Summary
Low molecular weight heparin (LMWH) appears to be a promising solution for reducing inflammatory post-burn episodes and results in improved healing. The clinical examination presented here includes patients with burn wounds ranging from 20 to 35% total body surface area (TBSA) who were categorized into two groups, of which one received subcutaneous LMWH treatment (10,000 units/day) and the other acted as control. The process of healing was assessed through regular examination of clinical features such as regression of erythema and oedema, eschar formation, and rate of re-epithelialization. Various studies have demonstrated an increase in levels of serum IL-6 indicating the severity of the morbid condition. In the present investigation, LMWH-treated patients exhibited a faster decline in levels of serum IL-6 (within 12 days) than control. Infiltration of inflammatory cells at the local wound site was assessed through a histological analysis of tissue samples taken on various days during the healing process. The LMWH-treated groups exhibited an organized healing pattern with better remodelling in a shorter duration (28 days), while control patients took more than 28 days for complete healing. A slight correlation was observed with TBSA to the inflammatory process, which subsided in patients treated with LMWH, favourably modulating the events involved in the inflammatory process of burn wound healing.
Keywords: molecular, weight, heparin-induced, pharmacological, modulation, burn
Abstract
L'héparine à bas poids moléculaire (HBPM) semble être une solution pleine de promesses pour la réduction des épisodes inflammatoires après les brûlures et pour l'amélioration de la guérison. L'examen clinique présenté ici comprend des patients atteints de brûlures qui oscillaient entre 20 et 35% de la surface corporelle totale (SCT), catégorisées en deux groupes, dont l'un recevait HBPM sous-cutanée (10.000 unités par jour) et l'autre servait comme groupe témoin. Le processus de la guérison a été évalué à travers un examen régulier des aspects cliniques comme la régression de l'érythème et de l'oedème, la formation de l'escarre et le taux de réépithélialisation. Diverses études ont démontré un incrément des niveaux de sérum IL-6 qui indiquent la sévérité de la condition de morbidité. Les Auteurs de cette étude ont trouvé que les patients traités avec HBPM présentaient une réduction plus rapide des niveaux de sérum IL-6 (entre 12 jours) par rapport au groupe témoin. L'infiltration des cellules inflammatoires au site local de la lésion a été évaluée moyennant une analyse histologique des échantillons tissulaires prélevés à divers moments pendant le processus de guérison. Les groupes HPBM présentaient un schéma organisé de guérison avec un remodelage meilleur en moins temps (28 jours), tandis que les patients du groupe témoin ont eu besoin de plus de 28 jours pour une guérison complète. Les Auteurs ont noté une légère corrélation entre la SCT et le processus inflammatoire qui diminuait dans les patients traités avec HPBM, ce qui modulait favorablement les événements intéressés au processus inflammatoire de la guérison des brûlures.
Introduction
Heparin has been extensively used in clinical practice as an anticoagulant for several decades. While most research studies pertaining to heparin have focused upon its role in the inhibition of blood coagulation, in more recent years the focus has broadened to include a range of antiinflammatory applications. 1, 2Despite increases in the understanding of the complete structural and functional interaction of heparin to elicit its wide range of activity, 1, 3, 4the synthesis of clinically useful heparin is a relatively recent achievement. Especially low molecular weight heparin (LMWH) preparations have a wider range of clinical applications than unfractionated heparin. 5, 6, 7Heparin in the treatment of burns with a complex local and systemic pathology has been used successfully over the years. 8, 9, 10Burned cells and tissue are destroyed as a result of direct thermal injury, and damage may progress from a secondary ischaemic process. Indirect destruction due to disturbances in the circulation, mediators of inflammation, increase in burn size and depth post-burn, and thrombosis causes burn wounds to heal slowly and imperfectly with scars and contractures. 11The effect of heparin in favourably modifying burn-induced inflammation is dose-dependent and dose-related. 12, 13The advantages of LMWH compared to standard unfractionated heparin include the improved pharmacokinetic profile, a half-life that is not dose-dependent, more constant anti-factor Xa inhibition, less platelet activity, and less inhibition of appropriate platelet aggregation; also, most importantly, their subcutaneous administration does not demand frequent coagulation determination. 14, 15Heparin consistently relieved burn pain and erythema and reduced the inflammatory increase in size with swelling or oedema in the local burn area. 16Moreover, heparin was shown to interfere with the key steps in leukocyte recruitment from the vasculature to the injury site, i.e. infiltration of inflammatory cells. 17, 18
During progressive healing of the burn wound, the production and activation of cells belonging to the immune system are regulated by a network of cytokines. 19Interleukin 6 (IL-6), a vital cytokine, has multiple functions, including modification of the regulatory effect of other cytokines, 20, 21and it reflects the severity of the morbid condition of the burn injury. 22It is well known that the interleukin signal proteins modulate the inflammatory response following trauma. 23, 24Modulation of these cytokine levels effectively regulates the interlinked pathways responsible for healing. Heparin has been investigated and proved to modulate several phases of wound healing, including angiogenesis. 25, 26, 27, 28It has a chemotactic effect on endothelial cells, with resultant stimulation of neovascularization 29and improvement of blood circulation subjacent to the burn, thus collectively inducing repair mechanisms.
We investigated the potential benefits of LMWH on burn wound healing in human patients with burns of different total body surface area (TBSA) by assessing the modulatory effect of LMWH on serum IL-6 levels. We also monitored several clinical parameters, including oedema, erythema, and rate of re-epithelialization after subcutaneous LMWH therapy.
Materials and Method
A controlled clinical assessment was carried out, after obtaining the patients' consent, performed in accordance with the ethical committee of Kilpauk Medical Collage and Hospital and with the Helsinki Declaration of 1975. The groups were picked by random trial and were subjective and non-objective. All clinical evaluations, including those of erythema and oedema, were recorded on a routine basis by regular clinical staff attending the patients.
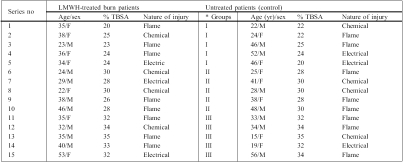
Immediately after admission, the patients were treated with a systemic antibiotic (ampicillin 1 g/twice daily) as chemoprophylaxis to prevent infection, and preliminary wound debridement was carried out, after which the patients were treated with LMWH. Further debridement was performed only in deep dermal injuries, if required. The investigation was carried out in 30 patients grouped in three categories based on their TBSA burned, i.e. superficial, partial, and deep partial-thickness burn wounds (group I: 25% TBSA; group II: 30% TBSA; group III: 35% TBSA) due to flame and chemical and electrical burns ( Table I ). In each group (n = 10), five patients were treated subcutaneously with 10,000 units/day of LMWH (average molecular weight, 4000 Daltons) for 10 days, while the others were treated as control and received routine medications. Blood samples were collected in the burn centre, and subsequent samples were taken on days 3, 5, 7, 10, and 12 from all patients. Serum was separated out and stored at -20 °C until further experiments. There was no sepsis either in LMWH-treated or control patients.
Table I. LMWH-treated and control burn patient demography.
Both groups of patients were observed form post-burn day 1 to 30.
* Groups were categorized on the basis of percentage TBSA:
Group I: 20-25%
Group II: 25-30%
Group III: 30-35%
Regression of oedema, absence of erythema, and eschar formation were assessed daily until they subsided. The rate of re-epithelialization was assessed by measuring the progression of the wound edge from the advancing edge, i.e. the distance between the weeping and the nonweeping surface edge expressed as the mean value in cm ± standard error of mean (mean ± SEM).
Punch biopsies (5 cm) were taken from the wound on post-burn days 5, 10, 15, 20, and 25 in both LMWH and control patients (group III). No single wound was biopsied more than twice so as not to affect the cosmetic outcome. Thin sections of 7m were cut and staining was performed using haematoxylin and eosin to identify inflammatory cell infiltration and to assess the progress of remodelling.
IL-6 levels in the serum samples collected on various days were assessed by enzyme-linked immunosorbent assay (ELISA) using the Human IL-6 ELISA kit of R&D systems (Minneapolis, USA). The tests were performed according to the manufacturer's instructions. The minimum detectable level of IL-6 with the assay kit was 0.70 pg/ml. Values were expressed as mean ± standard deviation pg/ml.
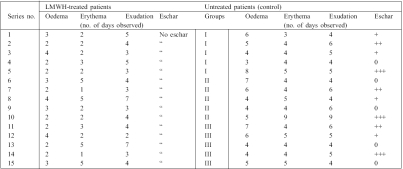
Table II. Clinical assessment of burn wound healing in LMWH-treated and control patients.
The difference between groups was considered significant if p < 0.05, evaluated by DMRT, one-way ANOVA.
Results
Percentage of burn area, depth of burn, and other presenting features such as oedema duration, the time taken for its regression, erythema surrounding the burn area, eschar formation, and rate of re-epithelialization were assessed. Oedema was more evident in the face, predominating over the eyelids and the narrowing of the palpebral fissures. In the extremities, measuring girth was the method of assessing oedema and swelling. In patients in the LMWH groups, oedema and erythema subsided within 5 days postburn, whereas in control patients these values were persistent for more than 5 days, declining after 8 days. Most interestingly, in all LMWH-treated groups, there were no signs or negligible signs of eschar formation, compared to control groups, where there was a positive sign of eschar formation (Table II).
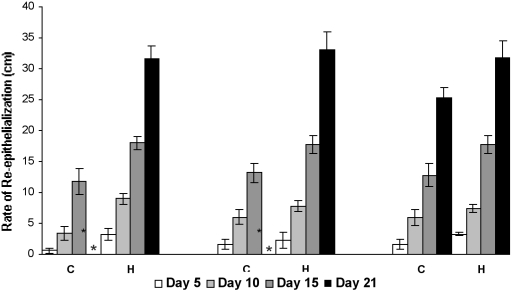
There was a prominent increase in the re-epithelialization rate in LMWH-treated groups compared to control patients. In all LMWH-treated groups, complete healing was observed within 28 days, whereas in control cases complete re-epithelialization took more than 28 days. In all LMWH-treated groups a two- to fourfold increase in the re-epithelialization rate was observed in all time intervals (Fig 1). Especially in group I of the LMWH-treated group, a five-fold increase in initial re-epithelialization (3.2 ± 0.95) was observed in comparison to group I control patients (0.6 ± 0.4).
Fig. 1. Rate of re-epithelialization in LMWH and control patients. Progression of wound edge from advancing edge expressed as mean value in cm ± standard error of mean (mean ± SEM). * Wounds in these patients (n = 4) took more than 28 days to heal completely.
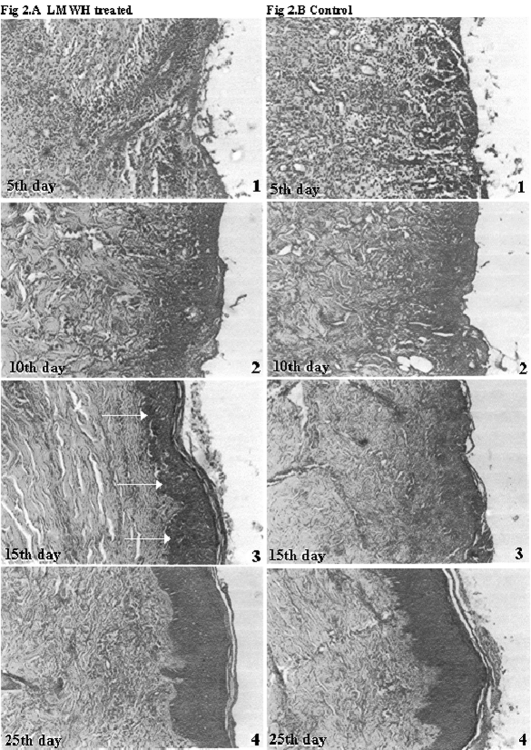
Tissue remodelling was observed in the histological sections (H & E stained) of biopsy samples. On post-burn day 5, control sections ( Fig. 2A ) showed an intense localized (granulomatous) inflammatory response, ulceration, and neutrophil cell accumulation, which persisted until day 15. Control sections taken on day 15 showed poor reorganization and epithelialization, with noticeable inflammatory cell infiltration. In LMWH-treated patients ( Fig. 2B ), on day 5, the presence of mild ulceration and fibrous exudate was evident, but on day 10 only sparse inflammatory cell distribution was seen compared to control. In contrast to control groups, a dense accumulation of fibroblasts was observed in LMWH-treated groups on day 15, with gradual replacement of the original matrix with newly synthesized collagen bundles. A complete remodelling with re-epithelialization was observed on day 25.
Fig. 2a,b. Histological sections represents healing pattern observed in LMWH-treated and control patients. Arrow in day 15 photograph of LMWH-treated group indicates margin of re-epithelialization.
Re-epithelialization and remodelling of epidermal and dermal compartments looked good in LMWH-treated patients in comparison to control patients.
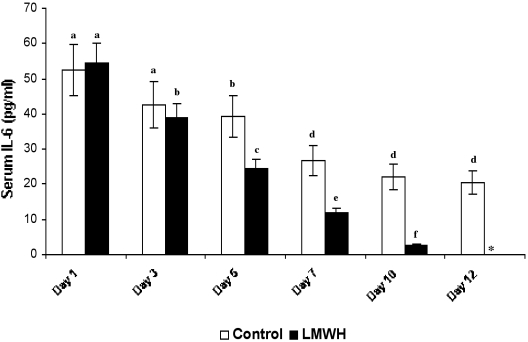
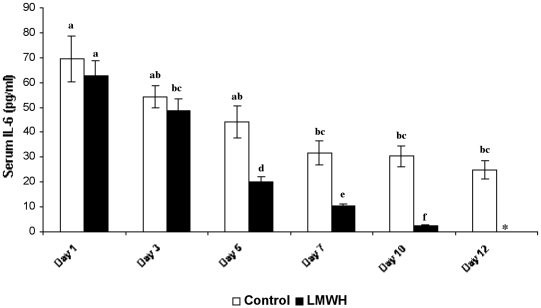
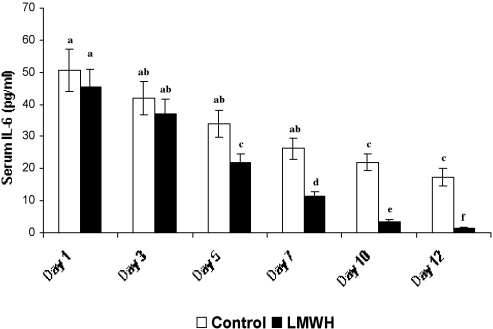
Serum IL-6 levels of both LMWH-treated and control groups were estimated under the detectable limits using the IL-6 kit provided by the manufacturer (sensitivity limit: minimum, 0.7 pg/ml). In patients with 25% TBSA ( Fig. 3 ), the initial circulating serum IL-6 levels were 52.64 ± 7.26 pg/ml and 54.62 ± 5.61 pg/ml in control and LMWHtreated patients respectively. No marked difference in IL 6 levels was observed between treated and untreated patients (observed in all groups) on day 3 post-burn. Figs. 4 and 5 show on day 5 a significant difference in levels of serum IL-6, especially with a considerable decline in LMWH-treated patients (24.29 ± 2.64 pg/ml, 21.98 ± 2.7 pg/ml and 20 ± 2.14 pg/ml in groups I, II, and III respectively) compared to control (39.24 ± 5.94 pg/ml, 33.96 ± 4.39 pg/ml, and 44.28 ± 6.42 pg/ml in groups I, II, and III respectively). After day 5 the IL-6 level in LMWHtreated patients progressively decreased, becoming undetectable after day 12 post-burn, whereas control patients still exhibited considerable levels of IL-6 levels even on day 12 post-burn (20.33 ± 3.30 pg/ml, 17.26 ± 2.7 pg/ml and 24.98 ± 3.14 pg/ml in groups I, II, and III respectively). Throughout our studies, Group III patients in both the control and the LMWH-treated group III exhibited maximum levels of serum IL-6.
Fig. 3. Serum IL-6 levels of LMWH-treated and control patients with 25% TBSA burns (n = 6). Similar letters in bar represent no significant difference at p < 0.05, evaluated by DMRT one-way ANOVA.
Fig. 4. Serum IL-6 levels of LMWH-treated and control patients with 30% TBSA burns (n = 6). Similar letters in bar represent no significant difference at p < 0.05, evaluated by DMRT one-way ANOVA.
Fig. 5. Serum IL-6 levels of LMWH-treated and control patients with 35% TBSA burns (n = 6). Similar letters in bar represent no significant difference at p < 0.05, evaluated by DMRT one-way ANOVA.
Discussion and conclusions
LMWH preparations, which have risen in popularity over the past decades, have a more attractive pharmacokinetic profile than unfractionated heparin. 30In humans, heparin decreased symptoms of pain and inflammation after injury. The average molecular weight of LMWH used in our investigation was 4,000 Daltons. Previous studies 31have shown that the efficiency of heparin to activate antithrombin towards the proteases depends on the chain length and sulphation pattern. It was also clearly demonstrated that a non-linear correlation existed between the strength of the AT-heparin complex and the molecular range, which is a good indicator of activation of factor Xa. Moreover, LMWH-treated patients have only a transient effect in the normal coagulation cascade, whereas its ability to modulate the inflammatory cascade is enhanced. In our LMWHtreatment investigation, erythema and oedema subsided by day 5 post-burn. It has been shown that LMWH significantly reduces oedema at the site of injury.1 Generally, burn size and depth increase post-burn, but in our investigations the size and depth of the burn never increased their initial burn size, which is in good correlation with earlier findings. 31It is well reported that heparin inhibits neutrophil rolling and accumulation at the site of injury. 33During the course of treatment with LMWH there were reduced celluare infiltration to areas of stasis and decreased local oedema at wound sites. LMWH does not affect coagulation immediately after destruction of vasculature - rather, it prevents further accumulation of inflammatory cells by preventing its infiltration at the site of injury. It also improves cellular patency by reducing the rolling and transmigration of inflammatory cells and thus reduces extravasation and further prevents occlusion of the microvasculature.
The histological pictures of day 15 in LMWH-treated patients show a well-defined margin of epidermal layer (bold arrows), whereas in control groups there is no clear demarcation between dermal and epidermal regions. The sparsely infiltrated inflammatory cells of LMWH-treated subjects, compared to control groups, indicates the enhanced healing process. Moreover, the dermal organization and epidermal layer are distinctly visible even at day 15, compared to control. Hence, the response of the host to LMWH actually improves the local cellular response towards necrotized sites. As a glycosaminoglycan, LMWH acts in vivo, improving tissue remodelling. It can be seen from the histological sections that the formation of collagen bundles is well organized and regularly oriented compared to control. The increased rate of re-epithelialization suggests that LMWH may well play a positive role in tissue remodelling, which is in good accordance with previous results. 27The early reduction in serum IL-6 levels in LMWH-treated patients, compared to control, indicates the involvement of LMWH in the modulation of the inflammatory pathway. The circulating serum IL-6 level is a good indicator of inflammatory progression in human burn patients, and this aspect has been addressed in some previous extensive studies. 23, 24, 34It has also been observed 35that IL-6 regulates the infiltration of various cells such as fibroblasts, endothelial cells, and especially leukocytes. The possible role of LMWH in the reduction of IL-6 levels may regard its ability to reduce the skin-homing of leukocytes to the site of injury. Since IL-6 is a pro-inflammatory cytokine, which acts as the prime modulator responsible for inducing changes in the expression of adhesion molecules, its occurrence during the course of the healing process could be detected until day till 12. 36The inflammatory reaction was marked during the time of admission, and a slight correlation between TBSA and circulating serum IL-6 levels was observable. Although patients with 25% and 30% TBSA did not show any remarkable difference between them in IL-6 levels, a significant difference could be seen compared to higher percentages of TBSA: patients with 25% TBSA showed 52.64 ± 7.26 pg/ml, while patients with 35% TBSA exhibited 69.6 ± 9.3 pg/ml of initial IL-6 levels. The observed values of serum IL-6 levels indicate that an increase in TBSA triggers an increased expression of IL-6, but the decline of its levels as the days progressed was less significant both in control and in LMWH-treated patients (in all groups), irrespective of the percentage of initial TBSA. It is quite evident that the initial decline of the major inflammatory cytokine level results in progressively better healing, with an improved clinical outcome. Moreover, earlier results 18indicate that heparin potentially inhibits neutrophil-derived elastase complement activation, TNF-a induced oedema, 37and neutrophil induced epithelial injury. 38TNF-a, IL-6, and IL-8 are known multifunctional markers relating the cellular and humoral mechanism involved in the inflammatory process. 39Hence, effective modulation of serum IL-6 levels through LMWH would have improved re-epithelialization by inhibiting inflammatory cell infiltration.
In our study design IL-6 was chosen as a marker to detect the inflammatory status, complemented with the histological study, which in combination gives a clear picture of the effect of LMWH in modulating the total inflammatory response to LWMH treatment. We have recently shown 40through our clinical experience with heparin that it favourably modulates burn-induced inflammation, consistently relieving pain. The current investigation clearly emphasizes the efficacy of LMWH in inducing faster healing of the burn wound through regulation of the proinflammatory cytokine IL-6 and by reducing the infiltration of inflammatory cells at the wound site, resulting in more organized remodelling of the burn wound than in control cases, which exhibited delayed regression of burn oedema, a decreased rate of re-epithelialization, and marked variation in IL-6 expression.
Acknowledgments
We express our sincere thanks to the Council of Scientific and Industrial Research, New Delhi, and to Dr T. Ramasami, Director, CLRI, for giving us the facilities and the opportunity to carry out this work.
References
- 1.Wang Q.L, Shang X.Y., Zhang S.L., Ji J.B., Cheng Y.N., Meng Y.J., Zhu Y.J. Effects of inhaled low molecular weight heparin on airway allergic inflammation in aerosol-ovalbumin-sensitized guinea pigs. Jpn. J. Pharmacol. 2000;82:326–30. doi: 10.1254/jjp.82.326. [DOI] [PubMed] [Google Scholar]
- 2.Campo C., Molinari J.F., Ungo J., Ahmed T. Molecular-weightdependent effects of non-anticoagulant heparins on allergic airway responses. J. Appl. Physiol. 1999;86:549–57. doi: 10.1152/jappl.1999.86.2.549. [DOI] [PubMed] [Google Scholar]
- 3.Lafuma C., Frisdal E., Harf A., Robert L., Hornebeck W. Prevention of leucocyte elastase-induced emphysema in mice by heparin fragments. Eur. Respir. J. 1991;4:1004–9. [PubMed] [Google Scholar]
- 4.Jaques L.B. Heparins - anionic polyelectrolyte drugs. Pharmacol. Rev. 1979;31:99–166. [PubMed] [Google Scholar]
- 5.Green D., Hirsh J., Heit J., Prins M., Davidson B., Lensing A.W. Low-molecular-weight heparin: A critical analysis of clinical trials. Pharmacol. Rev. 1994;46:89–109. [PubMed] [Google Scholar]
- 6.Lianchun W., Jillian R.B., Ajit V., Jeffrey D.E. Heparin's antiinflammatory effects require glucosamine 6-O-desulphation and are mediated by blockade of L- and p-selections. J. Clin. Invest. 2002;110:127–136. doi: 10.1172/JCI14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J.G., Mu J.S., Zhu H.S., Geng J.G. N-desulphated non-anticoagulant heparin inhibits leukocyte adhesion and transmigration in vitro and attenuates acute peritonitis and ischaemia and reperfusion injury in vivo. Inflamm. Res. 2002;51:1–9. doi: 10.1007/pl00012403. [DOI] [PubMed] [Google Scholar]
- 8.Wong G.C., Giugliano R.P., Antman E.M. Use of low-molecular weight heparins in the management of acute coronary artery syndromes and percutaneous coronary intervention. JAMA. 2003;289:331–42. doi: 10.1001/jama.289.3.331. [DOI] [PubMed] [Google Scholar]
- 9.Saliba M.J. Heparin efficacy in burns. II. Human thermal burn treatment with large doses of topical and parenteral heparin. Aerosp. Med. 1970;41:1302–6. [PubMed] [Google Scholar]
- 10.Saliba M.J. The effects and uses of heparin in the care of burns that improves treatment and enhances the quality of life. Acta Chir. Plast. 1997;39:13–16. [PubMed] [Google Scholar]
- 11.Saliba M.J. Heparin in the treatment of burns: A review. Burns. 2001;27:349–58. doi: 10.1016/s0305-4179(00)00130-3. [DOI] [PubMed] [Google Scholar]
- 12.Saliba M.J., Griner L.A. Heparin efficacy in burns. I. Significant early modification of experimental third-degree guinea pig thermal burn and ancillary findings. A double blind study. Aerosp. Med. 1970;41:179–87. [PubMed] [Google Scholar]
- 13.Saliba M.J. Jr, Dempsey W.C., Kruggel J.L. Large burns in humans. Treatment with heparin. JAMA. 1973;225:261–9. [PubMed] [Google Scholar]
- 14.Hull R.D., Raskob G.E., Brant R.F., Pineo G.F., Elliott G., Stein P.D., Gottschalk A., Valentine K.A., Mah A.F. Low-molecularweight heparin vs heparin in the treatment of patients with pulmonary embolism. American-Canadian Thrombosis Study Group. Arch. Intern. Med. 2000;16:229–36. doi: 10.1001/archinte.160.2.229. [DOI] [PubMed] [Google Scholar]
- 15.Hull R.D., Raskob G.E., Rosenbloom D., Pineo G.F., Lerner R.G., Gafni A., Trowbridge A.A., Elliott C.G., Green D., Feinglass J. Treatment of proximal vein thrombosis with subcutaneous lowmolecular weight heparin vs intravenous heparin. An economic perspective. Arch. Intern. Med. 1997;157:289–94. [PubMed] [Google Scholar]
- 16.Carr J. The anti-inflammatory action of heparin: Heparin as an antagonist to histamine, bradykinin and prostaglandin E1. Thromb. Res. 1979;16:507–16. doi: 10.1016/0049-3848(79)90097-5. [DOI] [PubMed] [Google Scholar]
- 17.Skinner M.P., Lucas C.M., Burns G.F., Chesterman C.N., Berndt M.C. GMP-140 binding to neutrophils is inhibited by sulphated glycans. J. Biological Chemistry. 1991;266:5371–4. [PubMed] [Google Scholar]
- 18.Fryer A., Huang Y.C., Rao G., Jacoby D., Mancilla E., Whorton R., Piantoadosi C.A., Kennedy T., Hoidal J. Selective O-desulphation produces non-anticoagulant heparin that retains pharmacological activity in the lung. J. Pharmacol. Exp. Ther. 1997;282:208–19. [PubMed] [Google Scholar]
- 19.Clark S.C., Kamen R. The human haematopoietic colony-stimulating factors. Science. 1987;236:1229–37. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- 20.Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S.C., Dinarello C.A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumour necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75:40–7. [PubMed] [Google Scholar]
- 21.Schluter B., Konig B., Bergmann U., Muller F.E., Konig W. Interleukin 6 - a potential mediator of lethal sepsis after major thermal trauma: Evidence for increased IL-6 production by peripheral blood mononuclear cells. J. Trauma. 1991;31:1663–70. doi: 10.1097/00005373-199112000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Yamada Y., Endo S., Inada K. Plasma cytokine levels in patients with severe burn injury - with reference to the relationship between infection and prognosis. Burns. 1996;22:587–93. doi: 10.1016/s0305-4179(96)00052-6. [DOI] [PubMed] [Google Scholar]
- 23.Drost A.C., Burleson D.G., Cioffi W.G., Jordan B.S., Mason A.D., Pruitt B.A. Plasma cytokines following thermal injury and their relationship with patient mortality, burn size, and time post-burn. J. Trauma. 1993;35:335–9. doi: 10.1097/00005373-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Guo Y., Dickerson C., Chrest F.J., Adker W.H., Munster A.M., Winchurch R.A. Increased levels of circulating interleukin-6 in burn patients. Clin. Immun. Immunopathol. 1990;54:361–71. doi: 10.1016/0090-1229(90)90050-z. [DOI] [PubMed] [Google Scholar]
- 25.Flint N., Cove F.L., Evans G.S. Heparin stimulates the proliferation of intestinal epithelial cells in primary culture. J. Cell Science. 1994;107:401–11. doi: 10.1242/jcs.107.2.401. [DOI] [PubMed] [Google Scholar]
- 26.Thomson R.C., Ludewia R.M., Wangensteen S.L., Rudolf L.M. Effects of heparin on wound healing. Surg. Gynecol. Obstet. 1972;134:22–26. [PubMed] [Google Scholar]
- 27.Kratz G., Back M., Arnander C., Larm O. Immobilized heparin accelerates the healing of human wounds in vivo. Scand. J. Plast. Reconstr. Hand Surg. 1998;32:381–5. doi: 10.1080/02844319850158462. [DOI] [PubMed] [Google Scholar]
- 28.Folkman J. Regulation of angiogenesis: A new function of heparin. Biochem. Pharmacol. 1985;34:905–9. doi: 10.1016/0006-2952(85)90588-x. [DOI] [PubMed] [Google Scholar]
- 29.Terranova V.P., Difloria R., Lyall R.M., Hic S., Friesel R., Maciag T. Human endothelial cells are chemotactic to endothelial cell growth factor and heparin. J. Cell Biol. 1985;101:2330–4. doi: 10.1083/jcb.101.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lever R., Page C. Novel drug development opportunities for heparin. Nature Rev. 2002;1:140–8. doi: 10.1038/nrd724. [DOI] [PubMed] [Google Scholar]
- 31.Sissi C., Naggi A., Torri G., Palumbo M. Effects of sulphation on antithrombin-thrombin/factor Xa interactions in semisynthetic low-molecular-weight heparins. Seminars in Thrombosis and Haemostasis. 2001;27:483–7. doi: 10.1055/s-2001-17959. [DOI] [PubMed] [Google Scholar]
- 32.Saliba M.J., Saliba R.J. Heparin in burns. Dose-related and dosedependent effects. Thromb. Diath. Haemorrh. 1975;33:113–23. [PubMed] [Google Scholar]
- 33.Nelson R.M., Cecconi O., Roberts W.G., Aruffo A., Linhardt R.J., Bevilacqua M.P. Heparin oligosaccharides bind L- and P-selection and inhibit acute inflammation. Blood. 1993;82:3253–8. [PubMed] [Google Scholar]
- 34.Ueyama M., Maruyama I., Osame M., Sawada Y. Marked increase in plasma interleukin-6 in burn patients. J. Lab. Clin. Med. 1992;120:693–8. [PubMed] [Google Scholar]
- 35.Rodriguez J.L., Miller C.G., Garner W.L., Till G.O., Guerrero P., Moore N.P., Corridore M., Normaolle D.P., Smith D.J., Remick D.G. Correlation of the local and systemic cytokine response with clinical outcome following thermal injury. J. Trauma. 1993;34:684–95. doi: 10.1097/00005373-199305000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Yeh F.L., Lin W.L., Shen H.D., Fang R.H. Changes in circulating levels of interleukin 6 in burned patients. Burns. 1999;25:131–6. doi: 10.1016/s0305-4179(98)00150-8. [DOI] [PubMed] [Google Scholar]
- 37.Hocking D., Ferro T.J., Johnson A. Dextran sulphate and heparin sulphate inhibit platelet-activating factor-induced pulmonary oedema. J. Appl. Physiol. 1992;72:179–85. doi: 10.1152/jappl.1992.72.1.179. [DOI] [PubMed] [Google Scholar]
- 38.Simon R.H., De Hart P.D., Todd R.F. Neutrophil-induced injury of rat pulmonary alveolar epithelial cells. J. Clinical Invest. 1986;78:1375–86. doi: 10.1172/JCI112724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Struzyna J., Pojda Z., Braun B., Chomicka M., Sobiczewska E., Wrembel J. Serum cytokine levels (IL-4, IL-6, IL-8, G-CSF, GMCSF) in burned patients. Burns. 1995;21:437–40. doi: 10.1016/0305-4179(95)00018-7. [DOI] [PubMed] [Google Scholar]
- 40.Ramakrishnan K.M., Babu M., Ramachandran K. Altered pattern of collagen synthesis in burns treated with both systemic and local heparin use that improved burn care. Burns:Multi-centre update. Proc. 10th Congress of International Society for Burn Injuries Jerusalem,Israel,Nov. 1998:11–21. [Google Scholar]