Summary
Scar management for the prevention of excessive scar formation has always been important but never so important as it is today. Optimal management continues to be an enigma for surgeons, and the best modality of treatment has been debated for many years. However, most studies have unfortunately been either retrospective or case report descriptions. Advances in scar management have been hampered by confusing or ambiguous terminology. There is no consensus on what amount of post-traumatic skin scar formation is "normal" and what should be considered "hypertrophic". In the World Health Organization's ICD-9, there is no diagnostic code for hypertrophic scar - only keloid is listed. Yet the medical and scientific literature distinguishes them as different conditions. This confusion results in inappropriate management of scar formation, and occasionally contributes to decision making related to elective or cosmetic surgery. Our experience suggests that there is no single treatment for scars that is adequate and that clinical judgement is very important when considering treatment and balancing the potential benefits of the various treatments available. The goal of treating scars is to restore functionality, provide relief of symptoms, enhance cosmetics, and prevent recurrence. This article is based on our scientific and clinical experiences and focuses on over-the-counter options to manage keloid and hypertrophic scars.
Keywords: MANAGEMENT, KELOID, HYPERTROPHIC, SCARS
Abstract
La gestion des cicatrices pour prévenir leur formation excessive a toujours été importante, mais jamais comme aujourd'hui. La gestion optimale reste une énigme pour les chirurgiens, et on discute depuis nombreuses années sur la meilleure modalité de traitement. Cependant, la plupart des études ont été malheureusement ou rétrospectives ou des descriptions de cas particuliers. Le progrès dans le champ de la gestion des cicatrices a été ralenti par une terminologie confuse ou ambiguë. On n'est pas d'accord sur la quantité de la formation de cicatrices cutanées post-traumatiques que l'on peut considérer comme "normale" et ce que l'on peut considérer comme "hypertrophique". Le ICD-9 de l'Organisation Mondiale e la Santé ne contient aucun code diagnostique pour la cicatrice hypertrophique - il cite seulement la cicatrice chéloïdienne. Mais la littérature médicale et scientifique les distingue comme des conditions différentes. Cette confusion porte à une gestion inappropriée de la formation des cicatrices, et en certains cas contribue au processus pour la prise de décisions pour ce qui concerne la chirurgie élective ou cosmétique. Selon l'expérience des Auteurs, il n'existe pas un seul traitement efficace, et le jugement clinique est très important quand on considère le traitement clinique et pèse les bénéfices potentiels des divers traitements disponibles. Le but du traitement des cicatrices est de restaurer la fonctionnalité, soulager les symptômes, améliorer l'aspect cosmétique et prévenir les récidives. Les Auteurs présentent leurs expériences scientifiques et cliniques et décrivent les options commerciales pour la gestion des cicatrices chéloïdiennes et hypertrophiques.
Introduction
Scars are a result of the natural healing process that occurs when the skin repairs itself after wounds, trauma, burns, surgical incision, or disease. Normal skin tissue is replaced with scar tissue to close open wounds and prevent infection. Scars can be painful, cause itching, and limit mobility. Many scars are primarily a cosmetic concern, but their presence may have a significant negative impact on the affected individual's self-esteem. Consequently, patients often seek treatment to reduce the visibility and discomfort of a scar.
There are several types of scars, two of the commonest being hypertrophic and keloid scars. In the medical literature, a hypertrophic scar is generally described as an overgrowth of scar tissue that remains within the boundaries of a wound. The wound boundary shrinks as more scar tissue forms. Currently, no objective diagnostic criteria have been formulated to indicate when a scar can be considered hypertrophic. Keloid scars are densely collagenous, nonencapsulated, benign connective tissue neoplasms. The size and shape of keloid scars have little correlation with the extent of the skin wound. Large disfiguring tumours often result from minimal skin trauma. Keloid scars, unlike hypertrophic scars, have a genetic aetiology.
A commonly taught but confusing concept is that keloids can be distinguished from hypertrophic scars by the extension of the scar beyond the wound border. This concept implies that a scar starts out as a hypertrophic scar and later becomes a keloid, when it has exceeded some vaguely defined wound boundary. Such a classification scheme sets the stage for confusion, particularly when one of the disorders is classified as an inheritable disease. Scientists investigating pathological scarring suggest that there are significant phenotypic differences between hypertrophic and keloid scars that may not be clinically obvious until they invade surrounding tissue.1 Therefore, while keloid scars are by definition hypertrophic, only a small percentage of large scars can be truly classified as keloid.
The wound healing process
In order to discuss hypertrophic and keloid scar pathogenesis and treatment, a review of the pertinent aspects of wound healing is essential. Wound healing is a very complex process that is tightly regulated to achieve wound repair. The process can be categorized in three distinct phases that have very different objectives: inflammation, proliferation, and maturation. Following the initial tissue injury, inflammatory mediators known as cytokines are released from the injured tissue cells and wound blood clot, after which the inflammatory phase initiates. The amount of blood released, the extent of devitalized tissue, and the bacterial content are important issues as the wound does not become sterile until it regains an epithelium.
If the wound healing process is uncomplicated, the proliferation stage (also called the "transitional repair stage") begins several days after injury.2 Platelet degranulation activates the coagulation cascade, and the resultant fibrin clot serves as a scaffold for the proliferation phase of wound healing. During the proliferative phase, the number and density of fibroblasts in the extracellular matrix increase, and the fibroblasts synthesize tissue components, such as proteoglycans, fibronectin, and collagen. New vessels and epithelium are formed as rapidly as possible to maximize the tissue replacement dynamics. All wound cells are maximally active and are sensitive to factors that regulate cell proliferation and protein biosynthesis. All the cells proliferate, and metalloproteinases are simultaneously released into the extracellular fluid to activate a matrix breakdown process. The balance between tissue degradation and biosynthesis permits remodelling of the provisional tissue and determines the net amount of scar tissue produced.
When enough provisional tissue is generated, a turnoff signal is received that initiates the final stage of wound healing, the maturation stage. This phase is characterized by cellular apoptosis and a shift in balance from scar remodelling towards scar degradation.3 The process is accompanied by extracellular matrix reorganization and reduction. Metalloproteinases synthesized during the proliferation stage continue to break down the extracellular matrix at a rate largely determined by physical and biochemical factors in the matrix. The amount of extracellular matrix biosynthesis is controlled by the need for tissue strength and other operational parameters. Mechanical stress is an important contributory parameter in net scar production.
The most important known determinates of scar production are the extent and duration of inflammation, the magnitude of mechanical tension acting on the scar, and the genetic phenotype of the individual concerned. Although other factors may be important - and some are yet unknown - scar management that is fundamentally based on these three critical parameters can be effective in limiting unnecessary scar formation in most cases.
Pathological scars
Hypertrophic scars
Several epigenetic causes of hypertrophic scarring have been identified. Basically, factors that increase or prolong wound inflammation or wound tension predispose to hypertrophic scar formation. Such factors include wound infection, prolonged healing by secondary intention, and immunologically foreign material present in the wound. Hypertrophic scars begin as the result of an injury to the deep dermis, and they are especially pronounced in wounds that have a prolongation of the inflammatory and proliferative phase of wound healing.2 The incidence of hypertrophic scars following surgery is about 40-70% - it is higher (up to 91%) following burn injury. Several reports conclude that there is a substantially increased risk of hypertrophic scarring in burn wounds that take longer than 21 days to heal.
Hypertrophic scarring also occurs as the result of dynamic mechanical skin tension acting on the healing wound. As a result of mechanical tension, scars located in certain areas of the body (e.g. sternum, deltoid, and upper back) are frequently hypertrophic. This anatomical dependency seems to correlate with a pattern of skin tension.4 The characteristic feature of hypertrophic scars is that they regress in time after injury, leaving behind, however, an unsightly wide gap of thinned dermis between the wound edges.
A familial pattern of hypertrophic scarring has not been reported. However, populations with a higher skin melanin content are known to have a higher incidence of hypertrophic scars. These populations include people of African, Asian, and Hispanic descent. Hormonal influences are also known to be a factor, with hypertrophic scarring often initiating at the onset of puberty or during pregnancy. Scar tissue cells are sensitive to the influences of the same growth factors that drive normal tissue growth and development. Schierle et al.5 reported an increase in testosterone receptors in hypertrophic scars, which may contribute to the formation of the scars during adolescence.
Keloid scars
Although the diagnosis of keloid scar is often inappropriately applied to hypertrophic scars, the two lesions can be differentiated at several levels. Unlike hypertrophic scars, the natural history of keloid scars is that they do not regress with time following injury. Keloid tumours grow to reach a certain size and may remain that size indefinitely. They are thought to be a localized reaction to cutaneous trauma, although in many cases no recollection of the inciting event can be discovered. While there is no doubt that trauma to the skin, including surgical incision, can cause keloids, a variety of factors may increase their incidence. People with darker skin, including Asians and, more commonly, blacks, are more susceptible to keloid formation than whites. The general population ratio may be as high as 15 keloids in the black population to one in the white population, which has led to at least one theory suggesting an abnormality in the metabolism of the melanocyte-stimulating hormone. This theory would seem to point to a higher incidence of keloids at times of increased melanocyte-stimulating hormone production, such as during puberty and pregnancy.
Keloids may affect virtually any surface on the body, with the central chest, deltoid region, and back having the highest frequency. This has led some to conclude that motion and tension play a large role in keloid formation. While this may be implicated in some cases, the earlobes, a site of countless keloids and recurrences, are subject to minimal motion and tension forces, even after previous keloid excisions. Conversely, the central face rarely has hypertrophic scars or keloids but is subject to extremes of motion and tension during the normal activities of daily living. Additionally, increased tension has been shown to increase fibroblasts and myofibroblasts in in vivo and in vitro studies.1
Clinical management
The management of scars should begin even before the surgical procedure and continue 12 to 24 months afterwards. The first consideration in the discussion of scar treatment is prevention. We should know how to prevent scars and we should learn how to identify them immediately and manage post-operative complications correctly. To do this, we should have a thorough understanding of wound healing and skin biomechanics.
In the surgical setting, events occurring during the management of the open wound are important. Careful and meticulous handling of tissue, evacuation of blood from the wound, and accurate wound edge alignment during closure, as well as precise suture placement, will reduce inflammation and therefore also scarring. The choice of suture material is important because some sutures trigger more immune reaction than others. Monofilament absorbable synthetic biopolymers produce the least reaction. Minimizing the tension acting on the wound by aligning the incision parallel to natural skin lines (when possible) or the use of local flaps will also help to reduce the incidence of post-surgical hypertrophic scars.
Over the past two decades, some fundamentally new therapeutic approaches to scar management have been reported. These new techniques have substantially augmented existing therapeutic approaches and continue to enhance them. The use of existing therapeutic approaches has been reviewed in detail elsewhere and will not to be covered in depth here. In the following paragraphs, we will attempt to briefly summarize some of these new concepts. We hypothesize that wound healing is evolutionarily optimized for speed of healing in dirty conditions, where a multiply redundant, compensating, rapid inflammatory response with overlapping cytokines and inflammatory cascades allows the wound to heal quickly in order to prevent infection and future wound breakdown.
Prevention of the extension of inflammation
Anti-inflammatory agents. The inhibition of ECM and inflammatory protein production by corticosteroids is one of the best-established approaches to scar management. Serial steroid injections do not eradicate keloids; they can however diminish pruritus and pain and soften the lesions. Corticosteroids are effective in the majority of noninfected scars that exhibit symptoms such as pain and pruritus. Intralesional corticosteroids are believed to act by inhibiting fibroblast growth and decreasing alpha2 macroglobulin levels, which lead to collagen degradation. Corticosteroid preparations used for intralesional injection have included hydrocortisone, triamcinolon, and dexametasone, with none proving to be particularly advantageous. Several concentrations between 10 and 40 mg/ml can be used. In steroid-sensitive areas (e.g. nose, eyelids, lips) that can undergo dermal atrophy, 10 mg/ml should be used initially. After the initial dose, the patient should return after 3 to 4 weeks, and the injection should be repeated at a similar or higher dose if required. The local adverse effects of steroid injections are associated with the dose given. These are hypopigmentation, dermal atrophy, teleangiectasia, necrosis, and ulceration. Doses of less than 120 mg per month in adults and less than 80 mg per month in children (6-10 years old) are recommended. The systemic effects of intralesional steroid therapy have been widely reported. A major disadvantage of using intralesional corticosteroid is the pain associated with the injection, which can lead to patient noncompliance in the follow-up.
Long-acting synthetic glucosteroids such as triamcinolone are commonly used in the treatment of hypertrophic and keloid scars. Synthetic glucosteroids, applied topically, are weakly soluble in water but well absorbed in the epidermis in time - they diffuse slowly into the scar. Alternatively, solutions of triamcinolone can be injected in 10-40 mg/ml concentrations.10 Both methods of delivery are effective in the majority of noninfected scars that exhibit symptoms such as pain and pruritis.11
Steroids are not often useful in the treatment of older scars, which are less metabolically active; they are also not routinely used as a scar preventative immediately after wound closure because of concern about wound infection or dehiscence.
The inflammatory response can be regulated at several different physiological levels before, during, and after inflammatory gene expression. Before gene expression there are transcription factors, such as NF-kB (nuclear factor kB) that are activated by cell injury. After gene expression there are inducible enzymes, such as cyclo-oxygenase (COX) regulation, and inhibition of histamine synthesis. The most widely used anti-inflammatory agents belong to the broad category of nonsteroid anti-inflammatory drugs (NSAIDs). These inhibit either the NF-kB pathway or COX activity, or both. It is noteworthy that corticosteroids exert a broad anti-inflammatory effect through inhibition of multiple inflammation transcription factors NF-kB, AP-1 (activator protein-1), and NF-AT (nuclear factor of activated T lymphocytes).12
NF-kB is a rapid-response transcription factor that is involved in the cellular stress response. It is involved in the upregulation of cell membrane receptors to inflammatory peptides. It is also involved in the production of cytokines, chemokines, and growth factors. NF-kB activation can be inhibited by several different agents. These agents include cyclosporin, tacrolimus, antioxidants, and salicylates (including aspirin). Salicylic acid indirectly inhibits NF-kB expression by blocking the enzymes that lead to dissociation of IKB (the activator of NF-kB) from the NFkB complex in the cytoplasm. This dissociation subsequently decreases the amount of inflammation. Aspirin not only inhibits the NF-kB pathway but also irreversibly inactivates both COX isoforms. Salicylates (2-5%) are commonly used to control certain causes of dermatitis and are routinely used in acne treatment products. Our experience increasingly suggests that topical application of salicylates to painful, itching scars is a practical approach to reducing inflammation. Topical aspirin should be used under a physician's guidance, because some patients - particularly asthmatics - may develop hypersensitivity. Vitamin D3, which is also used to treat dermatitis, is another inhibitor of NF-kB. The action of vitamin D3 is due to its affinity for the DNA promoter regions that transduce the NF-kB dissociation signals. To date, only one prospective uncontrolled study of topical salicylic acid has been reported.
Nonetheless, the number of physicians prescribing topical anti-inflammatory agents is increasing. Our personal clinical experience has been very encouraging of the effectiveness of topical 2% salicylic acid in managing pruritic growing scars.
Prostaglandins are a group of potent, hormone-like substances that mediate a wide range of physiological functions, such as the control of blood pressure and contraction of smooth muscle. The synthesis of prostaglandins is also central to the inflammatory response. The commonest strategy for prostaglandin synthesis blockade is pharmacological inhibition of cell membrane-bound COX.25 COX-1 is expressed constitutively throughout the body, especially in the stomach and kidneys. COX-2, however, is expressed constitutively only in the brain and kidneys.28 COX-2 synthesis is highly inducible by activation of NFkB, which occurs at sites of injury or infection and plays an important role in fibrosis. The adverse side effects of COX inhibition are minimized by the use of specific COX 2 inhibitors, making them agents of choice for prolonged usage. The therapeutic value of these agents in rheumatological disease is well established, but their potential value in the management of hypertrophic scarring has been a more recent consideration. Our reported experience suggests that COX-2 inhibitors reduce symptoms of pruritus and may induce scar maturation and involution.14 Some popular topical consumer scar treatment products are derived from onion extract, which has anti-inflammatory properties.
Antihistamines are commonly used to control symptoms of scar pruritus, but they also seem to exert an antifibrotic effect on scars. Antihistamines, particularly the H1 blockers, inhibit the inflammatory response, resulting in reduced scar formation and increased comfort. The inflamed scar is scratched less, which will most likely reduce the scar growth rate. Antihistamines are also known to inhibit collagen synthesis. Diphenhydramine and hydroxazine are the most commonly used antihistamines for scar management. We prefer the use of long-acting nondrowsy formulations such as loratadine or fexofenadine, which have the advantages of sustained action and fewer CNS side effects.
Inhibitors of gene transcription. The antimetabolities mitomycin-c and fluorouracil inhibit cell proliferation by blocking DNA synthesis and transcription through competitive inhibition of thymidylate synthesis.15 A single application in the first few days after wound closure seems to be effective. Antimetabolite-induced apoptosis has also been demonstrated in Tenon's capsule fibroblasts.16 These agents are thus likely to be helpful in scar control.
Growth factor inhibition. The transforming growth factor (TGF-b) family of proteins is centrally involved in regulating wound healing.8 The process of TGF-b synthesis and release is activated by injury and, along with the platelet-derived growth factor, it has a central and critical role in the induction of wound healing. The binding of TGF-b to its receptor on fibroblasts causes fibroblast proliferation ECM structural protein synthesis and TGF-b synthesis. Mannose-6-phosphate and several other disaccharides sterically limit TGF-b binding to its receptor and limit scar formation in experimental wound-healing models.17
The acceleration of scar degradation agents and growth factor inhibitors can limit scar production, and strategies to accelerate scar tissue degradation are also very useful. This approach may be the best for the management of older hypertrophic scars and older keloids. The rate of tissue breakdown can be increased by both pharmacological and physicochemical means.
Occlusive dressings. After elastic pressure wrap dressing for the healing of burn scars was observed by Larson et al. to be effective in the reduction of scar hypertrophy,20 24 mm Hg pressure garments became the mainstay of scar prevention. The action mechanism of pressure dressings is unknown, because they remain effective even when they lose their elasticity and pressure after several weeks of daily use. Measurements show a decrease in wound metabolism, with an increase in collagen activity. The drawbacks to their use are primarily related to thermal insulation and movement restriction. If continuous pressure (capillary pressure greater than 24 mm Hg) is applied to a keloid day and night for 6 to 12 months, this can help the keloid to regress. It is believed that constant pressure decreases soft-tissue cellularity, increases interstitial space, and results in histologically looser collagen bundles. However, since continuous pressure application is required (>23.5 h per day), this regimen is difficult to institute. Applying direct pressure to keloid sites has long been cited as clinically successful. We have had little success with this treatment because of the discomfort and prolonged duration required. Even pressure earrings are poorly accepted in our practice and are rarely worn continuously by our patient population.
Both hydrogel and silicon gel sheeting have also been used to control scar formation. It is clear, however, that the release of silicone into the scar is not the mode of action. Several reports have shown that hydrogel sheeting is equally effective as silicone and has fewer adverse sideeffects. 22,23 Hydrogel sheeting has recently been approved by the FDA as substantially equivalent to silicone for the treatment of hypertrophic scars. Hydrogels have the added advantage of their use as a hydration vehicle, as also a higher heat capacity for maintaining a continuous scar temperature. Silicone gels and sheeting have been successfully used to decrease the recurrence of keloids, hypertrophic scars, and post-burn scars.1,3 Several studies are currently under way to determine more accurately their mechanism of action, which is currently unclear. Importantly, this technique is relatively inexpensive and non-invasive, and in our experience has a high patient compliance and satisfaction rate.
We use silicone sheets for large flat-surface keloids as both an alternative and an adjunct to surgery.
Inducer of scar degradation
Calcium antagonists. Many organic calcium channel blockers (i.e. verapamil) induce collagenase production and scar tissue degradation. They induce changes in fibroblast gene expression, resulting in decreased collagen synthesis and increased collagenase production. These effects appear to be mediated by interruption of the basic cellular G-protein signal transduction pathway that is critical to regulation of fibroblast behaviour.24 This pathway can be interrupted at multiple points by a wide range of commonly used drugs.25 Verapamil, injected in a cream base applied topically on the scar, avoids rebound scarring from the trauma of intralesional injections. If an injection is necessary, the alternation of verapamil with cortisone injections at monthly intervals seems to generate an effective yet robust response.
Additional agents that may induce scar degradation include calcium channel blockers, calmodulin inhibitors (e.g. trifluoperazin), and specific protein kinase C (PKC) inhibitors (tamoxifen). These agents are promising as treatment for older, non-inflamed scars that are no longer actively remodelling.
Induction of remodelling
Apoptosis inducers. The density of keloid scar fibroblasts does not decrease as a scar ages. Instead, keloids continue to produce thick collagen bundles in a nodular array. They also produce high levels of the proteolytic enzyme caspase-3.3 Sayah et al. proposed that the downregulation of apoptosis-related genes in fibroblasts could alter the fibroblast phenotype, leading to increased scar tissue production.7 A pharmacological agent that could block the expression and ultimate production of caspase-3 could be very valuable in the treatment of hypertrophic and keloid scars.
Radiotherapy. The mechanism of radiotherapy involves the destruction of fibroblasts that are not replaced, leading to a reduction in otherwise excessive collagen synthesis. A common concern is the potential for radiationinduced malignancy. To avoid the risk, treatment portals were carefully designed to prevent scatter from affecting nearby body structures. Using this technique, virtually no radiation is received by the central nervous system or thyroid gland. Additionally, the radiation penetrates approximately 4 mm - about the thickness of an earlobe. In recurrent earlobe keloids that are not responsive to excision and steroid treatment, repeat surgical excision followed by radiation therapy within one week of excision should be an option, after careful discussion with the patient. In a patient who is less likely to follow up on a monthly basis for serial steroid injections over 6 months, postoperative radiotherapy to the earlobe keloid can be a useful option for adjunctive treatment for recurrent ear lobe keloids. In recurrent keloids in other head and neck sites, post-operative radiation is more controversial. If a recurrent keloid is present in the lower anterior neck, many surgeons would be more reluctant to institute post-operative radiotherapy because of the risk of radiation exposure to the thyroid gland. The mechanism of radiation-induced scar control seems to be the apoptosis of proliferating cells in the scar tissue.28 Low-dose ionizing radiation is most often reserved as the method of last resort for the treatment of intractable keloid scars. This therapy utilizes 15-20 Gy of orthovoltage radiation divided into five to six treatments. Although radiation therapy alone is not adequate, if it is used in conjunction with surgical intervention the reduction in recurrence may reach 25%, compared with a recurrence rate of up to 80% with surgery alone.
Cryotherapy has also been used to treat keloids and hypertrophic scars. Some researchers have used cryotherapy before intralesional steroid injection to allow for oedema and cellular injury from cryotherapy to subside. Others have used cryotherapy as an effective treatment for new keloids, reporting a good response in 74% of patients.5 An adverse effect of cryotherapy is hypopigmentation due to the cold sensitivity of melanocytes. Thus, darker-skinned patients are at increased risk for hypopigmentation. In addition, post-procedure healing can be prolonged, requiring several weeks.
Laser therapy (continuous-wave or short-pulsed carbon dioxide, 585-nm flashlamp pulsed dye) has been another approach to manage keloids and hypertrophic scars. However, the optimal time to institute laser treatment has not been determined. Some have proposed that when the laser treatment is initiated in the first few weeks post-injury, further scar proliferation can be abated. Currently, when laser therapy is used, it is usually instituted in combination with other therapy. Further studies with long-term follow-up are needed to fully evaluate keloid and hypertrophic scar recurrence rate with laser therapy.
Interferon therapy has also been used for treatment, on the basis of the finding that interferons alpha, beta, and gamma inhibit collagen synthesis (collagen types I and III) in dermal fibroblasts.
Surgical revision
The commonest indications for the surgical removal of scars are as follows: large scars that are unlikely to be completely managed by medical therapy in a reasonable timeframe, scars that harbour painful furuncles, and scar contractures that hamper musculoskeletal function.29 Although the outcome of surgery may be desirable in the short post-operative term, the surgical revision of hypertrophic or keloid scars is followed by a high recurrence rate. Adjunctive measures to reduce inflammation, skin tension, and other factors are essential to reduce recurrence. The protocol for patients that require elective surgical excision is to identify effective scar control medications beforehand, and then to restart the medications immediately following surgery. A gentle surgical technique is also critically important, because inflamed scar tissue produces a tremendous scar response to trauma. The use of lasers and other burning techniques for scar removal is very controversial.
Case report
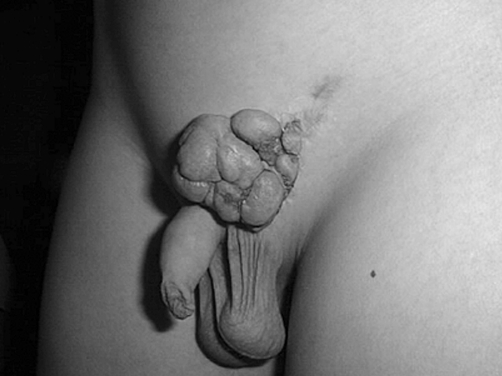
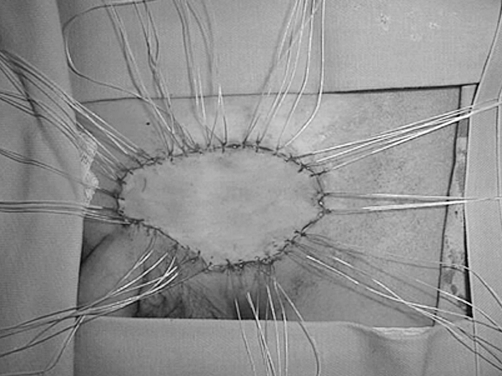
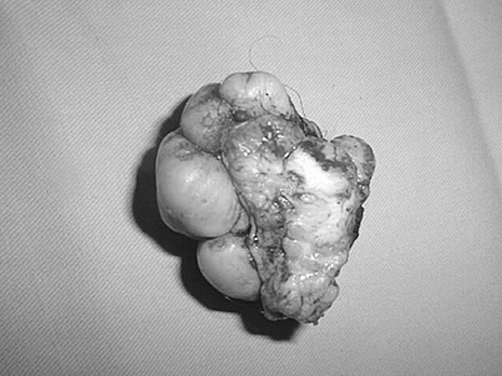
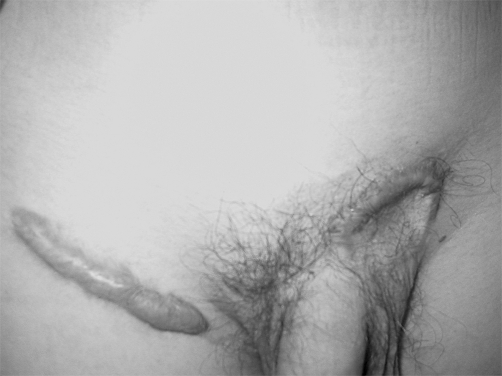
A 16-year-old boy (born in 1988) who developed a histologically documented keloid after left side hernioplasty (in 1996) twice underwent the surgical removal of a recurrent raised expanding keloid at the site of a previous excision within one year of surgical excision (1998). The last treatment option (by another surgeon) was surgical removal and full-thickness skin graft from the contralateral site (2001). It was possible to observe the extent of postoperative inflammation (partial dehiscence, redness). On admission, the patient was noted to have pruritus and pain. Within one year (2002) a keloid developed at the site of the full-thickness skin donation. According to our chart, the first step was to assess the size and symptoms of the keloid and the laxity of skin, in order to determine if the keloid could be excised in one stage or two stages. We noted that the defect would be too big to pull together and that it would be tight if we excised it in one stage, and we therefore decided to cut half out at a time, removing the other half after four months. Monthly corticosteroid intralesional injections (30 mg/ml) were used until the second part. After excision, the initial treatment included topical vitamin D3 (Diabonex) cream as an anti-inflammatory agent, and after checking for scar symptoms we used silicon gel (Dermatix Si Gel) and pressure therapy to accelerate scar maturation and degradation. The same steps were used in the second half. The final scar looked better than the original keloid.
Our charts were also used for hypertrophic scar prevention in patients with a predisposition for hypertrophic scarring who had undergone plastic operations.
Our charts were reviewed with regard to 10 patients who had undergone treatment for histologically documented keloid or hypertrophic scars and as prevention in 10 patients with post-plastic-operation risk factors. The initial treatments were individualized. The factors to consider when choosing the optimal treatment were the site and size of the lesions, scar duration, symptoms, and previous treatment (Fig. 1-5).
Fig. 1. Before first operation. Pre-operative view from anteroposterior (left) side.
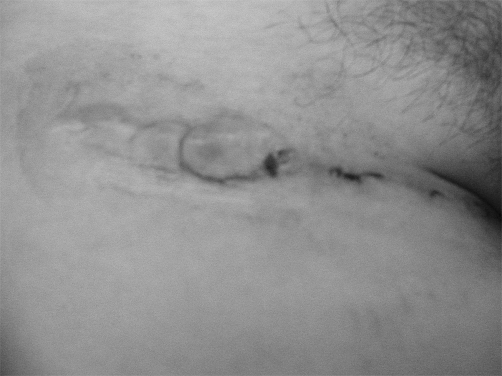
Fig. 5. After removal of suture (2 weeks post-op). Note the extent of inflammation.
Fig. 2. Before first operation. Pre-operative view from lateral (right) side.
Fig. 3. Intra-operative view. Full-thickness skin graft.
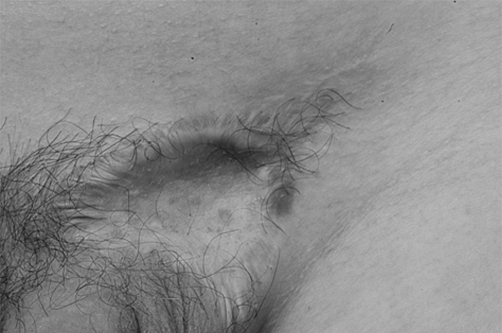
Fig. 4. Upside down view of excised keloid.
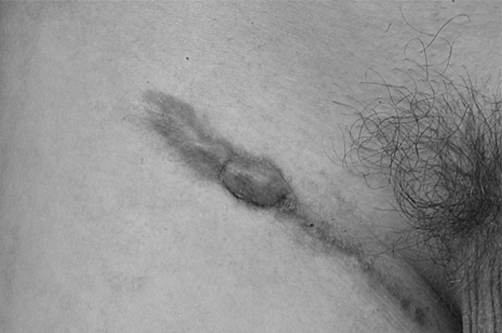
Fig. 6. Keloid recurrence one year post-op.
Fig. 7. After removal of suture, 2 weeks post-op and immediate treatment as described previously.
Fig. 8. Four weeks post-op. Surgical partial excision (according to our chart).
Fig. 9. Seven weeks post-surgical excision and other treatments as described.
Fig. 10. Showing use of silicon gel and pressure therapy.
Conclusion
As research continues on the initiation and control of the various stages of healing, it is important that we maintain up-to-date knowledge of the way advances in scientific research are being applied in the clinical setting. Pharmaceutical products are being incorporated into wound dressing, and we should be able to select and use these appropriately if we are to achieve some benefit for our patients. Despite recent scientific progress, scar-related disfigurement and disability remain major ongoing medical problems. Stripped of the conflicting and confusing terminology, hypertrophic and keloid scarring can be essentially reduced to inflammation-mediated dermal fibrosis. This suggests that much insight into effective management can be gleaned from the medical literature pertaining to dermatological and rheumatological conditions, which also have a large inflammatory gene expression component.
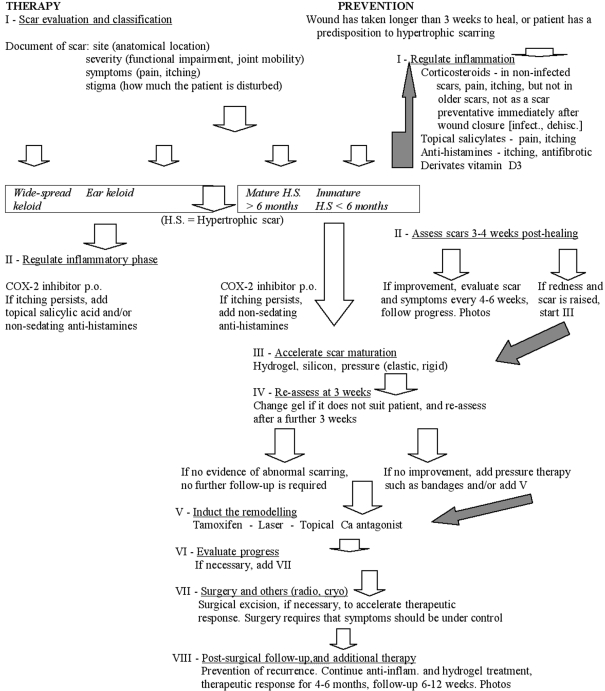
With these concepts in mind, we have designed several scar management protocols now under clinical evaluation. Under these protocols, summarized in the chart, patients with scars that are actively enlarging or itching are treated with either topical salicylates or oral COX-2 medications, or both, in order to control scar inflammation. The use of topical verapamil (5-10%) has shown great promise in inducing the degradation of older inactive scars and reduced the need of surgical intervention. Transepidermal delivery of these topical agents in scar management has made it possible to effectively treat scar conditions that previously were refractory to conventional therapy. We hope that this experience will lead to more effective therapeutic options for such patients.
In this article, we have attempted to briefly summarize and place into a conceptual framework the various existing and emerging therapies for hypertrophic scar disorders. We hope that this review will encourage the consistent use of less ambiguous terminology and more effective therapeutic strategies in the future.
It is probable that future developments in wound care will focus on the control mechanism involved in wounds and on the ways in which failures of these mechanisms impair healing. Control of wound healing is complex, and it is doubtful that any single solution to delayed healing will be found.
Chart. Keloid and hypertrophic scar management (by A. Edriss, based on scientific and clinical experience, but requiring a greater number of patients, long-time follow-up, and photographic documentation).
Acknowledgments
The Authors express their gratitude to Prof. R. Königová for her invaluable guidance
References
- 1.Santucci M., Borgognoni L., Reali U. Keloids and hypertrophic scars of Caucasians show distinctive morphologic and immunophenotypic profiles. Virchows Arch. 2001;438:457–463. doi: 10.1007/s004280000335. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph R., Vande Berg J., Ehrlich H.P. Cohen I.K., Diegelmann R.F., Lindblad W.J. Wound contraction and scar contracture. "Wound Healing: Biochemical and Clinical Aspects". W.B. Saunders Company; Philadelphia, PA: 1992. pp. 96–114. [Google Scholar]
- 3.Akasaka Y., Ishikawa Y., Ono I. et al.: Enhanced expression of Caspase-3 in hypertrophic scars and keloid: Induction of Caspase- 3 and apoptosis in keloid fibroblasts in vitro. Laboratory Investigation. 2000;80:345–347. doi: 10.1038/labinvest.3780039. [DOI] [PubMed] [Google Scholar]
- 4.Su C., Alizadeh K., Boddie A. et al. The problem scar. Clinics in Plastic Surgery . 1998;25:451–465. [PubMed] [Google Scholar]
- 5.Scherle H.P., Scholz D., Lemperle G. Elevated levels of testosterone receptors in keloid tissue: An experimental investigation. Plast. Reconstr. Surg. 1997;100:390–396. doi: 10.1097/00006534-199708000-00017. [DOI] [PubMed] [Google Scholar]
- 6.McCauley R.L., Chopra V., Li Y.Y. et al. Altered cytokine production in black patients with keloids. J. Clin. Immunol. 1992;12:300–308. doi: 10.1007/BF00918154. [DOI] [PubMed] [Google Scholar]
- 7.Sayah D.N., Soo C., Shaw W. et al. Downregulation of apopto-sis-related genes in keloid tissues. J. Surg. Res. 1997;87:209–216. doi: 10.1006/jsre.1999.5761. [DOI] [PubMed] [Google Scholar]
- 8.Tuan T.L., Nichter L.S. The molecular basis of keloid and hypertrophic scar formation. Molecular Medicine Today. 1998;4:19–24. doi: 10.1016/S1357-4310(97)80541-2. [DOI] [PubMed] [Google Scholar]
- 9.Boyce D.E., Ciampolini J., Ruge F. et al. Inflammatory-cell subpopulations in keloid scars. Brit. J. Plas. Surg. 2001;54:511–516. doi: 10.1054/bjps.2001.3638. [DOI] [PubMed] [Google Scholar]
- 10.Sclafani A.P., Gordon L., Chadha M. et al. Prevention of earlobe keloid recurrence with post-operative corticosteroid injection versus radiation therapy: A randomized, prospective study and review of the literature. Dermatol. Surg. 1996;22:569–574. doi: 10.1111/j.1524-4725.1996.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 11.Barnes P.J. Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clinical Sd. 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 12.Hawkes C.J. COX-2 inhibitors. Lancet. 1999;353 (9149):307–314. doi: 10.1016/s0140-6736(98)12154-2. [DOI] [PubMed] [Google Scholar]
- 13.McAdam B.F., Mardini I.A., Habib A. et al. Effect of regulated expression of human cyclo-oxygenase isoforms on eicosanod and isoeicosanoid production in inflammation. J. Clin. Invest. 2000;105:1473–1482. doi: 10.1172/JCI9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capelli-Schellpfeffer M., Lee R.C. Nonsteroidal anti-inflammatory (NSAID) medication reduces scar pruritus. Proceedings 3rd Meeting European Tissue Repair Society, 1999 Aug;:63–63. [Google Scholar]
- 15.Fitzpatrick R.E. Treatment of inflamed hypertrophic scars using intralesional 5-FU. Dermatol. Surgery. 1999;25:224–232. doi: 10.1046/j.1524-4725.1999.08165.x. [DOI] [PubMed] [Google Scholar]
- 16.Crowston J.G., Akbar A.N., Constable P.H. et al. Antimetaboliteinduced apoptosis in Tenon's capsule fibroblasts. Invest. Ophthalmol. Vis. Sci. 1998;39:449–454. [PubMed] [Google Scholar]
- 17.Ferguson M.W. Skin wound healing: Transforming growth factor beta antagonists decrease scarring and improve quality. J. Interferon Research. 1994;14:303–304. doi: 10.1089/jir.1994.14.303. [DOI] [PubMed] [Google Scholar]
- 18.Fulton J.E. Silicone gel sheeting for the prevention and management of evolving hypertrophic and keloid scars. Dermatol. Surg. 1995;21:947–951. doi: 10.1111/j.1524-4725.1995.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 19.Musgrave M.A., Umraw N., Fish J.S. et al. The effect of silicone gel sheets on perfusion of hypertrophic burn scars. J. Burn Care and Rehabil. 2002;23:208–214. doi: 10.1097/00004630-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Katz B.E. Silicone gel sheeting in scar therapy. Cutis. 1995;56:65–67. [PubMed] [Google Scholar]
- 21.Krieger L.M., Pan F., Doong H.et al. Thermal response of the epidermis to surface gels. Surgical Forum. 1993:738–740. [Google Scholar]
- 22.Ricketts C.H., Martin L., Faria D.T. et al. Cytokine mRNA changes during the treatment of hypertrophic scars with silicone and nonsilicone gel dressings. Dermatol. Surg. 1996;22:955–959. doi: 10.1111/j.1524-4725.1996.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 23.Philips T.J., Gerstein A.D., Lordan V.A. Randomized controlled trial of hydrocolloid dressing in the treatment of hypertrophic scars and keloids. Dermatol. Surg. 1996;22:775–778. doi: 10.1111/j.1524-4725.1996.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 24.Doong H., Dissanayake S., Gowrshankar T.R. et al. Calcium antagonists alter cell shape and induce procollagenase synthesis in keloid and normal human dermal fibroblasts. J. Burn Care and Rehabil. 1996;17:497–514. [PubMed] [Google Scholar]
- 25.Varedi M., Tredget E.E., Scott P.G. et al. Alteration in cell morphology triggers transforming growth factor B-1, collagenase, and tissue inhibitor of metalloproteinases-1 expression in normal and hypertrophic scar fibroblasts. J. Invest. Dermatol. 1995;104:118–123. doi: 10.1111/1523-1747.ep12613609. [DOI] [PubMed] [Google Scholar]
- 26.Lee R.C., Doong H., Jellema A. The response of burn scars to intralesional verapamil. Arch. Surg. 1994;129:107–111. doi: 10.1001/archsurg.1994.01420250119015. [DOI] [PubMed] [Google Scholar]
- 27.Norris J.E.C. Superficial X-ray therapy in keloid management: A retrospective study of 24 cases and literature review. Plast. Reconstr. Surg. 1995;95:1051–1055. doi: 10.1097/00006534-199505000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Watters D. Molecular mechanisms of ionizing radiation-induced apoptosis. Immunol. Cell Biol. 1999;77:263–271. doi: 10.1046/j.1440-1711.1999.00824.x. [DOI] [PubMed] [Google Scholar]
- 29.Sherris D.A., Larabee W.F. Management of scar contractures, hypertrophic scars, and keloids. Otolaryngol. Clin. North. Am. 1995;28:1057–1068. [PubMed] [Google Scholar]