Abstract
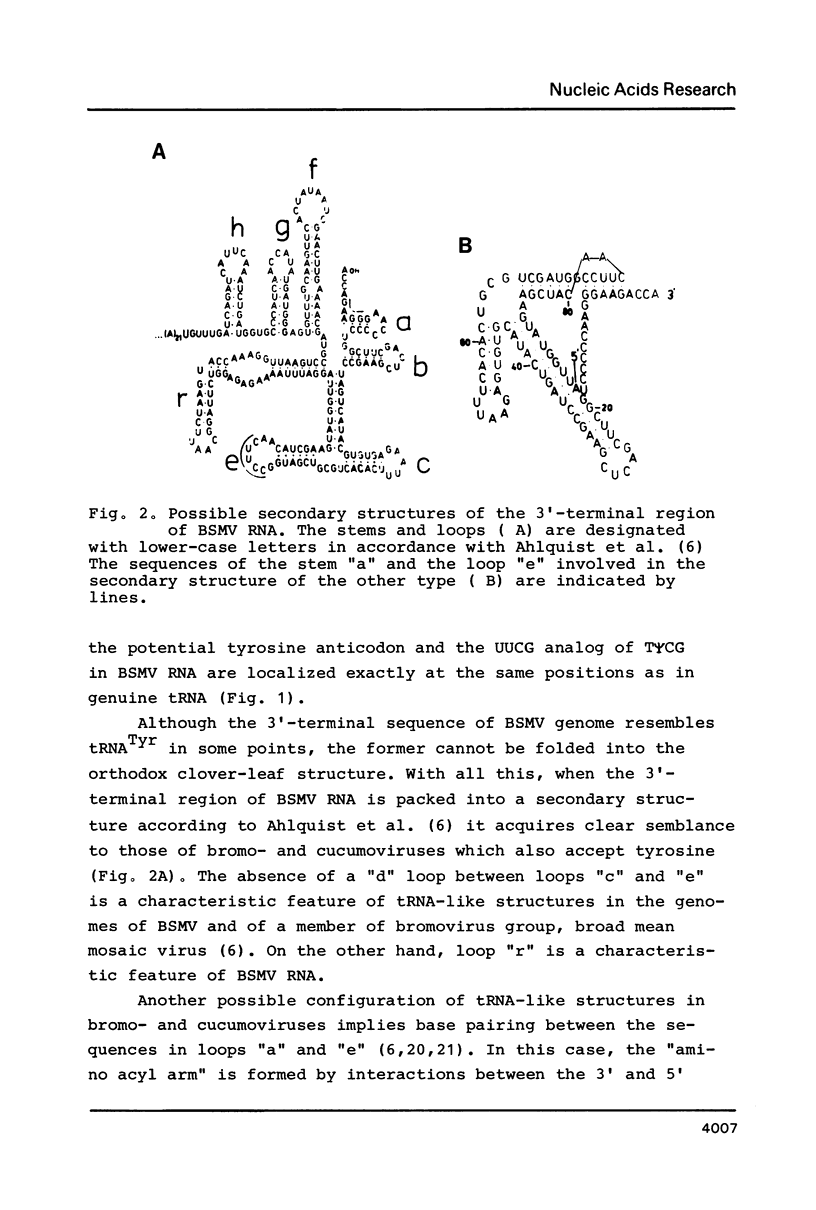
This paper describes the sequence of 257 nucleotides from the 3' end of RNA 2 of barley stripe mosaic virus ( BSMV , strain Argentina Mild) including an internal oligo (A) tract localized at a distance of 236 nucleotides from the 3' end, and the 3' terminal tRNA-like structure accepting tyrosine. This sequence is shown to be the same with RNAs 1,2 and 3 of another BSMV strain, Norwich , for at least the first 106 nucleotides from the 3' end. The 3' extremity of BSMV RNA bears some resemblance to tRNATyr from yeast in its primary structure. The possible secondary structures of the tRNA-like sequence in BSMV genome are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranovsky A. A., Dolja V. V., Kavsan V. M., Atabekov J. G. Detection of polyadenylate sequences in RNA components of barley stripe mosaic virus. Virology. 1978 Nov;91(1):95–105. doi: 10.1016/0042-6822(78)90358-6. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., Dasgupta R., Kaesberg P. Near identity of 3- RNA secondary structure in bromoviruses and cucumber mosaic virus. Cell. 1981 Jan;23(1):183–189. doi: 10.1016/0092-8674(81)90283-x. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., Luckow V., Kaesberg P. Complete nucleotide sequence of brome mosaic virus RNA3. J Mol Biol. 1981 Nov 25;153(1):23–38. doi: 10.1016/0022-2836(81)90524-6. [DOI] [PubMed] [Google Scholar]
- Darlix J. L. Stimultaneous purification of Escherichia coli termination factor rho, RNAase III and RNAase H. Eur J Biochem. 1975 Feb 21;51(2):369–376. doi: 10.1111/j.1432-1033.1975.tb03937.x. [DOI] [PubMed] [Google Scholar]
- Devos R., Van Emmelo J., Celen P., Gillis E., Fiers W. Synthesis by avian-myeloblastosis-virus RNA-dependent DNA polymerase of discrete reverse transcripts of bacteriophage RNA polyadenylated in vitro. Eur J Biochem. 1977 Oct 3;79(2):419–432. doi: 10.1111/j.1432-1033.1977.tb11824.x. [DOI] [PubMed] [Google Scholar]
- Dolja V. V., Negruk V. I., Novikov V. K., Atabekov J. G. A simple method for isolating pure RNA preparations after electrophoresis in polyacrylamide gel. Anal Biochem. 1977 Jun;80(2):502–506. doi: 10.1016/0003-2697(77)90673-x. [DOI] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Hall T. C. Transfer RNA-like structures in viral genomes. Int Rev Cytol. 1979;60:1–26. doi: 10.1016/s0074-7696(08)61257-7. [DOI] [PubMed] [Google Scholar]
- Joshi R. L., Joshi S., Chapeville F., Haenni A. L. tRNA-like structures of plant viral RNAs: conformational requirements for adenylation and aminoacylation. EMBO J. 1983;2(7):1123–1127. doi: 10.1002/j.1460-2075.1983.tb01556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., Chapeville F., Haenni A. L. Turnip yellow mosaic virus RNA is aminoacylated in vivo in Chinese cabbage leaves. EMBO J. 1982;1(8):935–938. doi: 10.1002/j.1460-2075.1982.tb01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld K., Pleij C. W., Bosch L. Three-dimensional models of the tRNA-like 3' termini of some plant viral RNAs. EMBO J. 1983;2(7):1079–1085. doi: 10.1002/j.1460-2075.1983.tb01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A. E. Purification and characterization of adenosine triphosphate: ribonucleic acid adenyltransferase from Escherichia coli. Eur J Biochem. 1973 Aug 1;37(1):31–40. doi: 10.1111/j.1432-1033.1973.tb02953.x. [DOI] [PubMed] [Google Scholar]
- Stern H., Westergaard M., Von Wettstein D. Presynaptic events in meiocytes of Lilium longiflorum and their relation to crossing-over: a preselection hypothesis. Proc Natl Acad Sci U S A. 1975 Mar;72(3):961–965. doi: 10.1073/pnas.72.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Palmieri M., Weissmann C. QB DNA-containing hybrid plasmids giving rise to QB phage formation in the bacterial host. Nature. 1978 Jul 20;274(5668):223–228. doi: 10.1038/274223a0. [DOI] [PubMed] [Google Scholar]


