Summary
Pseudomonas aeruginosa has a notorious characteristic of resistance to most antimicrobial compounds. This characteristic was subjected to verification in the present study, whereby 50 human isolates of the organism from different pathological sources were subjected to sensitivity tests against honey from three different sources by the agar-cup diffusion method. Gentamicin, an aminoglycoside antibiotic normally with activity against Gram-negative bacteria, was used alongside honey. The 50 isolates of P. aeruginosa showed 100% sensitivity to each of the three types of honey tested in their undiluted form. This was not the case with gentamicin used in 8 and 4 µg/ml concentrations, both of which varied in their antipseudomonal activity, like the 1:2 aqueous dilution of each honey which failed to appreciably inhibit a lower number of pseudomonal isolates than either of the two concentrations of gentamicin. Honey is suggested as an effective natural product in overcoming the widespread antibiotic resistance of P. aeruginosa.
Keywords: antipseudomonal, property, honey, gentamicin
Abstract
Pseudomonas aeruginosa possède la caractéristique notoire de la résistance à la plupart des composés antimicrobiens. Les Auteurs, pour vérifier cette caractéristique, ont soumis 50 isolats humains de l'organisme, provenant de diverses sources pathologiques, à des tests de sensibilité contre du miel provenant de trois sources différentes, utilisant la méthode de la diffusion «agar cup». La gentamicine, un antibiotique aminoglycoside qui normalement est actif contre les bactéries à gram négatif, a été utilisé conjointement avec le miel. Les 50 isolats de P. aeruginosa démontraient une sensibilité de 100% à chacun des trois types de miel testé en forme non diluée. Ce n'était pas le cas de la gentamicine utilisée dans les concentrations de 8 et 4 µg/ml, qui variaient toutes les deux pour ce qui concerne leur activité antipseudomonale, comme aussi la dilution l:2 aqueuse de chaque miel qui ne réussissait pas à inhiber en manière appréciable un numéro inférieur d'isolats de type pseudomonal par rapport à l'un ou l'autre des deux concentrations de gentamicine. Le miel peut être proposé comme produit efficace naturel pour surmonter la résistance antibiotique diffuse de P. aeruginosa.
Introduction
Knowledge of the antimicrobial property of honey, primarily known as a nutritive food source, can be traced back to the early nineteenth century. This property was initially attributed to inhibine, 1but later hydrogen peroxide was identified as the inhibitory agent. 2Other antimicrobial factors subsequently suggested were low protein content, high C/N ratio, acidity, low redox potential, viscosity, and high osmotic pressure. 3, 4
The carbohydrate contents of glucose and fructose in honey account for its traditional use as a sweetener, as also its suitability for diabetics, athletes, and the elderly 5-hence honey's wide recognition as a food supplement owing to its higher rate of absorption than table sugar, its nutritive property, and its easy digestibility. 6, 7, 8, 9This explains the ubiquity of honey harvesters, collectors, and hawkers and their significant increase in Nigeria.
Honey's curative and antimicrobial effects against various diseases and infections have been well documented. 10, 11, 12Comparatively, it has been ranked higher in antibacterial effect on burn wounds than silver sulphadiazine. 13
Pseudomonas aeruginosa is a Gram-negative rod recognized as being amongst the "problem" bacteria on account of its resistance to most antimicrobial compounds. 14The organism is an opportunistic pathogen and has been isolated from pus, wounds, ears, and burns. It is involved in the aetiology of conjunctivitis, endocarditis, meningitis, and urinary tract infections.
Amongst the aminoglycosides, gentamicin, in combination with vancomycin or a penicillin, provides a good remedy in Gram-negative bacterial infections due particularly to P. aeruginosa, facilitated by enhanced drug uptake coupled with inhibition of cell wall synthesis. 15At a concentration of 4 µg/ml, gentamicin was observed in an in vitro experiment to effectively inhibit P. aeruginosa. 16Similarly, honey was reported to cause a rapid decline in bacteria and higher fungi such as Aspergillus niger. 4Specifically, P. aeruginosa was among three laboratory isolates that had their growth inhibited by honey. 12
Available reports do not indicate deliberate comparative studies on honey's antibacterial activity and standard antibiotics, a prerequisite before offering or suggesting a novel product as a therapeutic remedy. This work was designed along these lines with respect to the action of honey and gentamicin against clinical strains of P. aeruginosa.
Materials and methods
Bacteriology
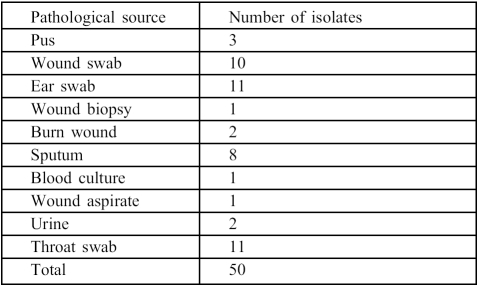
Fifty isolates of P. aeruginosa from various pathological sources ( Table I ) were obtained on sterile nutrient agar (Oxoid) slants from the routine section of the Medical Microbiology Laboratory, University College Hospital, Ibadan, Nigeria. They were re-isolated on cetrimide agar and subjected to conventional tests 17and then preserved on fresh nutrient agar slants in a refrigerator at 40 °C.
Table I. Pathological sources of P. aeruginosa.
Honey
Honey was obtained from three pure natural honey collection centres in Ibadan and Abeokuta, South West Nigeria. Each stock was used undiluted and also as 1:2 aqueous (aq.) dilution against each isolate of P. aeruginosa.
Gentamicin
Gentamicin sulphate BP, a product of Medreich, India, was obtained in ampoule vials (2 ml) from a local pharmacy store. It was used in 8 and 4 µg/ml (aq.) alongside honey against every pseudomonal isolate.
Sensitivity test
The agar-cup diffusion method 12was employed to obtain the susceptibility pattern of the respective pseudomonal isolates against each undiluted honey and its 1:2 aq. dilution, as also the 8 and 4 µg/ml of gentamicin. Considerations on the sensitivity and resistance of isolates were based on the extent or absence of zones of growth inhibition. 18
Results
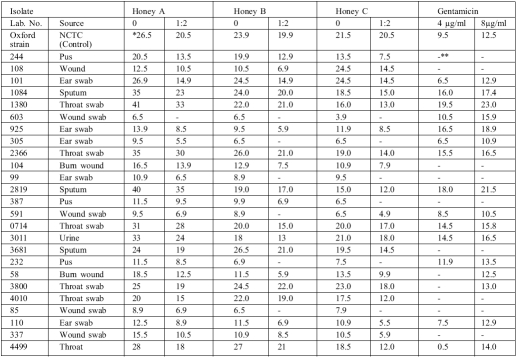
Undiluted honey from the three honey samples A, B, and C produced zones of growth inhibition for every pseudomonal isolate, varying from 5.5 to 41 mm and indicating 100% sensitivity of the clinical strains of P. aeruginosa to undiluted honey. However, gentamicin and the 1.2 aq. dilutions of the three honey samples varied in their growth inhibition ( Table II ).
Table II. Some results of the sensitivity test on honey and gentamicin against clinical isolates of P. aeruginosa.
NCTC = National Collection of Typed Culture (UK)
0 = indiluted honey
* = zone of inhibition in mm, indicating sensitivity of isolate
** = no zone of inhibition, indicating resistance of isolate
In honey sample A, only one isolate failed to be inhibited (2% resistance) by 1.2 aq. dilution, compared to nine isolates (18% resistance) in honey sample B, while honey sample C did not inhibit 10 isolates of P. aeruginosa (20% resistance) in its 1:2 aq. dilution. Comparatively, 4 µg/ml of gentamicin failed to inhibit 23 of the 50 pseudomonal isolates (46% resistance), while 21 isolates persisted in their growth against 8 µg/ml of gentamicin (42% resistance) ( Table III ).
Table III. Relative percentage resistance of clinical isolates of P. aeruginosa to honey and gentamicin.
* = undiluted honey
1:2 = diluted honey (1 ml honey mixed with 1 ml sterile distilled water)
0% = no resistant isolate
Discussion and conclusion
Pseudomonas aeruginosa has long been recognized as a major burn pathogen. 19 20It has increased its presence not only in burns but also in other forms of trauma. 21Of all the Gram-negative aerobic rods, Pseudomonas species are the most repeatedly encountered and are chronic or acute. 22
Previous reports 3 23on the inhibitory activity of honey on bacteria, particularly the Gram-negatives including P. aeruginosa, find support in the present study. This is evident in the 100% sensitivity of the pseudomonal isolates to the undiluted stock of the three honey samples tested. This activity was also shared by the 1:2 aq. dilution of each honey which, however, recorded a number of resistant isolates but fewer than the number recorded by either of 4 and 8 µg/ml of gentamicin. These contrasting results in favour of honey find analogies in the report of Molan 13on a higher antibacterial activity for honey than silver sulphadiazine in the treatment of bacterial infections of burn wounds. Variations in the inhibitory activity of 1:2 dilutions of the honey samples could be a reflection of differences in honey's antibacterial activity. 24It has been observed that honey is a sound topical wound-healing agent and that honey compound has equal and even better results as regards its antibacterial and antifungal properties and its wound healing promotion effects. 16With honey, the healing of burn wounds is faster and presents less scar formation. 25Honey has been described as a nectar of life and recommended as a therapy for wounds. It has been proved to be beneficial if applied immediately after a burn injury. It is cost-effective and free of toxicity and allergy. 26Notably, the fact that the strains of P. aeruginosa tested came from different human pathological sources lends credence to honey's therapeutic value.
In conclusion, honey - a natural product - could effectively complement standard antibiotics, especially in cases of recalcitrant infections due to P. aeruginosa in wounds in general and in burn wounds in particular, with beneficial healing effects.
Acknowledgments
We acknowledge the technical assistance rendered by Mr O.P. Ojo and Miss E.I. Okpekpe in the collection of bacterial isolates and some benchwork.
References
- 1.Sackett W.G. Honey as a carrier of intestinal diseases. International J. Food Microbiology. 1919;11:18–21. [Google Scholar]
- 2.White J.W., Kushnir I., Riethiof M.L., Subers M.H. Composition of American Honey. Agricultural Research Service, United States Department of Agriculture, Washington DC. 1962:1261. [Google Scholar]
- 3.Allen K.L., Molan P.C., Reid G.M. A survey of the antibacterial activity of some New Zealand honeys. J. Pharm. Pharmacology. 1991;43:817–822. doi: 10.1111/j.2042-7158.1991.tb03186.x. [DOI] [PubMed] [Google Scholar]
- 4.Tysett C., de Rautlin de la Roy Y. Assays on the study of osmophilic yeasts, organisms causing fermentation of honey collected in France. Faculty of Pharmacology, Univ. of Nancy Bull. 1993;134:1–26. [Google Scholar]
- 5.Bonvehi J.S., Jorda R.E. The microbiological quality of honey as determined by aerobic colony counts. J. Food Protec. 1993;56:336–7. doi: 10.4315/0362-028X-56.4.336. [DOI] [PubMed] [Google Scholar]
- 6.Crane E. The Archaeology of Beekeeping. Duckworth; London: 1983. pp. 11–13. [Google Scholar]
- 7.Swensson N., Sugivama H., Kuo J. Honey as food. J. Bees, FTP working paper. 1991;183 [Google Scholar]
- 8.Molan P. Antibacterial Activity and the Bee World. Waikato University Press; New Zealand: 1992. pp. 5–28. [Google Scholar]
- 9.Willix D.J., Molan P., Harfoot C.G. A comparison of the sensitivity of wound-infecting species of bacteria to the antibacterial activity of manuka honey and other species of honey. J. Appl. Bacteriol. 1999;73:388–94. doi: 10.1111/j.1365-2672.1992.tb04993.x. [DOI] [PubMed] [Google Scholar]
- 10.Obi C.L., Ugoji E.O., Edun S.A., Lawal S.F., Aniyiwo C.E. The antibacterial effect of honey on diarrhoea-causing bacterial agents isolated in Lagos, Nigeria. Afr. J. Med. Sci. 1994;23:257–60. [PubMed] [Google Scholar]
- 11.Cliver D.O., Snowdown J.A. Micro-organisms in honey. International J. Food Microbiology. 1996;31:1–26. doi: 10.1016/0168-1605(96)00970-1. [DOI] [PubMed] [Google Scholar]
- 12.Allen K.I., Radwan S., Reid G.M. Antimicrobial potential of honey on some microbial isolates. J. Medical and Pharmaceutical Sciences. 2000;2:75–9. [Google Scholar]
- 13.Molan P. The Curative Property of Honey: The Nature of Antibacterial Activity and the Bee World. Waikato University Press; New Zealand: 2000. pp. 10–15. [Google Scholar]
- 14.Geddes A.M. Ciprofloxacin Product Monograph. First printing, ADIS Press; New Zealand: 1986. Antibiotics and drug therapy in hospital. pp. 14–18. [Google Scholar]
- 15.Klika L.J., Goodman J.N. Antibiotic interactions. J. American Medical Association. 1982;248:1309. [Google Scholar]
- 16.Osman O.F., Mansour J.S., El-Hakim S. Honey compound for wound care: A preliminary report. Annals of Burns and Fire Disasters. 2003;16:131–4. [Google Scholar]
- 17.Cowan S.T. "Cowan and Steel's Manual for the Identification of Medical Bacteria" (2nd edition) Cambridge University Press; London: 1974. pp. 1–30. [Google Scholar]
- 18.Singleton P. “Bacteria in Biology, Biotechnology and Medicine” (4th edition) John Wiley & Sons Ltd; New York: 1999. pp. 333–8. [Google Scholar]
- 19.Artz C.P., Moncrief J.A. The Treatment of Burns. W.B. Saunders Co.; Philadelphia: 1969. p. 585. [Google Scholar]
- 20.Teplitz C. The pathology of burn and fundamentals of burn wound sepsis. In: Artz C.P., Moncrief J.A., Pruitt B.A. jr, editors. "Burns: A Team Approach". W.B. Saunders Co.; Philadelphia: 1979. pp. 45–94. [Google Scholar]
- 21.Heggers J.P., Barnes S.T., Robson M.C., et al. Microbiological flora of orthopaedic war wounds. Milit. Med. 1969;134:602. [PubMed] [Google Scholar]
- 22.Heggers J., Linares H.A., Edgar P., et al. Treatment of infections in burns. In: Herndon D.N, editor. Total Burn Care. W.B. Saunders Co.; Philadelphia: 1996. pp. 98–135. [Google Scholar]
- 23.Singh N., Parminder K. Quality evaluation of different types of Indian honey. Food Chemistry. 1996;58:129–33. [Google Scholar]
- 24.Smith M.R., McCaughey W.F., Kemmerrer A.R. Biological effects of honey. J. Api. Res. 1969;8:99–110. [Google Scholar]
- 25.Subrahmanyam M., Shahapure A.G., Nagame N.S., et al. Effects of topical application of honey on burn wound healing. Annals of Burns and Fire Disasters. 2001;14:3–5. [Google Scholar]
- 26.Subrahamanyam M., Shahapure A.G., Nagame N.S., et al. Free radical control - the mechanism of the action of honey in burns. Annals of Burns and Fire Disasters. 2003;16:135–7. [Google Scholar]