Summary
Many studies have reported that zinc plasma levels significantly decrease after a burn, leading to zinc deficiency, and that increased free radical generation and decreased natural antioxidant may negatively affect wound healing and burn outcome in general. Targeting of these changes is considered an important strategy in the treatment of burns in an attempt to improve burn outcome in the clinical setting. Zinc was given orally in a nutritional dose (15 mg elemental zinc) as a zinc sulphate capsule to burn patients in order to improve post-burn zinc deficiency and burn outcome. The study was carried out in 58 burn patients of different age groups, sex, and occupation with different burn size. The patients were allocated to two groups: group A patients (43 in number) were treated with topical povidone-iodine ointment for the first four days post-injury followed by topical silver sulphadiazine cream 1% until discharge in addition to other prescribed drugs according to our burn unit policy; group B patients (15) received the same treatment as group A plus a single daily oral dose of zinc sulphate in a 66 mg capsule, equivalent to 15 mg elemental zinc. In each group, using standard methods, we considered plasma zinc and copper levels, oxidative stress parameters, thyroid, liver, and renal function tests, microbiological factors, mortality rate, healing time, and cost effectiveness. The administration of zinc in dietary doses significantly increased the plasma zinc level in burn patients to around normal control levels and improved the antioxidant status, as represented by elevation of the natural antioxidant level (glutathione), in addition to improving healing time, the incidence of eschar formation, and the mortality rate, compared with the zinc-nonsupplemented group. We conclude that dietary zinc supplementation in zinc-deficient burn patients led to great improvements in their outcome and that zinc deficiency was as an important goal to target during treatment; also, that the use of a combination of topical and systemic antioxidants (povidoneiodine ointment and zinc sulphate, respectively) represented a good strategy for improving results in burn patient treatment.
Keywords: zinc, supplement, prognosis, burn, patients, iraq
Abstract
Beaucoup d'études ont trouvé que le niveau plasmatique du zinc diminue en manière significative après une brûlure, ce qui cause une déficience de zinc, et que la génération augmentée des radicaux libres et la réduction des antioxydants naturels peuvent influencer négativement la guérison des lésions et les résultats des brûlures en général. Prendre pour cible ces modifications est considéré une stratégie importante dans le traitement des brûlures dans la tentative d'améliorer les résultats des brûlures dans le cadre clinique. Les Auteurs ont administré à des patients brûlés le zinc par voie orale en dosage nutritionnel (15 mg zinc élémentaire) en forme de capsule de sulfate de zinc dans le but d'améliorer la déficience post-brûlure du zinc et les résultats finals. L'étude a pris en considération 58 patients brûlés de différent âge, sexe et occupation atteints de brûlures de dimension diverses. Les patients ont été divisés en deux groupes: les patients du groupe A (43) ont été traités avec l'onguent de povidone-iode topique pendant les quatre premiers jours après la brûlure, suivi par la crème de sulfadiazine d'argent topique 1% jusqu'à la sortie de l'hôpital, en plus des autres médications prescrites par le protocole de l'unité des brûlures; les patients du groupe B (15) ont reçu le même traitement que le groupe A avec en outre une dose orale quotidienne de sulfate de zinc dans une capsule de 66 mg, équivalente à 15 mg de zinc élémentaire. Dans chacun des deux groupes, les Auteurs ont considéré, en utilisant les méthodes standard, les niveaux de zinc et de cuivre plasmatique, les paramètres de stress oxydatif, les tests de fonction thyroïdienne, hépatique et rénale, les facteurs microbiologiques, le taux de mortalité, le temps de guérison et l'efficacité coût. L'administration de zinc en dosage diététique a augmenté en manière significative le niveau plasmatique de zinc chez les patients brûlés jusqu'à approximativement les niveaux normaux témoins et a amélioré l'état antioxydant, représenté par l'élévation du niveau antioxydant naturel (glutathion), en plus d'améliorer le temps de guérison, la fréquence de la formation des escarres et le taux de mortalité par rapport au groupe non traité avec le zinc. Les Auteurs concluent que l'addition du zinc diététique, chez les patients qui présentent une déficience de zinc, a porté à de grandes améliorations dans les résultats et que la déficience de zinc est un but important d'attaquer pendant le traitement; en outre, l'emploi d'une combinaison des oxydants topiques et systémiques (povidone-iode topique et sulfate de zinc, respectivement) constitue une bonne stratégie pour l'amélioration des résultats dans le traitement des patients brûlés.
Introduction
It is well known that burn patients present increased nutritional requirements associated with their resultant hypermetabolic state. 1Among the nutrients required for supplementation are trace elements, which require greater attention because stress alters their level owing to alterations in intestinal absorption, in body losses, in the distribution of body proteins, and in protein concentration. 2The various trace elements have a specific role to play during healing and perform vital functions in the body. They are directly involved in free radical scavenging and are potentially important in burns. Trace element status is altered after major burns, especially during the first week postinjury, when the serum level is severely decreased. 3
Burn wound healing is a complicated process that requires several steps, and any alteration at any point may delay the healing process. In cutaneous thermal injury, several factors can increase tissue damage. Among these, the oxygen free radicals are important. 4Oxidative stress contributes to secondary tissue damage and impaired immune functions after burn injury. 5
As a consequence of these considerations, the targeting of changes occurring after burn injury (e.g. increase in free radical generation, decrease in natural antioxidants, immune suppression, and decreased levels of trace elements, which collectively may lead to delay in wound healing) is an important strategy in burns treatment in attempts to improve outcomes in the clinical setting. One of the famous trace elements widely used in the clinical setting for this purpose is zinc.Zinc is an essential trace element in the body, present in all organs, tissues, and body fluids. 6Zinc is needed for more than 300 enzymes used by the body that are responsible for conducting diverse functions such as wound healing, fertility, protein synthesis, cellular reproduction, vision, immunity, free radical protection, DNA replication, and gene transcription. At the cellular level, zinc is critical for cell survival, affecting signal transduction, transcription, and replication. 7Zinc thus plays an essential role in growth immune function, antioxidant defence, and wound healing. 8
Zinc deficiency leads to many pathological signs, e.g. growth failure, neuropathy, diarrhoea, dermatitis, hair loss, bleeding tendency, hypotension, and hypothermia. Zinc deficiency states are associated with anorexia and alterations of the epidermal, gastrointestinal, central nervous, immune, skeletal and reproductive systems. 9Zinc deficiency has been shown to reduce osteoblastic activity, collagen, and proteoglycan synthesis, as well as alkaline phosphatase activity. 10
In burn patients, many studies have reported that the zinc plasma level is significantly decreased post-injury, 11and that this is associated with increased urinary zinc excretion. 12It has been reported that zinc is normally transported in plasma bound to proteins, while post-burn these proteins are broken down owing to an increased catabolic rate and tissue damage. 13Without proteins to bind it, zinc may be lost in the urine. 14
Conversely, Voruganti et al. 15showed that in burn patients zinc concentration in wound exudates exceeded plasma concentration, suggesting that the primary reason for the low plasma zinc level might be due to elevated loss of zinc through wound exudates. In contrast, Agay et al. 16showed that serum zinc level significantly decreased 6 h after burn injury owing to redistribution to the liver.
Zinc’s antioxidant effect
Zinc's ability to retard oxidative processes has long been recognized. In general, the mechanism of antioxidation can be divided into acute effects and chronic effects. The chronic effects involve exposure of an organism to zinc on a long-term basis, resulting in induction of some other substance that is the ultimate antioxidant, such as the metallothioneins. Chronic zinc deprivation generally results in increased sensitivity to oxidative stress. The acute effects involve two mechanisms: protection of protein sulphydryls or reduction of OH formation from H 2O 2through the antagonism of redox-active transition metals, such as iron and copper. Protection of protein sulphydryl groups is thought to involve reduction of sulphydryl reactivity through one of three mechanisms: 1. direct binding of zinc to the sulphydryl; 2. steric hindrance as a result of binding to some other protein site in close proximity to the sulphydryl group; 3. a conformational change from binding to some other site on the protein. Antagonism of redox active, transition metal-catalysed, site-specific reactions has led to the theory that zinc may be capable of reducing cellular injury that might have a component of sitespecific oxidative damage, such as post-ischaemic tissue damage. Zinc is capable of reducing post-ischaemic injury to a variety of tissues and organs through a mechanism that may involve the antagonism of copper and iron reactivity. 17, 18
In our study zinc was given orally in a nutritional dose (15 mg elemental zinc) as zinc sulphate salt to burn patients to improve their post-burn zinc deficiency. This may improve burn outcome as a result of its antioxidant and immunomodifier effect and its beneficial effect on wound healing.
Patients and methods
This study was conducted on 58 patients (27 males and 31 females) of varying age (6-67 yr) (mean, 35.6 ± 19.4, ± SD), with a burn percentage ranging from 15 to 70% estimated according to the rule of nine and a burn degree of first to third. The causes of the burns were direct flame in 45 patients (77.5%) and hot water in 13 patients (22.5%).
The patients admitted to the burn unit in the Department of Surgery in Baquba General Hospital, Diyala, Iraq over a period of six months.
The patients were allocated to two groups:
Group A: 43 patients (20 males and 23 females), treated according to the hospital's therapeutic policy with topical povidone-iodine ointment 10% (PVP-I) for the first four days of admission followed by topical silver sulphadiazine cream 1% (SSD) until discharge day, in addition to other prescribed drugs determined by burn unit policy.
Group B: 15 patients (7 males and 8 females), treated with the same treatment as Group A, plus a single daily oral dose of a capsule containing 66 mg zinc sulphate, equivalent to 15 mg elemental zinc (which is the recommended dietary allowance). 19This was taken from the first day of admission until discharge day, in the morning with a cup of water.
In addition, 12 healthy subjects (5 males and 7 females), with the same age range as that of the patients, were selected to serve as control for basic comparison.
Sample collection and preparation
Blood samples were collected from all subjects by venipuncture. Ten millilitres were taken on admission (zero time) to the burns unit and then every two days of treatment and on discharge day to check changes in the parameters studied. All blood samples were collected in a heparinized plain tube. Erythrocytes were separated by centrifugation at 3000 rpm for 10 min at 4 °C; the plasma obtained was used for biochemical analysis, which included:
1. Determination of plasma zinc and copper
For the measurement of plasma zinc levels, the plasma sample was diluted fivefold with deionized water and aspirated into an atomic absorption spectrophotometer. Calculations were performed by comparison with a standard zinc chloride solution prepared for the purpose. 20For copper determination, the plasma sample was diluted with an equal volume of deionized water and directly aspirated into the atomic absorption instrument, and the amount of copper was calculated by comparison with a standard copper sulphate solution treated in the same manner. 21Results were expressed as mg/dl.
2. Measurement of oxidative stress parameters
• measurement of the plasma malondialdehyde (MDA) level: MDA is a by-product of lipid peroxidation and its measurement is based on the reaction of thiobarbituric acid (TBA) with MDA, forming TBA2-MDA adducts, according to the standard method of Stocks and Dormandy 22modified by Gilbert et al.; 23
• measurement of the plasma glutathione (GSH) level: GSH contents (measured as total sulphydryl groups) were measured according to the method of Godin et al. 24
3. Thyroid function test: in each subject triiodothyronine (T3) and thyroxin (T4) plasma levels were measured by enzyme immunoassay according to the methods of Utiger 25and Wistom 26respectively; a ready-made kit was used for the purpose (BioCheck, Burlingame).
4. Liver function test: alkaline phosphatase activity was measured colorimetrically according to the method of Kind et al., 27utilizing a ready-made kit for the purpose (BioMerieux, France). Also, the activities of glutamate-pyruvate transaminase (SGPT) and glutamate-oxaloacetate transaminase (SGOT) enzymes were evaluated colorimetrically according to the method of Reitman 28utilizing a ready-made kit for the purpose (RANDOX, UK).
5. Renal function test
• blood urea: the determination of serum urea level was performed using the urease-modified Berthelot reaction 29utilizing a ready-made kit for the purpose;
• serum creatinine: serum creatinine was evaluated utilizing a ready-made kit for the purpose (Biomaghreb, Tunisia), according to the method of Henry. 30
6. Wound swabs for microbiological examination 31and characterization of the invading micro-organism, if present, were taken on admission and at two-day intervals during follow-up until discharge.
7. Mortality rate, 32healing time (reported as the time required for complete healing of the burn wound without any sign of infection), 33and cost of treatment were calculated on a per course of treatment basis. 34
8. Statistical analysis
• results were expressed as mean ± SD;
• the Student t-test was used to examine the degree of significance, and a p value of less than 0.05 was considered significant.
Results
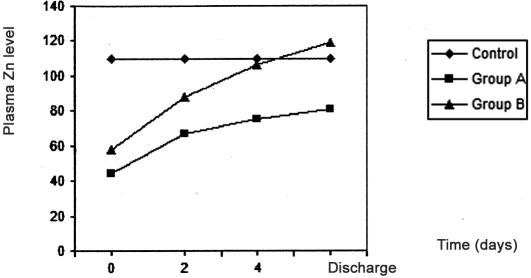
As shown in Table I and Fig. 1 the plasma zinc levels in burn patients were significantly reduced (p < 0.05) compared with healthy control subjects; the lowest plasma zinc value was at zero time (time of resuscitation) in both group A and group B compared with control. In group A, plasma zinc levels increased nonsignificantly after two days, four days, and at discharge time, while in group B (the zinc-supplemented group) plasma zinc levels significantly increased (p < 0.05) four days after initiation of treatment and at discharge time, reaching a highest value that was slightly more than that of control ( Fig. 1 ).
Table I. Effects of treatment in group A and group B on zinc and copper plasma levels and oxidative stress parameters in burn patients.
Group A: burn patients treated with topical povidone-iodine ointment for the first 4 days after admission followed by topical silver sulphadiazine cream in addition to other prescribed drugs.
Group B: burn patients treated as in group A with the addition of single daily oral dose of 66 mg zinc sulphate (15 mg elemental zinc).
Results represent mean ± standard deviation.
Results with non-identical superscripts were considered significantly different (p < 0.05).
Zero time represents time of resuscitation.
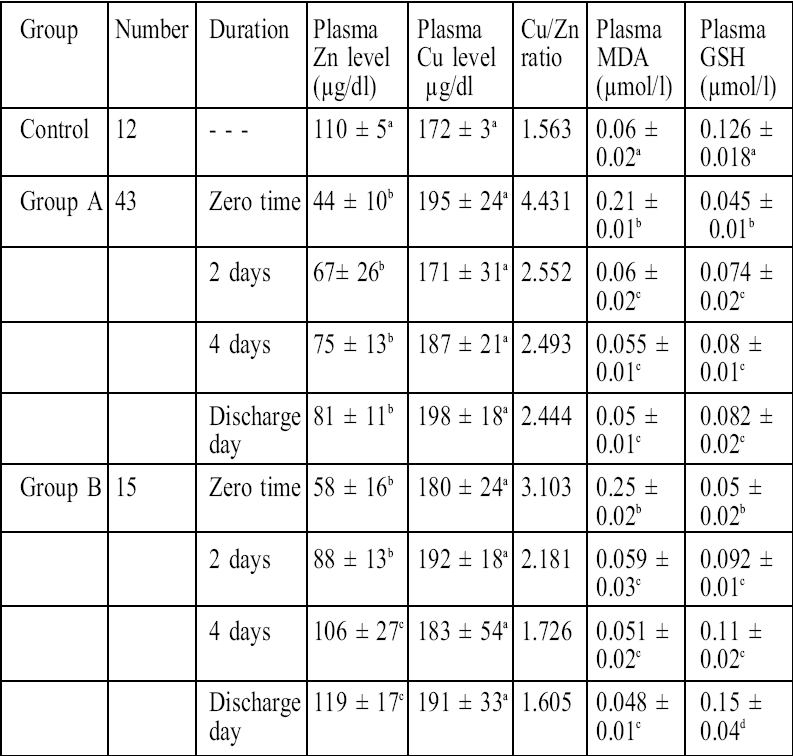
Fig. 1. Effects of treatment in group A and group B on plasma zinc level in burn patients.
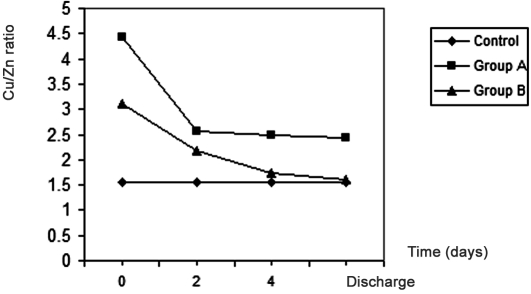
In contrast, plasma copper level showed nonsignificant changes in burn patients compared with control in both groups. The Cu/Zn ratio showed a 183% increase in group A burn patients at zero time, an increment that became 56% at discharge time compared with the control value, while in group B (the zinc-supplemented group) the elevation in the Cu/Zn ratio dropped from 98% at zero time to 2% at discharge time, which was the normal ratio Fig. 2 .
Fig. 2. Effects of treatment in group A and group B on Cu/Zn ratio in burn patients.
Table I also shows the effects of treatment in group A and group B on plasma MDA levels; the end product of lipid peroxidation in group A MDA plasma levels was significantly higher (p < 0.05) than control values at zero
time and decreased significantly after two days, four days, and at discharge time, when it was close to control values; the same finding occurred in group B. The other parameter that expresses oxidative stress is the plasma GSH level, which significantly decreased in burn patients compared with control, significantly increasing after two days, four days, and at discharge time in group A. In group B GSH levels increased significantly (p < 0.05) after two days and four days compared with pre-treatment values and increased significantly (p < 0.05) at discharge time compared with the four-day value in group B ( Fig. 3 ). The GSH level correlated positively with the plasma zinc level (r = 0.97, p < 0.05).
Fig. 3. Effects of treatment in group A and group B on plasma GSH level in burn patients.
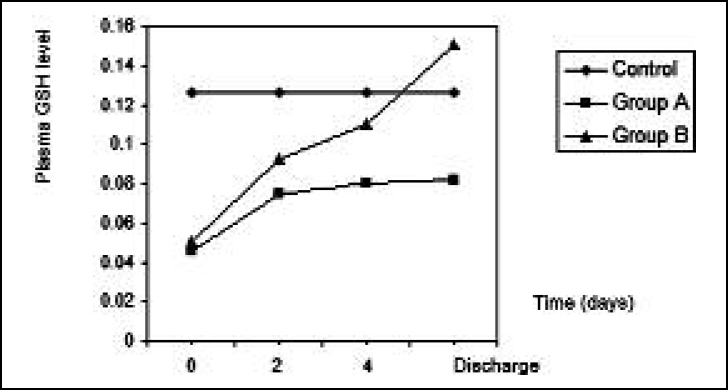
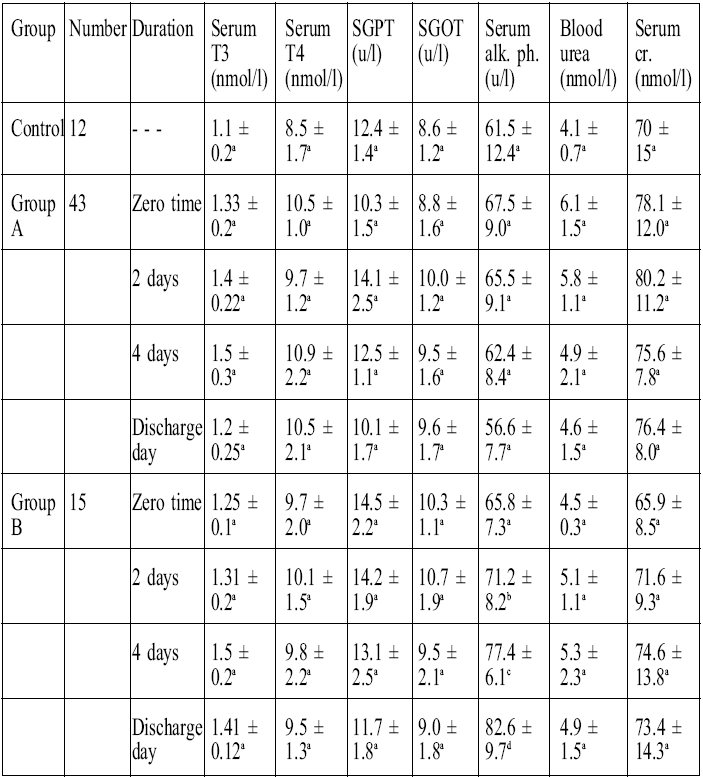
Table II clearly shows that in the groups treated there were no significant changes with regard to thyroid hormones T3 and T4, blood urea, and serum creatinine, which
Table II. Effects of treatment in group A and group B on T3 and T4 serum levels, liver function tests, and renal function tests in burn patients.
Group A: burn patients treated with topical povidone-iodine ointment for the first 4 days after admission followed by topical silver sulphadiazine cream in addition to other prescribed drugs.
Group B: burn patients treated as group A with the addition of a single daily oral dose of 66 mg zinc sulphate (15 mg elemental zinc).
Results represents mean ± standard deviation.
Results with non-identical superscripts were considered significantly different (p < 0.05).
Zero time represents time of resuscitation.
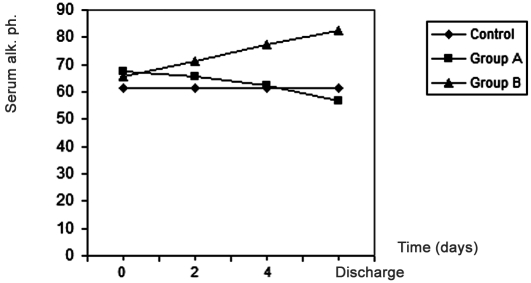
represent the renal function tests, or in the liver enzyme activities of SGOT and SGPT, except for alkaline phosphatase which showed a significant increase (p < 0.05) in group B (the zinc-supplemented group) after two days and four days the start of treatment, compared with pre-treatment values, and a significant increase at discharge time compared with values on day 4 day after the start of treatment ( Fig. 4 ). It was also found that there was a positive correlation (r = 0.98, p < 0.05) between plasma zinc levels and serum alkaline phosphatase.
Fig. 4. Effects of treatment in group A and group B on serum alkaline phosphatase in burn patients.
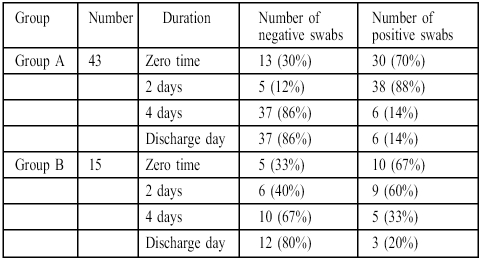
Table III showed that the incidence of invading bacteria isolated from burn patients expressed as a percentage of the total number decreased with time in both groups.
Table III. Effects of treatment in group A and group B on the incidence of invading micro-organisms isolated from burn patients.
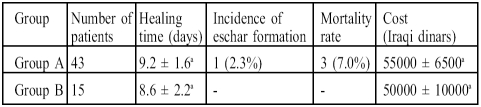
While Table IV shows that healing time decreased nonsignificantly (p < 0.05) in group B compared with group A and that the incidence of eschar formation decreased from 2.3% in group A to zero in group B, the most important result in this study is the reduction in the mortality rate from 7% in group A to zero in group B.
Table IV. Effects of treatment in group A and group B on healing time, incidence of eschar formation, mortality rate, and cost in burn patients.
Group A: burn patients treated with topical povidone-iodine ointment for the first 4 days after admission followed by topical silver sulphadiazine cream in addition to other prescribed drugs.
Group B: burn patients treated as in group A with the addition of a single daily oral dose of 66 mg zinc sulphate (15 mg elemental zinc).
Results represent percentage of total or mean ± standard deviation.
Results with non-identical superscripts were considered significantly different (p < 0.05).
Zero time represents time of resuscitation.
Discussion
The results presented Table I clearly show that plasma zinc levels were significantly reduced post-burn compared with control, while plasma copper levels remain unchanged.Pochon 35showed that plasma zinc and copper decreased post-burn, presenting data that are compatible with our data regarding plasma zinc levels but incompatible regarding plasma copper levels, while Agay et al. 16showed there was an increase in plasma copper levels post-burn, which is also incompatible with our result.
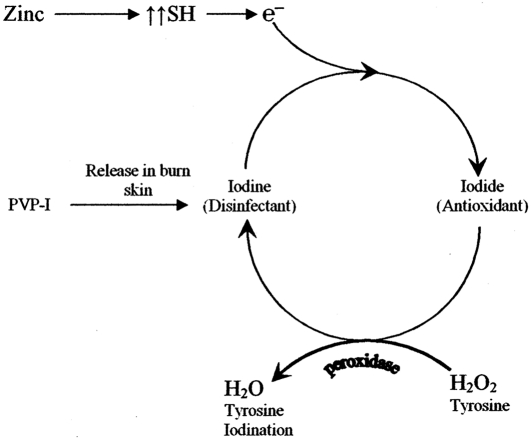
Topical treatment with PVP-I for the first four days post-burn, followed by topical SSD cream in addition to other prescribed drugs according to our burn unit policy, resulted in an increase in plasma zinc levels but nonsignificantly led to a slight improvement in the Cu/Zn ratio, while in group B, in which zinc sulphate was administered orally to burn patients in a nutritional dose in addition to the same treatment as group A, Table I shows a significant elevation in the plasma zinc level four days after start of treatment, without any effects on the plasma copper level - there was an improvement in the Cu/Zn ratio, which returned to normal at discharge time, a result compatible is with that obtained by Li et al. 36Voruganti et al. 15showed in burn patients that zinc concentrations in wound exudates exceeded plasma concentrations, suggesting that the primary reason for low plasma zinc levels was the elevated loss of zinc through wound exudates. Al-Kaisy and Sahib 37found that the use of topical PVP-I i treated groups for the first four days decreased oozing and fluid loss from the skin of burn patients and also decreased fluid requirements, meaning decreased wound exudates and zinc loss, which may explain the elevation in plasma zinc levels in both groups. It has been shown that after use of topical PVP-I ointment for burned skin the first event is the release of iodine, 38leaving the PVP polymer alone; the second event is the uptake of water by the polymer from the wound skin, 39leading to swelling of the polymer and cross-linking when exposed to more water, 40, 41causing the formation of a layer 42, 43that is impermeable to water and prevents fluid losses from burned skin. The released iodine, which acts as a disinfectant, 44has the ability to oxidize sulphydryl groups and other molecules; it reacts with sites of unsaturation in lipids 45and converts to iodide. At this stage the action of iodide is completely different - it now acts as an electron donor in the presence of H2O2 and peroxidase, and the remaining iodine readily iodinates tyrosine. Its action changes from antibacterial to antioxidant, 46which has a very important role in decreasing microvascular permeability and again fluid loss by antagonizing the reactive oxygen species (ROS) responsible for this process. 47ROS is widely generated in burns - the important pathway is through the xanthine oxidase enzyme, which leads to formation of H 2O 2. 48
This may also explain the reduction in MDA levels and the elevation in GSH levels in group A, indicating an improvement in oxidative stress parameters.
Zinc supplementation in group B also acted as an antioxidant with various mechanisms, 17, 18which also led to decreased ROS and decreased microvascular permeability and fluid loss. At the same time one of the antioxidant effects of zinc is the protection of protein sulphydryl groups,17 considered to be a substrate for iodine to convert it to iodide and act as an antioxidant ( Fig. 5 ).
Fig. 5. Proposed mechanism demonstrates the conversion of iodine to iodide and the antioxidant effect of iodide when used topically as PVP-I on burn skin in combination with oral zinc supplementation.
In group B zinc acted as an antioxidant and reduced MDA levels significantly after two days, four days, and at discharge time, while GSH increased significantly after two days and four days, compared with pre-treatment, and at discharge time compared with the four-day value; also, there was a strong positive correlation (r = 0.97, p < 0.05) between plasma zinc levels and plasma GSH, owing to thiol protection by zinc. 17
The results shown in Table II indicate that burns treatment in both groups had no effect on thyroid function, as represented by serum T 3and T 4, a result compatible with that obtained by Balogh et al. 49With regard to liver enzymes, Table II shows no significant changes in the activity of SGOT and SGPT except for alkaline phosphatase, which was significantly elevated (p < 0.05) in group B after two and four days and at discharge time compared with pre-treatment and control values. Enzyme alkaline phosphatase is a zinc-dependent enzyme that is sensitive to dietary zinc levels and is considered a useful tool for the detection of mild zinc deficiency. 50Our results show there was a strong positive correlation (r = 0.98, p < 0.05) between plasma zinc levels and serum alkaline phosphatase.
Regarding blood urea and serum creatinine levels, which were measured as a renal function test, the results of treatment in both groups indicated no change in these values compared with control values. This finding is consistent with that of other research. 51
Table III presents the incidence of wound infection in the treated groups. These show the same incidence profile, as represented by a high percentage at zero time and after two days; the incidence percentage decreased after four days and at discharge time to a nonsignificant value.
Table IV shows that healing time in group B decreased nonsignificantly compared with group A, in which zinc sulphate was not administered. It was found that zinc deficiency increased the time necessary for wound closure and decreased wound strength. 52The role of zinc in wound healing has been investigated since the 1950s 53but the mechanism by which zinc affects the healing process is still not clear.
Wound healing is a complicated process that involves various cell types, structural proteins, cytokines, and ROS. 54In general, it presents three major stages: inflammation, proliferation, and remodelling. Inflammation is considered to be a critical stage for establishing an environment that facilitates the subsequent stages of the healing process. 55The initial event during this stage is triggered by ROS, 56and antioxidant levels have been shown to be depleted in healing cutaneous wounds, 57and it has also been shown that the inflammatory stage is regulated by the nuclear factor NFkB, a critical transcription factor that regulates the expression of inflammatory mediators. Hence NFkB plays a pivotal role during the inflammatory stage: NFkB activation is thought to be a critical event in early wound healing. 58NFkB is normally maintained in an inactive form in the cytoplasm, bound to the inhibitory protein IkB; during activation IkB is phosphorylated and degraded, facilitating the translocation of NFkB to the nucleus, where it regulates the expression of immune and inflammatory genes. It has been found that zinc deficiency reduces NFkB-binding activity in vivo and impairs its translocation, and that NFkB specifically requires zinc for optimal DNA binding. 59Yunsook et al. 58have therefore suggested that zinc deficiency may delay wound healing as a result of impaired NFkB-binding activity and that high doses of zinc have the same effect on wound healing; they believe that dietary zinc has an optimal effect on the healing process, which may explain the results presented in Table IV .
Table IV also shows that the treatment in group B (the zinc-supplemented group) reduced the incidence of eschar formation from 2.3% in group A to zero. Mauviel 60showed that zinc was an important co-factor for metalloproteinases, which are likely to play a significant role in the tissue remodelling that occurs after burn injury.
Finally, the results in Table IV show that the mortality rate fell from 7% in group A to zero in group B. As the primary cause of death in burns is the development of postburn multiple organ failure, 61and as one of the main factors involved in the genesis and development of multiple organ failure is ROS, 62the use of a topical antioxidant like PVP-I37 and systemic antioxidants like oral zinc sulphate may have a great benefit in the reduction of mortality rates. The results presented in Table IV can be explained on this basis.
Conclusion
This study showed that burn patients had significantly lower plasma zinc levels than control and that zinc supplementation significantly increased plasma zinc levels and improved the Cu/Zn ratio in burn patients. The study also showed that zinc supplementation significantly improved the antioxidant status of burn patients, as represented by a significant elevation of the GSH level (the natural antioxidant); this improvement led to a decrease in healing time, in the incidence of eschar formation, and in the mortality rate (to zero) in burn patients, compared with the non-zinc-supplemented group, thus indicating the great benefit of using a combination of topical PVP-I and a systemic (zinc sulphate) antioxidant to improve burn outcome. The importance of regarding oxidative stress as an important goal for therapeutic targeting in burn treatment protocols is also considered.
References
- 1.Gudaviciene D., Rimdeika R., Adamonis K. Nutrition of burned patients. Medicina. 2004;40:1–8. [PubMed] [Google Scholar]
- 2.Beisel W.R., Pekarek R.S., Wannemacher R.W. The impact of infectious disease on trace element metabolism of the host. In: Hoekstra W.G., Suttie J.W., Ganther H.E., Mertz W., editors. Trace Element Metabolism in Animals (2nd ed.) University Park Press; Baltimore: 1974. pp. 217–40. [Google Scholar]
- 3.Berger M., Cavadini C., Bart A., Mansourian R., Guinchard S., Bartholdi I. Cutaneous zinc and copper losses in burns. Burns. 1992a;18:373–80. doi: 10.1016/0305-4179(92)90035-s. [DOI] [PubMed] [Google Scholar]
- 4.Rasik A.M., Shukla A. Antioxidant status in delayed healing type of wounds. Int. J. Exp. Path. 2000;81:257–63. doi: 10.1046/j.1365-2613.2000.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rock C.L., Dechert R.E., Khilnani R., Parker R.S., Rodriguez J.L. Carotenoids and antioxidant vitamins in patients after burn injury. J. Burn Care Rehabil. 1997;18:269–78. doi: 10.1097/00004630-199705000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 7.Coleman J.E. Zinc proteins: Enzymes, storage proteins, transcription factors, and replication proteins. Annu. Rev. Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- 8.Tapiero H., Tew K.D. Trace elements in human physiology and pathology: Zinc and metallothioniens. Biomedicine & Pharmacotherapy. 2003;57:399–411. doi: 10.1016/s0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 9.Sandstead H.H. Zinc deficiency. A public health problem? Am. J. Dis. Child. 1991;145:853–9. doi: 10.1001/archpedi.1991.02160080029016. [DOI] [PubMed] [Google Scholar]
- 10.Calhoun N.R., Smith J.C., Becker K.L. The role of zinc in bone metabolism. Orthopedics. 1974;103:212–34. doi: 10.1097/00003086-197409000-00084. [DOI] [PubMed] [Google Scholar]
- 11.Boosalis M.G., Solem L.D., McCall J.T., Ahrenholz D.H., Mc Clain C.J. Serum zinc response to thermal injury. J. Am. Coll. Nutr. 1988;7:69–76. doi: 10.1080/07315724.1988.10720222. [DOI] [PubMed] [Google Scholar]
- 12.Dong Y.L., Zhu Z.M, Li J. Copper and zinc content of the serum of burned patients. J. Zhongua Wai Ke Za Zhi. 1988;26:34–6. [PubMed] [Google Scholar]
- 13.Davies M.I., Fell G.S. Tissue catabolism in patients with burns. Clin. Chim. Acta. 1974;51:83–92. doi: 10.1016/0009-8981(74)90064-3. [DOI] [PubMed] [Google Scholar]
- 14.Carr G., Wilkinson A.W. Zinc and copper urinary excretions in children with burns and scalds. Clin. Chim. Acta. 1975;61:199–204. doi: 10.1016/0009-8981(75)90315-0. [DOI] [PubMed] [Google Scholar]
- 15.Voruganti V.S., Klein G.L., Hong-Xing Lu, Thomas S., Freeland Graves J.H., Herndon D.N. Impaired zinc and copper status in children with burn injuries: Need to reassess nutritional requirements. Burns. 2005;31:711–6. doi: 10.1016/j.burns.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Agay D., Anderson R.A., Sandre C., Bryden N.A., Alonso A., Roussel A.M., Chancerelle Y. Alterations of antioxidant trace elements (Zn, Se, Cu) and related metallo-enzymes in plasma and tissues following burn injury in rats. Burns. 2005;31:366–71. doi: 10.1016/j.burns.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Powell S.R. The antioxidant properties of zinc. J. Nutr. 2000;130:1447S–1454S. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- 18.Zago M.P., Oteiza P.I. The antioxidant properties of zinc: Interactions with iron and antioxidants. Free Radical Biology & Medicine. 2001;31:266–74. doi: 10.1016/s0891-5849(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 19.National Research Council. Zinc. National Research Council, editor. Recommended Dietary Allowances. National Academy of Sciences Washington, D.C. 1980:144–7. [Google Scholar]
- 20.Taylor and Briggs R.Z. J. Anal. Atom. Spectrosc. 1986;1391:394. [Google Scholar]
- 21.Taylor and Bryant T.N. Clin. Chem. Acta. 1981;110:83–90. doi: 10.1016/0009-8981(81)90304-1. [DOI] [PubMed] [Google Scholar]
- 22.Stocks J., Dormandy T.L. The auto-oxidation of human red cell lipids induced by hydrogen peroxide. Brit. J. Haematol. 1971;20:95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert H.S., Stemp D.D., Roth E.F. A method to correct errors caused by generation of interfering compounds during lipid peroxidation. Anal. Biochem. 1984;173:282–6. doi: 10.1016/0003-2697(84)90086-1. [DOI] [PubMed] [Google Scholar]
- 24.Godin D.V., Wahaieb S.A., Garnett M.E. Antioxidant enzyme alteration in experimental and clinical diabetes. Mol. and Cell. Bioch. 1988;84:223–31. doi: 10.1007/BF00421057. [DOI] [PubMed] [Google Scholar]
- 25.Utiger R.D. Serum triiodothyronine in man. Ann. Rev. Med. 1974;25:289–302. doi: 10.1146/annurev.me.25.020174.001445. [DOI] [PubMed] [Google Scholar]
- 26.Wistom G.B. Enzyme-immunoassay. Clin. Chem. 1976;22:1243. [PubMed] [Google Scholar]
- 27.Kind R.R.N., King E.J. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J. Clin. Path. 1954;7:322–6. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reitman S., Frankel S. GOT/GPT procedures. Am. J. Clin. Path. 1957;28:56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 29.Fawcett J.K., Scott J.E. Determination of urea in blood or serum. J. Clin. Path. 1960;13:156–9. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry J.B. "Clinical Diagnosis and Management" (17th ed.) Sanders Publisher; 1984. [Google Scholar]
- 31.Revathi G., Puri J., Jain B.K. Bacteriology of burns. Burns. 1998;24:347–9. doi: 10.1016/s0305-4179(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 32.O'Mara M.S., Caushaj P., Goldfarb I.W., Slater H. Treatment and mortality trends among massively burned patients. Annals of Burns and Fire Disasters. 2000;13:73–6. [Google Scholar]
- 33.Williams R.L., Armstrong D.G. Wound healing: New modalities for a new millennium. Clin. Pediatr. Med. Surg. 1998;15:151–4. [PubMed] [Google Scholar]
- 34.Lofts J.A. Cost analysis of a major burn. N.Z. Med. J. 1991;16:488. [PubMed] [Google Scholar]
- 35.Pochon J.P. Zinc- and copper-replacement therapy - a must in burns and scalds in children? Prog. Pediatr. Surg. 1981;14:151–72. [PubMed] [Google Scholar]
- 36.Li L., Guo Z., Zhao L. Effects of supplement Zn on levels of Zn in serum, growth hormone, and hydroxyproline. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi. 1998;14:425–8. [PubMed] [Google Scholar]
- 37.Al-Kaisy A.A., Salih Sahib A. Role of the antioxidant effect of vitamin E with vitamin C and topical povidone-iodine ointment in the treatment of burns. Annals of Burns and Fire Disasters. 2005;18:19–30. [PMC free article] [PubMed] [Google Scholar]
- 38.Lavelle K.J., Doedens D.J., Kleit S.A., Forney R.B. Iodine absorption in burn patients treated topically with povidone-iodine. Clin. Pharmacol. Ther. 1975;17:355–62. doi: 10.1002/cpt1975173355. [DOI] [PubMed] [Google Scholar]
- 39.Yasushi M., Tomoya N., Isao I. Hydration and phase behaviour of poly(N-vinylcaprolactam and poly(N-vinylpyrrolidone) in water. Macromolecules. 2002;35:217–22. [Google Scholar]
- 40.Jan P., Jiri L., Antonin M., Cestmir K. Translational diffusion of paramagnetic tracers in poly(1-vinylpyrrolidone) hydrogel and concentrated aqueous solutions by 1D electron spin resonance imaging. Macromolecules. 1999;32:8230–3. [Google Scholar]
- 41.Suvegh K., Zelko R. Physical aging of poly(vinylpyrrolidone) under different humidity conditions. Macromolecules. 2002;35:7950–800. [Google Scholar]
- 42.Ebube N.K., Owusu-Ababio G., Adeyeye C.M. Preformulation studies and characterizations of the physicochemical properties of amorphous polymers using artificial neural networks. Int. J. Pharm. 2000;196:27–35. doi: 10.1016/s0378-5173(99)00405-6. [DOI] [PubMed] [Google Scholar]
- 43.Masayo H., Ken-ichiro Y., Fukashi K., Tsutomu U., Yoshiyuki U., Hiroyuki S., Ichiro I., Kiyotaka S. Nanoscopic behavior of polyvinylpyrrolidone particles on polysulfone/polyvinylpyrrolidone film. Biomaterials. 2004;25:1019–28. doi: 10.1016/s0142-9612(03)00629-x. [DOI] [PubMed] [Google Scholar]
- 44.Arthur K., Loretta P. Dilute povidone-iodine solutions inhibit human skin fibroblast growth. Dermatol. Surg. 2002;28:210–4. doi: 10.1046/j.1524-4725.2002.01161.x. [DOI] [PubMed] [Google Scholar]
- 45.Gottardi W. Iodine and iodine compounds. In: Block S.S., editor. Disinfection, Sterilization, and Preservation. Lea & Febiger; Philadelphia: 1983. pp. 183–96. [Google Scholar]
- 46.Venturi S., Venturi M. Iodide, thyroid, and stomach carcinogenesis: Evolutionary story of a primitive antioxidant. Europ. J. Endocrinol. 1999;140:371–2. doi: 10.1530/eje.0.1400371. [DOI] [PubMed] [Google Scholar]
- 47.Youn Y.K., La Londe C., Demling R. Oxidants and the pathophysiology of burn and smoke inhalation injury. Free Rad. Biol. Med. 1992;12:409. doi: 10.1016/0891-5849(92)90090-4. [DOI] [PubMed] [Google Scholar]
- 48.Latha B., Babu M. The involvement of free radicals in burn injury: A review. Burns. 2001;27:309–17. doi: 10.1016/s0305-4179(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 49.Balogh D., Bauer M., Riccabona G. The influence of povidoneiodine ointment on thyroid hormones in severe burns. J. Hosp. Infec. 1985;6:147–53. doi: 10.1016/s0195-6701(85)80061-x. [DOI] [PubMed] [Google Scholar]
- 50.Kasarskis E., Scuna A. Serum alkaline phosphatase after treatment of zinc deficiency in humans. Am. J. Clin. Nutr. 1980;33:2609–12. doi: 10.1093/ajcn/33.12.2609. [DOI] [PubMed] [Google Scholar]
- 51.Vanholder R., Van Den Bogaerde J., Vogelaers D., Colardyn F. Renal function in burns. Acta Anaesthesiol. Belg. 1987;38:367–71. [PubMed] [Google Scholar]
- 52.Sandstead H.H., Lanier V.C. jr, Shephard G.H., Gillespie D.D. Zinc and wound healing. Effects of zinc deficiency and zinc supplementation. Am. J. Clin. Nutr. 1970;23:514–9. doi: 10.1093/ajcn/23.5.514. [DOI] [PubMed] [Google Scholar]
- 53.Okada A., Takagi Y., Nezu R., Lee S. Zinc in clinical surgery- a research review. Jpn. J. Surg. 1990;20:635–44. doi: 10.1007/BF02471026. [DOI] [PubMed] [Google Scholar]
- 54.Rahmat A., Norman J.N., Smith G. The effect of zinc deficiency on wound healing. Br. J. Surg. 1974;61:271–3. doi: 10.1002/bjs.1800610405. [DOI] [PubMed] [Google Scholar]
- 55.Hubner G., Brauchle M., Smola H., Madlener M., Fassler R., Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996;8:548–56. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- 56.Steiling H., Munz B., Werner S., Brauchle M. Different types of ROS-scavenging enzymes are expressed during cutaneous wound repair. Exp. Cell Res. 1999;247:484–94. doi: 10.1006/excr.1998.4366. [DOI] [PubMed] [Google Scholar]
- 57.Shukla A., Rasik A.M., Patnaik G.K. Depletion of reduced glutathione, ascorbic acid, vitamin E, and antioxidant defence enzymes in a healing cutaneous wound. Free Radic. Res. 1997;26:93–1. doi: 10.3109/10715769709097788. [DOI] [PubMed] [Google Scholar]
- 58.Yunsook M.L., Tammy M.B. Dietary zinc alters early inflammatory responses during cutaneous wound healing in weanling CD 1 mice. J. Nutr. 2004;134:811–6. doi: 10.1093/jn/134.4.811. [DOI] [PubMed] [Google Scholar]
- 59.Kudrin A.V. Trace elements in regulation of NF-kB activity. J. Trace Elements Med. Biol. 2000;14:129–42. doi: 10.1016/s0946-672x(00)80001-2. [DOI] [PubMed] [Google Scholar]
- 60.Mauviel A. Cytokine regulation of metalloproteinase gene expression. J. Cell Biochem. 1993;53:288–95. doi: 10.1002/jcb.240530404. [DOI] [PubMed] [Google Scholar]
- 61.Yang Z.C. Clinical study of the pathogenesis of multiple organ failure after burns. Zhonghna Zheng Xing Shao Shang Wai Ke Za Zhi. 1992;8:8–12. [PubMed] [Google Scholar]