Summary
The aim of this paper is describe the application of desamidated collagen as a biomaterial in order to evaluate the utility of collagen, particularly as a drug delivery device, as a haemostat, and as a wound cover. We also evaluated the utility of vitamin A, vitamin C, 50% glucose, and gentamicin locally applied onto the open wound. Any biomaterial should ensure non-toxicity to the biological environment where it is applied. We point out the utility of vitamin A, vitamin C, 50% glucose, and gentamicin locally applied onto the open wound with a biological covering. Collagen sheet is becoming evident in our ongoing studies. We are convinced that collagen sheets are very useful in first- and second-degree burns. The cost factor comes down and the pain associated with dressing can be avoided. Especially in children it is very useful since the trauma of dressing can be avoided.
Keywords: collagen, sheet, wound, healing
Abstract
Dans cet article les Auteurs se proposent de décrire l’application du collagène désamidé comme biomatériau pour évaluer l’utilité du collagène, particulièrement comme mécanisme de livraison des médicaments, comme hémostatique et comme couverture des lésions. Ils ont en outre évalué l’utilité de la vitamine A, la vitamine C, du glucose à 50% et de la gentamicine appliquée localement sur les lésions ouvertes. Tous les biomatériaux devraient garantir la non-toxicité de l’environnement biologique où ils sont appliqués. Les Auteurs soulignent l’utilité de la vitamine A, de la vitamine C, du glucose à 50% et de la gentamicine appliquée localement sur les lésions ouvertes avec une couverture biologique. La feuille de collagène est toujours plus évidente dans leurs études actuelles, et ils sont convaincus que la feuille de collagène est très utile dans les brûlures de premier et deuxième degré. Le facteur économique est moins pesant et la douleur associée aux pansements peut être évitée. Cette méthode est particulièrement indiquée pour les enfants puisqu’elle évite l’expérience douleureuse des pansements.
Introduction
There are occasions in surgery that require use of a temporary cover for raw wounds. These include skin loss secondary to burns, trauma, amputation, chronic ulcer, leprosy, and skin graft sites. The body needs its own regeneration time, while complications consequent to loss of skin cover can always occur.
The orderly ingrowth of epithelium over denuded areas needs a layer of collagen sheet to act as the scaffold on which to grow and arrange itself. Denuded areas are unable to provide this effectively, leading to formation of extensive scars and even keloids.
It is for these purposes that denuded areas need a temporary cover until such time that the body is able to manufacture a cover of its own. 1
Desamidation of bovine collagen is a novel idea for preparing chemically modified collagen, which is largely unutilized. Desamidation of these wastes offers an alternative source of solubilized collagen with a much higher yield. 2
Our aim is to apply desamidated collagen as a biomaterial to evaluate the utility of collagen, particularly as a drug delivery device, as a haemostat, and as a wound cover. We evaluated the utility of vitamin A, vitamin C, 50% glucose, and gentamiicin locally applied onto the open wound. Any biomaterial should ensure non-toxicity to the biological environment where it is applied.
Case study
The patient, S.S., 80 years old, was admitted for hospitalization owing to pain in the toes of both feet, the pain being specifically acute in the second toe of the left foot and the middle toe of the right foot. The patient did not state any vascular system discomforts, in terms of circulation malfunctioning in the lower extremities. The pains started after chiropodist treatment, i.e. after cutting and treatment of the nails on the patient’s toes. The patient was examined by a surgeon in another hospital and subsequently admitted into our healthcare facility.
On examination, we ascertained:
insufficient circulation in the second toe of the left foot and the middle toe of the right foot
distal necrosis and infection on the tips of the toes
Therapy and observation
Surgical treatment of the wound
Debridement was performed up to the healthy tissue together with resection of the tips of the distal phalanges on the affected toes using local anaesthesia.
The wound was left open.
The following were locally applied onto the open wound: vitamin A (1 ml), vitamin C (5 ml), 50% glucose (10 ml), and gentamicin, subsequently followed by a collagen sheet-biological cover (layer).
The rest of the vitamin A, vitamin C, 50% glucose, and gentamicin was applied over the biological cover.
The wound was then covered by five-layer gauze.
The patient was discharged from hospital and carried on his normal working activities, but with some restrictions in terms of walking, with the aim of preventing mechanical sliding of the collagen cover on the wound.
Wound bandaging in the first month was performed every second day with the addition of the specified medicaments.
After a month, wound bandaging continued every fourth day.
Collagen cover sliding occurred only in the case of adhesive surface disturbance between the wound and a collagen layer itself (collagen layer sterility was disturbed by contact with the greater surface of the skin layer).
Adhesive surface disturbance was initiated by the occurrence of exudate or mechanical sliding in the process of the patient moving.
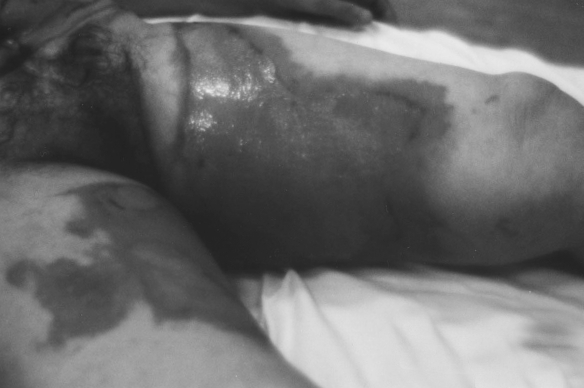
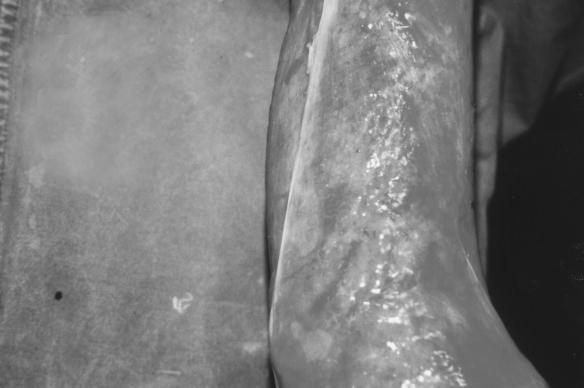
Fig. 1a. Fresh burn injury-before collagen application.
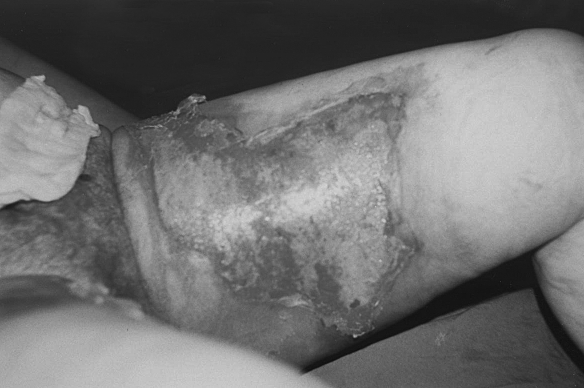
Fig. 1b. Three days after collagen application.
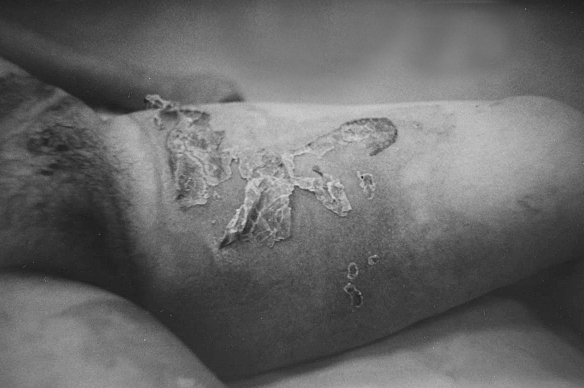
Fig. 1c. Ten days after collagen application.
In the course of subsequent treatment, additional debridement was performed, further spreading of the necrotic area not having been ascertained.
A vascular surgeon was consulted. Doppler colour DE was also carried out.
Diagnosis: Occlusio arteriarum cruris bil. suspecta. Leriche syndrome. Therapy: Trental (3 x 1), Acetysal Ph 8 (2 x 1). On the recommendation of the vascular surgeon, no additional surgical operation on the toes of the feet was performed
The patient’s wounds were regularly bandaged and eventually, apart from some lesser debridement, including also bone debridement, following regular wound bandaging and therapy, gradual healing occurred of the defects in the toes per secundam in the middle of the toe (the area of the middle phalanx), without any subjective discomfort for the patient.
The dressings were removed, the sites were inspected for infection, and reapplication of collagen sheet was subjected to inspection (7 times for 4 months).
In the subsequent course of time a similar change occurred in the little toe of the right foot, which was being treated in the same way.
In the process of applying the collagen layers and of locally applied therapy with vitamins, glucose, and antibiotics, the role of the agents was demonstrated in terms of the initiation of rapid epithelialization and wound healing - a common defect after amputation. A better and faster process of healing was achieved in the surgical interventions: in our case minimal necrectomies occurred without any inflammatory response, with solid local findings, which led, albeit in a somewhat longer time period (4 months), to the elimination of the defects at the site of the toe amputation.
Discussion
1. Why choose collagen sheet?
For the following reasons, collagen sheet provides multiple benefits that cannot be provided by conventional dressings:
Chemically, bovine collagen is very similar to the human form. This is crucial, as the human immune system will reject everything that deviates too much from its own proteins.
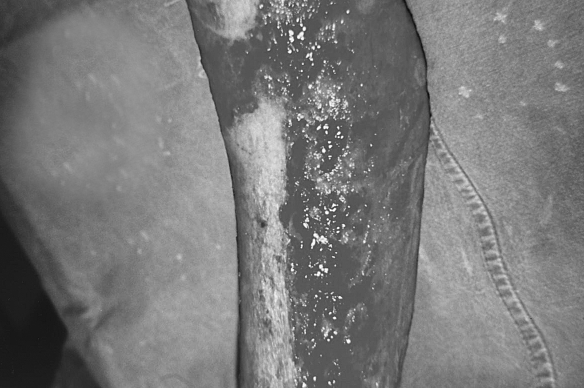
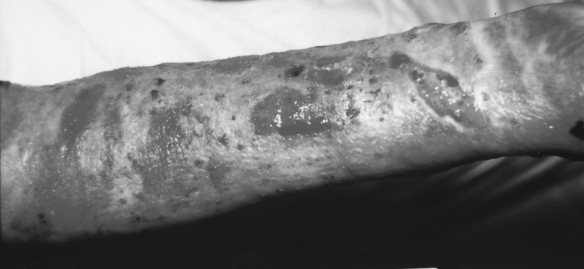
Fig. 2a. Two weeks after burn injury - classic treatment.
For these reasons, collagen sheets are well qualified for use as an effective wound cover. 3
Collagen sheet is prepared from bovine collagenous tissue by treating tissues with a series of chemical and enzymatic procedures. This is followed by chemical cross-linking, packing, and sterilization.
Collagen sheet is retained in the tissue and gradually absorbed by inflammatory cellular activity; the fibrous tissue is replaced by fibroblasts. Granulation tissue developed at a normal rate and the cellular events were precisely the same as those occurring in normal wounds. Collagen is well recognized as a useful and important biopolymer in the field of biomaterial engineering. In order to gain satisfactory results from collagen when used as a biomaterial, its physical and biological properties prove necessary. Desamidation of collagen is one such approach that develops better biomaterial with minimal alterations in collagen’s native properties.
Desamidation of hide trimmings and Achilles tendons resulted in a modified form of collagen. It improved the solubility as well as the swelling properties of the biomolecule, which are prerequisites for a biomaterial. Collagen contains moderate amounts of positively and negatively charged groups derived from the diamino dicarboxylic acids built into the molecule. The collagen molecule contains 45 aspartic acids, 72 glutamic acids, and 37 lysine and 45 arginine residues. These residues are polar in nature and, on dissociation, the carboxyl groups are negatively charged. In the isoelectric state and in equilibrium with water in the pH range from 5.5 to 9.5, these polar groups are completely ionized. Collagen is an amphoteric ionic structure attaining the highest degree of charge, both positive in the isoelectric pH range, i.e. around 7.0 for collagen. Desamidation causes progressive hydrolysis of amide groups of asparagines and solubility of collagen. Desamidation collagen has high viscosity and a very high hydroxyproline content, like native collagen. 1
Fig. 2b. Collagen applied.
Fig. 2c. Ten days after collagen application.
Any biomaterial should ensure non-toxicity to the biological environment where it is being applied. Owing to its greater swelling, desamidated collagen presents a greater surface area for cell growth and interaction. 4
Collagen sheets are very useful in first- and second-degree burns. The cost factor comes down, and the pain associated with dressing can be avoided. It is especially useful in children since the trauma of dressing can be avoided.
Composition of the material (collagen sheets)
Collagen sheets are produced from bovine tissues comprising mostly type I and III collagen, packed in a neutral glass vial containing sterile preserving liquid medium (a mixture of isopropyl alcohol and water) sterilized with ethylene oxide.
Basic principles of technological treatment
Collagen is a biological skin substitute, i.e. natural, easily available, ready to use, non-immunogenic, and non-pyrogenic. Biological dressings are the logical best candidate for the management of wounds since they create the most physiological interface between the wound surface and the environment. This enables the body’s reparative and immune systems to function most effectively. 5
On the basis of work carried out in our department, we have summarized the physical properties of collagen sheet as follows:
a. Biological
Collagen sheet is non-inflammatory
Collagen sheet facilitates migration of fibro-blasts and microvascular cells
Collagen sheet helps in the synthesis of neodermal collagen matrices
Collagen sheet has low antigenicity
Collagen sheet has minimal biodegradation
Collagen sheet is non-toxic
Collagen sheet helps in minimizing scarring
b. Physiological
Collagen sheet is impermeable to bacterial migration
Collagen sheet modulates fluid flux from the wound
Collagen sheet is elastic, soft, and supple
Collagen sheet has good tear strength
Collagen sheet has good suturing characteristics
Collagen sheet has enough strength to be peeled off the wound
c. Adverse effects
Collagen sheet has been found to be well tolerated in clinical trials. There have been no reports of clinically significant immunological or histological responses to the implementation of collagen sheet, and no reports of rejection of collagen sheet.
Collagen serves as a template for the infiltration of fibroblasts, macrophages, and lymphocytes and attracts additional monocytes to the wound, thus increasing the amount of debris removed and capillaries forming the neovascular network. As healing progresses, collagen is deposited by the fibroblasts, replacing the collagen portion of the collagen sheet. 6
Before healing can begin, haemostasis must occur. Collagen applied at the time of haemostasis acts as an auxiliary mechanism to augment clotting. Collagen actually increases platelet adherence to the endothelial vessel wall, thus sealing it off. 7
There is no threat of HIV or hepatitis infections, as bovine material is obtained from countries free of bovine spongiform encephalopathy (BSE) and possesses a long shelf-life under normal storage conditions.
2. Why glucose sugar?
Fibroblasts combine and build amino acids into a sugar protein structure to form collagen at the wound site.
3. Why choose gentamicin or Garamycin?
Both are effective and cheap antibiotics.
4. Why choose vitamin A?
Vitamin A is a family of fat-soluble vitamins. Retinol is one of the most active and usable forms of vitamin A and is found in animal foods such as liver and eggs. 8
Vitamin A plays an important role in eyesight, bone growth, reproduction, cell division, and cell differentiation, which is the process by which a cell decides what it is going to become. It also maintains the surface linings of the eyes and the respiratory, urinary, and intestinal tracts. 9When the linings break down, bacteria can enter the body and cause infection. 10Vitamin A also helps the body to regulate its immune system. The immune system helps to prevent or fight off infections by making white blood cells that destroy harmful bacteria and viruses. Vitamin A may help lymphocytes - a type of white blood cell that fights infections - to function more effectively. Vitamin A may also help to prevent bacteria and viruses from entering the body by maintaining the integrity of skin and mucous membranes. 11
5. Why choose vitamin C?
Vitamin C contains L-ascorbic acid as its primary ingredient.
The choice of this particular form of vitamin C is based on long-established medical and biological knowledge that L-ascorbic acid is the only form of vitamin C that the body can recognize and utilize to stimulate collagen synthesis. Vitamin C, in the form of L-ascorbic acid, is the one vitamin that doctors and biologists agree can accelerate wound healing, protect fatty tissues from oxidation damage, and play an integral role in collagen synthesis. L-ascorbic acid, at 10% concentration applied directly to the skin, stimulates the growth of collagen and produces astounding rejuvenating effects.
The production of collagen is highly dependent on the availability of vitamin C to fibroblasts in the stratum basale cells.11
The trouble with this priority system is that the skin is very sensitive to any shortfall in vitamin C availability.
Vitamin C aids in the absorption of iron and the formation of red blood cells and converts folic acid to active folinic acid. It has histamine-reducing properties and increases lymphocyte formation.
Vitamin C is antioxidant and provides optimal nutritional support to all physiological functions, notably including vascular and capillary integrity in support of the circulatory system.
Vitamin C is utilized in all organs of the body, including the skin, and research has shown that whenever there is a shortfall in the body’s overall requirements vitamin C is selectively withdrawn from the skin to make up the shortfall in any of the other organs.
As the human system does not manufacture vitamin C, it must be taken into the body from outside sources. Environmental conditions cause damage to the fragile capillary pathways that feed the skin. Nutrients circulating in the bloodstream can no longer reach the skin in adequate amounts. Without these essential components, the skin cells cannot make the healthy collagen needed for healing.13
If we are not able to maintain a base level of vitamin C in the body pool, we must - if we are to compensate for vitamin C deficiency - deliver the nutrients directly to the skin to provide support to the stratum basale.
The problem with the oral supplementation approach is one of both absorption and selective delivery. For example, if we take one standard aspirin tablet we deliver three molecules of aspirin to every cell of the body. This is obviously a problem if we only need the aspirin for a sore toe. To get sufficient vitamin C into the system orally to deliver 100 milligrams of vitamin C to the skin of the face you would need to ingest more than one kilogram of vitamin C.
It has been shown that collagen production is increased by up to 300% in fibroblast cells exposed to vitamin C.14
Collagenase is a destructive enzyme secreted by cells to degrade the extracellular matrix protein, collagen.
Vitamin C is a potent inhibitor of collagenase.
Some people are allergic to vitamin C. If they experience an allergic reaction, blotches, or itching, they should wash the area with cool water and not reapply it.
Conclusion
Collagen sheet provides multiple benefits that cannot be provided by conventional dressings.
We point out the utility, when locally applied onto an open wound, of vitamin A, vitamin C, 50% glucose, and gentamicin with a biological covering.
By controlling infection in poor vascular profusion, this report shows that collagen sheet functions as a drug delivery device.15
The collagen mesh provides a blueprint, a road map, and the right way.15
Collagen serves as a template for the infiltration of fibroblasts, macrophages, and lymphocytes and attracts additional monocytes to the wound, thus increasing the amount of debris removed and the capillaries forming the neovascular network. As healing progresses, collagen is deposited by the fibroblasts, replacing the collagen portion of the collagen sheet.
There is no threat of HIV or hepatitis infections; bovine material is obtained from countries free of BSE, and has a long shelf-life (5 years) under normal storage conditions.
Collagen sheet has been found to be well tolerated in clinical trials. There are no reports of clinically significant immunological or histological responses to the implantation of collagen sheet, and no reports of collagen sheet rejection.
If we are not able to maintain a base level of vitamin C, vitamin A, antibiotics, and glucose in the body pool, then - in order to compensate for a deficiency - we deliver the nutrients directly to the skin to provide support to the stratum basale.
Without these essential components, the skin cells cannot make the healthy collagen needed for healing.
In our opinion the use of collagen, vitamin A, vitamin C, gentamicin, and 50% glucose resulted in better quality wound healing and overall wound treatment.
We are convinced that collagen sheet is very useful in first- and second-degree burns. The cost factor is reduced and the pain associated with dressing can be avoided. It is especially useful in children since the trauma of dressing can be avoided.
Any biomaterial like collagen sheet should ensure non-toxicity to the biological environment where it is applied.
References
- 1.Stenzel K.H., Miyata T., Rubin A.L. Collagen as a biomaterial. Ann. Rev. Biophys. Bioeng. 1974;3:231–53. doi: 10.1146/annurev.bb.03.060174.001311. [DOI] [PubMed] [Google Scholar]
- 2.Radhika M., Sehgal P.K. Studies on desamidation of bovine collagen. J. Biomed. Mater. Res. 1997;35:4. doi: 10.1002/(sici)1097-4636(19970615)35:4<497::aid-jbm9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.McKenzie R.C., Sauder D.N. The role of keratinocyte cytokines in inflammation and immunity. J. Invest. Dermatol. 1990;95:105–7. doi: 10.1111/1523-1747.ep12874955. [DOI] [PubMed] [Google Scholar]
- 4.Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J. Cell Biol. 1972;54:626–7. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chvapil M., Kronenthal R.L., Van Vinkle W. Medical and surgical applications of collagen. Int. Rev. Connect. Tissue Res. 1973;6:1–61. doi: 10.1016/b978-0-12-363706-2.50007-6. [DOI] [PubMed] [Google Scholar]
- 6.Chvapil M. Fundamental aspects of biocompatibility. “CRC Series in Biocompatibility”. 1981:87–104. [Google Scholar]
- 7.Doillon C.J., Silver F.H. Collagen-based wound dressings: Effects of hyaluronic acid and fibronectin. Biomaterials 7L. 1986:3–8. doi: 10.1016/0142-9612(86)90080-3. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Agriculture; U.S. Department of Health and Human Services. Your Health: Dietary Guidelines for Americans (4th ed.): Home and Garden Bulletin. Vol. 232 U.S. Government Printing Office; Washington, DC: 1985. [Google Scholar]
- 9.Recommended Dietary Allowances (10th ed.) Academy Press; Washington, DC: 1987. Subcommittee on the 10th Edition of the RDAs, Food and Nutrition Board, Commission on Life Sciences, National Research Council. [Google Scholar]
- 10.Third Report on Nutrition Monitoring in the United States: Volumes 1 and 2. U.S. Government Printing Office Washington DC. U.S. Government Printing Office; Washington, DC: 1995. Federation of American Societies for Experimental Biology, Life Sciences Research Office. Prepared for the Interagency Board for Nutrition Monitoring and Related Research. [Google Scholar]
- 11.Browne M.B. Label Facts for Healthful Eating. Mazer Corporation; Dayton, Ohio: 1993. [Google Scholar]
- 12.Occsin J., et al. Ascorbic acid specifically increased type I and III procollagen messenger RNA levels in human skin fibroblasts. J. Invest. Dermatology. 1988;90:420. doi: 10.1111/1523-1747.ep12460849. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan D., et al. A new stabilized ascorbic acid solution: Percutaneous absorption and effect on relative collagen synthesis. J. Cutaneous Ag. and Cosm. Derm. 1988;1:88–92. [Google Scholar]
- 14.Murad S., et al. Regulation of collagen synthesis by ascorbic acid. Nat. Ac. Sc. USA. 1981;78:22870. doi: 10.1073/pnas.78.5.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin A.L., Stenzel K.H., Miyata T., White M.J., Dunn M. Collagen as a vehicle for drug delivery. Preliminary report. J. Clin. Pharmacol. 1973;13:309. doi: 10.1002/j.1552-4604.1973.tb00217.x. [DOI] [PubMed] [Google Scholar]
- 16.Kleinman H.K., Klebe R.J., Martin G.R. Role of collagenous matrices in the adhesion and growth of cells. J. Cell Biol. 1981;88:473–85. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silver F.H., Pins G. Cell growth on collagen: A review of tissue engineering using scaffolds containing extracellular matrix. J. Long-Term Effects of Medical Implants. 1992;2:67–80. [PubMed] [Google Scholar]
- 18.Tajima S., et al. Regulation of collagen synthesis by ascorbic acid. Biochem. Biophys. Res. Commun. 1982;106:632. doi: 10.1016/0006-291x(82)91157-3. [DOI] [PubMed] [Google Scholar]
- 19.Yang J., Nandi S. Growth of cultured cells using collagen as a substrate. Int. Rev. Cytol. 1983;81:249–86. doi: 10.1016/s0074-7696(08)62340-2. [DOI] [PubMed] [Google Scholar]