Summary
Burn injury is associated with immune suppression and the subsequent development of sepsis. Severe burn injury is associated with depressed immune response, including a functional impairment of Th1 lymphocytes and natural killer cells and a decrease in interferon-a production. Dendritic cells (DCs) are potent antigen-presenting cells and play a key role in T cell activation; they are essential in coordinating the host response to pathogens. Using three-colour flow cytometry, we determined the percentage of lineage-negative LIN-DR+ DCs in burn patients and healthy subjects. The percentages of DCs were lower in the circulation of septic than in nonseptic patients and healthy subjects at all times examined (14 days) after burn injury. In contrast, the DC percentage in nonseptic patients was low at day 1, increased from day 3 to day 10, and reverted to normal levels at day 14. The data from the present study suggest that the DC percentage decreased early after burn injury. In addition, in the presence of severe sepsis, the DC percentage remained lower until day 14. This DC reduction may contribute to the immunosuppression observed after burn injury.
Keywords: DECREASE, CIRCULATING, DENDRITIC, CELLS, BURN, PATIENTS
Abstract
Les brûlures sont associées à la suppression immunitaire et au développement successif du sepsis. Les brûlures sévères sont associées à une réponse immunitaire déprimée, avec une altération fonctionnelle des lymphocytes Th1 et des cellules natural killer comme aussi une diminution de la production d'interféron-a. Les cellules dendritiques (CDs) sont des cellules puissantes présentatrices d'antigène qui jouent un rôle important dans l'activation des cellules T; elles sont essentielles pour la coordination de la réponse de l'hôte aux pathogènes. Utilisant la cytométrie en flux en trois couleurs, nous avons déterminé le pourcentage de CDs à lignage négatif LIN-DR+ chez les patients brûlés et les sujets sains. Les pourcentages des CDs étaient inférieurs dans la circulation des patients septiques par rapport à celle des patients non septiques et des sujets sains toutes les fois qu'ils ont été examinés (14 jours) après la brûlure. Par contre, le pourcentage des CDs chez les patients non septiques était bas le premier jour, augmentait du troisième au dixième jour et retournait aux niveaux normaux le quatorzième jour. Les données de cette étude suggèrent que le pourcentage des CDs diminuait bientôt après la brûlure. En outre, en présence d'un sepsis sévère, le pourcentage des CDs restait plus bas jusqu'au quatorzième jour. Cette réduction des CDs pourrait contribuer à l'immunosuppression observée après une brûlure.
Introduction
Immune suppression and the subsequent development of sepsis have been well recognized as a major consequence of burn injury.
Severe burn injury is associated with depressed immune response, including functional impairment of Th1 lymphocytes, natural killer (NK) cells and a decrease in IFN-a production.1-3 Dendritic cells (DCs) are the most potent antigen (Ag)-presenting cells (APCs) and play a role in the activation of both the innate and the adaptive immune responses. Immature DCs capture and process Ag in inflammatory tissues and subsequently they present the processed Ags to naive T cells in lymphoid tissues to generate effector T cells.
In vivo murine studies increasingly point to the importance of DCs in the resistance to burn wound infection, but little is known about the percentage of circulating DCs in burn patients.5 The purpose of this study was to compare the percentage of DCs in burn patients and in healthy subjects and to relate the number of DCs to the occurrence of sepsis. We show in this study that septic patients have a significant decrease in the percentage of DCs compared with nonseptic patients and healthy subjects.
Materials and methods
Patients
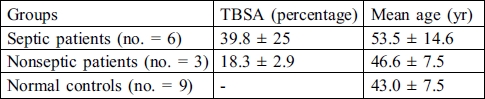
Peripheral blood was obtained from nine burn patients (eight males, one female; range of TBSA, 15-80%; mean age, 51.4 ± 13.6 yr) and nine healthy subjects (four males, five females; mean age, 43 ± 7.5 yr). Venous blood was collected into heparinized tubes from the patients and the healthy subjects. Patient samples were collected at 1, 3, 7, 10, and 14 days and processed for flow cytometry in order to study the percentage of DCs.
Antibodies
The MoAb used for staining of DCs was combined to prepare a lineage cocktail with FITC-labelled anti-CD3, anti-CD14, anti-CD19, anti-CD20, anti-CD56 MoAb, and PerCP-labelled anti-HLA-DR MoAb (all from Becton Dickinson, Mountain View, CA).
Flow cytometry analysis
After washing twice with PBS containing 1% BSA, 1 mM EDTA (Euroclone, UK), and 0.1% sodium azide (Sigma-Aldrich, St Louis, MO), the cells were resuspended in flow buffer, blocked with CD16/CD32 Fc Ab (Becton Dickinson, Mountain View, CA), and then stained. A cocktail of antibodies was used to identify LIN- cells, which lack CD3, CD19, CD14, CD20, and CD56 markers and which are DR+. Within the LIN- DR+ cells are the DC population.
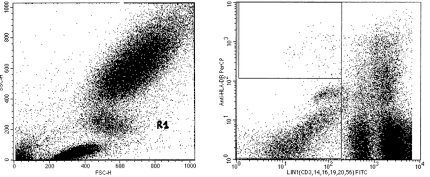
The gating strategy used to identify and quantify LIN- DR cells was as follows: cells in the lymphocyte-monocyte light scatter gate were evaluated for the expression of lineage markers LIN- FITC and DR- PerCP.
Three-colour flow cytometry analysis was performed using a FACS (Becton Dickinson). At least 500,000 events were acquired for each sample. DC percentages are expressed as a percentage of total mononuclear cells. The acquired data were analysed using the CellQuest software program (Becton Dickinson, Mountain View, CA).
Statistical analysis
Differences were analysed using unpaired Student's t-tests. Differences were considered significant at p < 0.05.
Results
Patient characteristics
The clinical characteristics of the burn patients and the characteristics of the control group are summarized in Table I.
Table I. Clinical characteristics of burn patients and healthy subjects.
The patients were classified as septic on the basis of one of the following criteria (signs and symptoms):
positive blood or tissue culture for bacteria or fungi
hyperthermia (> 38 °C)
impaired mental status
haemodynamic instability, usually requiring vasopressors
No differences in mean age were observed in the studied group. There was a significant difference in the patients' TBSA percentage associated with the development of sepsis. At the time of admission and for three days none of patients had been treated with antibiotics.
Percentage of LIN-DR+ DCs
The proportion of LIN-DR+ DCs was estimated as the percentage of cells in the R1 gate (Fig. 1).
Fig. 1. The gating strategy used to identify LIN– DR+ cells. Peri-pheral blood mononuclear cells obtained from patients and healthy subjects were stained with a lineage cocktail of FITC-conjugated MoAb (CD3, CD14, CD19, CD20, and CD56) plus a PerCP-conjugated anti-HLA DR MoAb. Cells in the lymphocyte-monocyte light scatter gate (A) were evaluated for expression of lineage markers LIN (FL-1) and DR (FL-2; B).
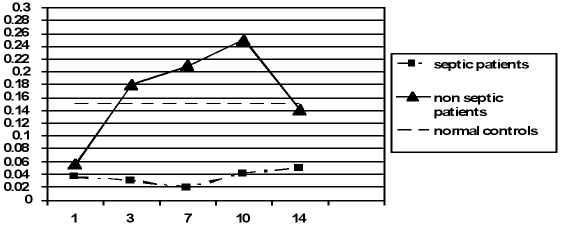
Fig. 2 shows the percentages of LIN-DR+ cells in the patients and healthy subjects at the indicated times. At day one a significant decrease in DC percentage was found in patients versus controls (p < 0.001), but no significant difference was observed between septic and nonseptic patients (0.036 ± 0.006 and 0.056 ± 0.01; p nonsignificant). The DC percentage increased from day 3 to day 10 only in nonseptic patients, indicating that these cells were present and able to induce the immune response; after day 10 the DC percentage in nonseptic patients decreased and at day 14 the DC levels were comparable to those of healthy subjects (0.14 ± 0.005 vs 0.15 ± 0.01; p nonsignificant). In contrast, septic patients showed a marked decrease in DCs in the peripheral blood at days 1, 3, and 7 after the burn injury. The DC percentage was significantly lower in septic patients than in nonseptic patients and healthy subjects (p < 0.001) and, when examined, was lower at all times after the burn injury.
Fig. 2. DC percentage in studied groups at the indicated days.
Taken together, these data suggest that the persistent lack of immunocompetent cells was the likely explanation for the defective immune response in the septic burn patients.
Discussion
In this study we observed a loss of circulating DCs in the burn patients compared to the healthy subjects. In particular, in septic patients the DC percentage was lower at all times in our study. In contrast, in nonseptic patients, these cells increased from day 3 to day 10, reverting to base levels at day 14.
Sepsis is a leading cause of mortality in severely burned patients and is associated with dysregulation of the immune system. Defects in innate and acquired immunity have been reported in septic patients7 and several reports have indicated that the Th1 immune response is compromised after burn injury.1-3 While DCs are clearly important in immune activation, little is known about their role in sepsis-induced immunosuppression.
DCs are potent APCs, in which they serve as a critical link between the innate and the acquired immune systems. DCs migrate throughout the body and act as sentinels by constantly sampling their environment. After encountering a stimulus/antigen, activation of DCs leads to their migration to peripheral lymph nodes, as well as their maturation, resulting in functional and phenotypic changes. Many of these phenotypic changes result in the DCs becoming a potent stimulator of lymphocytes.
Considering that DCs are the major initiators of a specific immune response to pathogens, we hypothesized that an alteration in the number of DCs might account for the immunosuppression associated with sepsis. To address this question, we evaluated the DC percentage of peripheral blood from burn patients.
We found that DCs in the peripheral blood were reduced after burn injury and remained lower in septic patients from day 1 to day 14. In contrast, in nonseptic patients this cell population was restored and even more abundant from day 3 to day 10 compared with healthy subjects. At day 14 the DC levels in nonseptic patients did not differ from those in healthy subjects.
In vivo murine and human studies increasingly point to the importance of the loss of DCs during sepsis. Previous studies have illustrated a specific decrease in the splenic DC population in septic human patients when compared with patients who have suffered trauma.10,11 Other research groups have demonstrated that there is a loss of DCs in local and distant lymph nodes. These investigations suggest that this contributes to the alteration in the acquired immune status of septic mice.
The overall loss of DCs during the progression of sepsis may contribute to suppression of the immune response - the loss of DCs may impair the host's capacity to contain and/or respond to the microbial infection and may contribute to the development of sepsis.
Apoptosis may be responsible for the decline in the DC population in peripheral blood from septic burn patients.14 In addition, it is possible that a lack of functional precursors contributes to the DC decline. It was reported in a murine burn and sepsis model that there was a significant decline in DC precursors after injury.15 Human studies have demonstrated a similar phenomenon, with peripheral blood monocytes having a decreased capacity to differentiate into immature DCs in trauma patients.
Conclusion
The data in the present study suggest that the DC percentage decreased soon after burn injury. In addition, in the presence of severe sepsis, the DC percentage remained lower until day 14. This DC reduction could contribute to the immunosuppression observed after burn injury. Our experiments did not address the role of DCs in the development of sepsis. However, on the basis of recent reports that indicate a role for DCs in the defence against bacterial infections and sepsis, it is feasible that DCs are required for the maintenance of the immune response and it is possible that strategies aimed at maintaining DC numbers can improve the final outcome.
References
- 1.O'Sullivan S.T., Lederer J.A., Horgan A.F., Chin D.H., Mannick J.A., Rodrick Major M.L. Injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann. Surg. 1995;222:482–482. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender B.S., Winchurch R.A., Thupari J.N., Proust J.J., Adler W.H., Munster A.M. Depressed natural killer cell function in thermally injured depressed natural killer cell function in thermally injured adults: successful in vivo and in vitro immunomodulation and the role of endotoxin. Clin. Exp. Immunol. 1988;71:120–120. [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki F., Pollard R.B. Alterations of interferon production in a mouse model of thermal injury. J. Immunol. 1982;129:1806–1806. [PubMed] [Google Scholar]
- 4.Banchereau J., Briere F., Caux C. et al. Immunobiology of dendritic cells. Ann. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 5.Toliver-Kinsky T.E., Cui W., Murphey E.D., Lin C., Sherwood E.R. Enhancement of dendritic cell production by FMS-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J. Immunol. 2005;174:404–410. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 6.O'Doherty U., Peng M., Gezelter S., Swiggard W.J., Betjes M., Bhardwaj N., Steinman R.M. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunobiology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- 7.Oberholzer A., Oberholzer C., Moldawer L.L. Sepsis syndrome: Understanding the role of innate and acquired immunity. Shock. 2001;16:83–83. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 8.Moll H. Dendritic cells and host resistance to infection. Cell Microbiol. 2003;5:493–500. doi: 10.1046/j.1462-5822.2003.00291.x. [DOI] [PubMed] [Google Scholar]
- 9.Efron P., Moldawer L.L. Sepsis and dendritic cell. Shock. 2003;20:386–386. doi: 10.1097/01.SHK.0000092698.10326.6f. [DOI] [PubMed] [Google Scholar]
- 10.Lipscomb M.F., Masten B.J. Dendritic cells: Immune regulators in health and disease. Physiol. Rev. 2002;82:97–97. doi: 10.1152/physrev.00023.2001. [DOI] [PubMed] [Google Scholar]
- 11.Hotchkiss R.S., Tinsley K.W., Swanson P.E., Grayson M.H., Osborne D.F., Wagner T.H., Cobb J.P., Coopersmith C., Karl I.E. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J. Immunol. 2002;168:2493–2493. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 12.Efron P.A., Martins A., Minnich D., Tinsley K., Ungaro R., Bahjat F.R., Hotchkiss R., Clare-Salzler M., Moldawer L.L. Characterization of the systemic loss of dendritic cells in murine lymph nodes during polymicrobial sepsis. J. Immunol. 2004;173:3035–3043. doi: 10.4049/jimmunol.173.5.3035. [DOI] [PubMed] [Google Scholar]
- 13.Tinsley K.W., Grayson M.H., Swanson P.E. et al. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J. Immunol. 2003;171:909–914. doi: 10.4049/jimmunol.171.2.909. [DOI] [PubMed] [Google Scholar]
- 14.Wesche D.E., Lomas-Neira J.L., Perl M., Chung C., Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J. Leukocyte Biol. 2005;78:325–337. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- 15.Sen S., Muthu K., He L.K., Daud A., Jones S., Gamelli R., Shankar R. Thermal injury and sepsis deplete precursor dendritic cells and alter their functions. J. Amer. Coll. Surg. 2003;197:S39–S39. [Google Scholar]
- 16.De A.K., Laudanski K. Failure of monocytes of trauma patients to convert to immature dendritic cells is related to preferential macrophage-colony-stimulating factor-driven macrophage differentiation. J. Immunol. 2003;170:6355–6355. doi: 10.4049/jimmunol.170.12.6355. [DOI] [PubMed] [Google Scholar]