/00004630-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Gilardi G.L. Pseudomonas and related genera. In: Balows A., Hausler W.J., Herrmann K.L., Isenberg H.D., Shadomy H.J., editors. Manual of Clinical Microbiology. 5. American Society for Microbiology; Washington DC: 1991. pp. 429–429. [Google Scholar]
- 25.Barry A.L., Thornsberry C. Susceptibility tests: Diffusion test procedures. In: Balows A., Hausler W.J., Herrmann K.L., Isenberg H.D., Shadomy H.J., editors. Manual of Clinical Microbiology. 5. American Society for Microbiology; Washington DC: 1991. pp. 1120–1120. [Google Scholar]
- 26.Epi Info Version 6.04. The Division of Surveillance and Epidemiology, Epidemiology Program Office, Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA in collaboration with the Global Programme on AIDS, World Health Organization (WHO), Geneva, Switzerland. [Google Scholar]
- 27.Tredget E.E., Shankowsky H.A., Mark Joffe A. et al. Epidemiology of infection with Pseudomonas aeruginosa in burn patients: The role of hydrotherapy. Clin. Infect. Dis. 1992;15:941–949. doi: 10.1093/clind/15.6.941. [DOI] [PubMed] [Google Scholar]
- 28.MacManus A.T., Mason A.D. jr, MacManus W.F., Pruitt B.A. jr. Twenty-five years review of Pseudomonas aeruginosa bacteraemia in a burn centre. Eur. J. Clin. Microbiol. 1985;4:219–223. doi: 10.1007/BF02013601. [DOI] [PubMed] [Google Scholar]
- 29.Richard P., Le Floch R., Chamoux C. et al. Pseudomonas aeruginosa outbreak in a burn unit: Role of antimicrobials in the emergence of multiple resistant strains. J. Infect. Dis. 1994;170:377–383. doi: 10.1093/infdis/170.2.377. [DOI] [PubMed] [Google Scholar]
- 30.Al-Akayleh A.T. Invasive burn wound infection. Ann. Burns and Fire Disasters. 1999;12:204–206. [Google Scholar]
- 31.Husain M.T., Karim Q.N., Tajuri S. Analysis of infection in a burn ward. Burns. 1989;15:299–302. doi: 10.1016/0305-4179(89)90006-5. [DOI] [PubMed] [Google Scholar]
- 32.Atoyebi O.A., Sowemimo G.O.A., Odugbemi T. Bacterial flora of burn wounds in Lagos, Nigeria: A prospective study. Burns. 1992;18:448–451. doi: 10.1016/0305-4179(92)90175-t. [DOI] [PubMed] [Google Scholar]
- 33.Pandit D.V., Gore M.A., Saileshwar N., Deodhar L.P. Laboratory data from the surveillance of a burns ward for the detection of hospital infection. Burns. 1993;19:52–55. doi: 10.1016/0305-4179(93)90101-d. [DOI] [PubMed] [Google Scholar]
- 34.Sharma S., Hans C. Bacterial infections in burns patients: A three years study at RML hospital, Delhi. J. Commun. Dis. 1996;28:101–106. [PubMed] [Google Scholar]
- 35.Revathi G., Puri J., Jain B.K. Bacteriology of burns. Burns. 1998;24:347–349. doi: 10.1016/s0305-4179(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 36.Arslan E., Dalay C., Yavuz M., Göcenler L., Acartürk S. Gramnegative bacterial surveillance in burn patients. Ann. Burns and Fire Disasters. 1999;12:81–83. [Google Scholar]
- 37.Shankowsky H.A., Callioux L.S., Tredget E.E. North America survey of hydrotherapy in modern burn care. J. Burn Care Rehabil. 1994;15:143–146. doi: 10.1097/00004630-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Cremer R., Ainaud P., Le Bever H., Faber N., Carsin H. Nosocomial infections in a burns unit. Results of a prospective study over a year. Ann. Fr. Anesth. Reanim. 1996;15:599–607. doi: 10.1016/0750-7658(96)82125-3. [DOI] [PubMed] [Google Scholar]
- 39.Calvario A., Di Lonardo A., Larocca A.M.V., Parisi D., Montagna M.T., Ressa M., Silvestri A., Maggio G. Microbiological monitoring of severely burned patients admitted to the burns centre in Bari (Italy) in the period 1989-92. Ann. Burns and Fire Disasters. 1994;7:73–79. [Google Scholar]
- 40.Donati L., Scammazo F., Gervasoni M., Magliano A., Stankov B., Fraschini F. Infection and antibiotic therapy in 4000 burned patients treated in Milan, Italy, between 1976 and 1988. Burns. 1993;19:345–348. doi: 10.1016/0305-4179(93)90125-r. [DOI] [PubMed] [Google Scholar]
- 41.Taylor G.D., Kibsey P., Kirkland T., Burroughs E., Tredget E. Predominance of staphylococcal organisms in infections occurring in a burns intensive care unit. Burns. 1991;18:332–335. doi: 10.1016/0305-4179(92)90158-q. [DOI] [PubMed] [Google Scholar]
- 42.Pegg S.P. Multiple resistant Staphylococcus aureus. Ann. Acad. Med. Singapore. 1992;21:664–666. [PubMed] [Google Scholar]
- 43.Lesseva M.I., Hadjiiski O.G. Staphylococcal infections in the Sofia Burn Centre, Bulgaria. Burns. 1996;22:279–282. doi: 10.1016/0305-4179(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 44.Shannon T., Edgar P., Villarreal C., Herndon D.N., Philips L.G., Heggers J.P. Much ado about nothing: Methicillin-resistant Staphylococcus aureus. J. Burn Care Rehabil. 1997;18:326–331. doi: 10.1097/00004630-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Appelgren P., Bjornhagen V., Bragderyd K., Jonsson C.E., Ransjo U. A prospective study of infections in burn patients. Burns. 2002;28:39–46. doi: 10.1016/s0305-4179(01)00070-5. [DOI] [PubMed] [Google Scholar]
- 46.Camilleri I.G., Pedler S.J., Murphy O., Reid C.A. Cross infection on a combined paediatric plastic surgery and burns unit: A clinical and microbiological audit. Burns. 1999;25:655–658. doi: 10.1016/s0305-4179(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 47.Pruitt B.A. jr, MacManus A.T., Kim S.H., Goodwin C.W. Burn wound infections: Current status. World J. Surg. 1998;22:135–145. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- 48.Dayoub A., Zeidan F., Radidy S. Infection in burns: Experience of a teaching hospital in Syria. Ann. Burns and Fire Disasters. 1995;8:17–19. [Google Scholar]
- 49.Holder I.A., Volpel K., Ronald G., Paranchych W. Studies on multiple Pseudomonas aeruginosa isolates from individual burn patients by RFLP, O antigen serotyping and antibiogram analysis. Burns. 1995;21:441–444. doi: 10.1016/0305-4179(95)00012-z. [DOI] [PubMed] [Google Scholar]
- 50.Xu W.S. Effect of the use of human hyperimmune plasma against Pseudomonas protein for protection against Pseudomonas sepsis in burn mice. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi. 1993;9:52–55. [PubMed] [Google Scholar]