Summary
We report our experience in using remifentanil as sole agent for the analgesia of spontaneously breathing non-intubated burn patients during dressing changes. Sixty procedures were collected and analysed. Remifentanil was used during monitoring of vital functions, with oxygen inhalation throughout the procedure, at the bedside in the intensive care unit ward. Infusion speed was varied by the nurse in charge, depending on pain, analgesia, and adverse effects. The dosage of continuous infusion ranged from 0.125 to 1 mg.kg-1.mn-1 (average, 0.42). All patients received intravenously morphine 30 min before the end of the procedure (average, 10 mg). The main side effects were hypoxia and drowsiness, always quickly reversed when the doses were reduced. All patients had low levels of pain during and after the procedure, and were satisfied with the analgesia protocol. We conclude that remifentanil is another possible manner of analgesia in the dressing of burn patients, but that it must be used in an "anaesthesiological" environment.
Keywords: remifentanil, analgesia, during, dressing, spontaneously, breathing, burn, patients
Abstract
Nous rapportons notre expérience de l'utilisation du rémifentanil comme agent unique d'analgésie durant le pansement des patients brûlés, non intubés, ventilant spontanément. Soixante procédures ont été analysées. Le rémifentanil a été utilisé sous monitorage des fonctions vitales chez des patients respirant de l'air enrichi en oxygène, au lit du patient en chambre de réanimation. La posologie était adaptée par l'infirmière réalisant le pansement selon le niveau de douleur ou d'analgésie et l'apparition d'effets adverses. Les posologies variaient de 0,125 à 1 mg.kg-1.mn-1, en moyenne 0,42, par voie intraveineuse. Les principaux effets adverses observés étaient la somnolence et l'hypoxie, toujours rapidement réversibles avec la diminution des doses. Tous les patients recevaient de la morphine 30 min avant la fin du pansement, en moyenne 30 mg IV. Tous les patients ont eu des niveaux de douleur bas, pendant et après la procédure, et se disaient satisfaits de cette technique. Nous concluons que le rémifentanil est une autre possibilité d'analgésie pour le pansement des brûlés, mais qu'il doit être utilisé dans un environnement de type "anesthésie".
Introduction
Management of pain is an important part of burns treatment, and a complete chapter is devoted to the subject in "Total Burn Care". 1Pain at rest seems quite easy to treat, especially since long-lasting oral morphine sulphate has been available. On the other hand, pain during dressing changes is more difficult to treat, and some burn patients report that during their hospitalization it is their worst pain experience. 2In some patients, pain remains unbearable, even if they receive acetaminophen as well as high doses of morphine before the procedure and nitrous protoxide during it. Since repeated general anaesthesia for these procedures is not possible in our unit (besides the fact that the fasting necessary for general anaesthesia is not desirable for patient nutrition and cicatrization), the use of a powerful, quick-acting, and lasting opioid such as remifentanil (RF) is a possibility. After using RF to dress patients under mechanical ventilation, when the morphine used for sedation proved to be insufficient (even in large amounts) for dressing-related pain, we decided to use RF in conscious, non-intubated patients. We report here our experience of 60 procedures in a five-month period.
Pharmacology of remifentanil
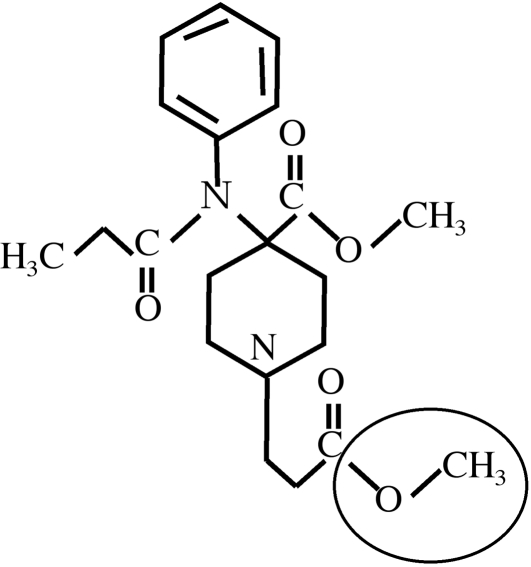
Remifentanil 3is a 4-anilidopiperidine derived from fentanyl. The methyl ester function circled in Fig. 1 is the target of non-specific esterases, present in numerous tissues that transform RF to G190291, which is 1,000 times less powerful than RF and therefore does not provide analgesia or respiratory side effects, even if its urinary elimination is impaired by renal insufficiency.
Fig. 1. Structure of remifentanil.
The metabolism of RF is not influenced by age or by kidney or liver failure.This metabolism explains the very short half-life (initial 1', terminal 30') of RF. Contrary to fentanyl, no redistribution occurs with RF, and its action does not last longer if the duration of infusion increases.
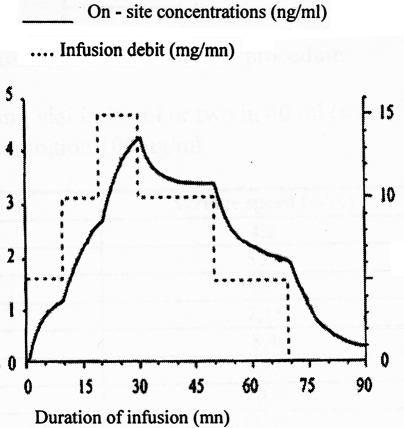
Fig. 2. Evolution of onsite concentrations with various infusion debits.
The very high tissue diffusion index explains why the peak concentration at the site of action is obtained within 1'30'' and the balance of concentrations, when used in continuous infusion, is reached within 10'. The onsite concentrations are therefore quite parallel to infusion speed (Fig. 2). 4, 5The intrinsic analgesic power is evaluated in the same order as fentanyl, i.e. eight 3to forty 5times that of alfentanil. The context sensitive halftime is 3' and is independent of the duration of the infusion, and the clinical effects last 3 to 5'. RF does not provide any post-procedural analgesia. It has no hypnotic effect, respiratory depression is parallel to its analgesic power, and muscular rigidity (also involving the respiratory muscles) contraindicates use of RF boluses in non-intubated patients. Hypotension and deep bradycardia are likely if used in hypovolaemic patients.
Finally, RF provides a powerful analgesia, achieved in a few minutes and lasting some minutes in any patient (although the elderly are more susceptible to RF, as also to other opioids), with a respiratory depression that is as deep as its analgesic effect is high.
Patients and methods
Between November 2004 and March 2005, 60 procedures of dressing changes were compared. They all concerned patients who had no tracheal tube.
The patients selected included some whose tracheal tube had been removed the day before, since intubated patients receive RF for the procedure. It should be noted that these patients - when having dressing changes during mechanical ventilation - received 0.2 to 0.3 mg.kg -1.mn -1RF and that these dosages were anaesthetic dosages. Some other selected patients had suffered considerable pain during previous procedures although they had received high doses of oral morphine sulphate (50 to 70 mg), plus 1 g acetaminophen and usually 10 mg midazolam (or 100 mg hydroxyzin) before the procedure, and N 2O during it. Some others were patients for whom the scheduled procedure was known to be notably painful (e.g. removing greased gauze for first dressing after hospitalization, first dressing after excision or on donor sites in large areas, removing numerous staples from extensive grafted areas, etc.).
Once selected, the patients were informed the day before of the scheduled protocol. Also the day before the anaesthesiologist prepared a personalized table ( Fig. 3 ) providing the nurse with some warnings, an explanation of the dilution of remifentanil, a dosage table, instructions on the use of remifentanil infusion, a prescription of morphine, the date, the patient's name, and the name of the prescribing doctor who signed the form.
Fig. 3. Example of remifentanil prescription form.
On the day of the procedure, the nurse in charge made sure that an anaesthesiologist would be available at all times during the procedure.
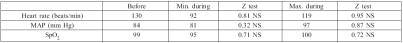
Table I. Variation of vital parameters.
If the patient was not already hospitalized in the intensive care unit (ICU) ward, he was taken there for the duration of the procedure and for one hour thereafter, in order to perform monitored surveillance. Before the procedure the patient had to fast. The dressing was changed "early" (9 a.m.) in order to let the patient have breakfast soon after.
The patient received oxygen via a disposable mask, in order to obtain 40% of the inspired fraction. If the patient did not already have one, a peripheral venous route was installed. If the patient already had a multi-lumen catheter, one of the lumina was dedicated to remifentanil. An antireflux device was installed in this route. RF infusion was initiated after at least 5' of oxygen inhalation at a dose of 0.1 mg.kg -1.mn -1. The procedure started 5' later. If the patient expressed pain of over 4 on a verbal rating scale (VRS) from 0 to 10, the procedure was discontinued and the dose of RF was increased by 0.025 mg.kg -1.mn -1, with the procedure being continued 3' later, and so on if the VRS remained over 4. If the patient exhibited drowsiness, the dose was reduced in steps of 0.025 mg.kg -1.mn -1, repeated every 3' if necessary. In the event of reduced pulse oxygen concentration, the dose was reduced in steps of 0.05 mg.kg -1.mn -1, also repeated every 3' if necessary. 30' before the scheduled end of the procedure, the patient received the prescribed dose of morphine via IV route. If the patient was in pain after the procedure, he received additional 3 mg doses of morphine every 5' until he rated his pain under 4. Monitored surveillance went on for 1 h after the last dose of morphine.
We recorded the following: pulse oxymeter value (SpO 2), mean arterial pressure (MAP), and heart rate (HR) before the procedure, as well as the same values at the highest and lowest levels reached during the procedure. We also recorded the highest and lowest VRS values during the procedures. The values of SpO 2, MAP, and HR were compared, using the Z test. Any side effect was also recorded.
Results
Twenty-seven patients underwent 60 dressing changes under an analgesia with RF, and solely with RF. No hypnotic or anxiolytic or analgesic drug of any kind was added except morphine, as described before. The average dosage of RF at which the patients had pain evaluated below 4 as the procedure was in progress was 0.42 ± 0.22 mg.kg -1.mn -1.
The duration of the procedure length was 93 ± 30'. A total number of 88 vials of RF were opened.
The highest VRS during the procedure was 5.92 ± 2.38; the lowest was 1.07 ± 1.51. No patient rated his pain at continuously over 4. Even those patients who at some time of the procedure expressed high levels of pain were very satisfied with the analgesia provided. Table I shows the variations in vital signs during the procedure compared with before. None of these was statistically significant. The patients were given 10.11 ± 7.25 mg morphine, and 67 10-mg vials were used.
A total number of 38 adverse effects were noted, concerning 19 procedures - some occurred more than once during the same procedure. There were 14 episodes of drowsiness and 18 falls of SpO 2below 95% (never below 90% except during three apnoeas, of which the deepest was 75%, all three due to a random bolus after folding of the infusion tube during mobilization of the patient). There were three episodes of decrease in MAP (between 65 and 70). All these episodes were rapidly reversed with a reduction or brief interruption of infusion of RF. The anaesthesiologist never had to prescribe anything or provide anything else, such as artificial ventilation, vascular filling, or RF reversal with naloxone.
Discussion
According to Joly, writing in 2003, "the use of RF in spontaneously breathing patients is not nowadays recommended", 6but it is possible to find data attesting its use.
As early as 1999, Torres 7stated that studies were needed to assess the possibility and safety of RF in spontaneously breathing patients, as Gold had done in 1997. 8
However, RF has infrequently been used for analgesia (and more often for sedation in an ICU, which is beyond our purpose). Papers on the subject are even more uncommon as regards adults. RF has been used in continuous infusion for shock wave lithotripsy, at a rate of 0.05 mg.kg -1.mn -1. 9For the fiberoptic intubation of conscious patients, dosages ranged from 0.1 to 0.5 mg.kg -1.mn -1. 10For colonoscopy, 11administered at 0.2 mg.kg -1.mn -1, RF provided a good analgesia, with a better haemodynamic profile and a faster recovery than propofol, although hypoventilation was frequent (55% of the patients). In radiology, 12RF was successfully used at very low doses of 0.01 to 0.05 mg.kg -1.mn -1, without marked respiratory depression.
With regard to burns, though mentioned by Latarjet 13after his work with alfentanil, 14we have not found any papers related to RF.
In our study, the mean dosage was much higher that that seen in the literature, apart from Gold's paper. 8This probably explains the frequency (about one-third) of cases of respiratory depression, albeit less than that observed by Calvo, 12even if our dosages were higher and the respiratory depression did not seem to be correlated to the average dosage of RF. This has to be compared with its efficacy, and the balance turns in favour of its positive effects, since all patients had good analgesia and the side effects - even the three apnoeas - reversed spontaneously, rapidly, and without medical intervention. The nurses in the unit found it easy to learn to manipulate RF in this manner for the following reasons: 1. they were used to its utilization in intubated patients; 2. the anaesthesiologists provided training about RF and its practical use; 3. guidelines were written on the prescription form for each patient. The nurses were soon asking us to use RF because the quality of the analgesia permitted improvement in the quality of the dressing itself and provided a good experience for the patient.
Until now, over 150 dressings have been changed using this technique, which has become routine in our unit.
Conclusion
The infusion of remifentanil in conscious non-intubated patients is a new weapon against pain during dressing change in burn patients. Like any weapon, it has its side effects (i.e. respiratory depression), which can be minimized with preoxygenation and anaesthetic-type monitoring (with an anaesthesiologist on standby). Nurses and anaesthesiologists should be trained in the treatment of intubated patients before starting on non-intubated ones. Nurses too should have courses and training before the onset of such a protocol.
Acknowledgments
To the nurses of the Unit for their involvement in this study, and to Dr Jean François Arnould. The idea of using remifentanil in this indication is his.
References
- 1.Meyer W., Marvin J., Patterson D., Thomas C., Blakeney P. Management of pain and other discomforts in burned patients. In: Herndon D., editor. Total Burn Care (2nd edition) W.B. Saunders; Philadelphia: 2002. pp. 747–65. [Google Scholar]
- 2.Choinière M. The pain of burns. In: Wall P., Melzack R., editors. Textbook of Pain. Churchill Livingstone; London: 1994. pp. 523–41. [Google Scholar]
- 3.Chauvin M. Pharmacologie des morphiniques et des agonistes de la morphine: Rémifentanil. Encycl. Méd. Chir., Anesthésie-Réanimation. 2000:1–8. [Google Scholar]
- 4.Shafer S.L. New intravenous anesthetic. Remifentanil. ASA Annual Refresher Courses Anesthesiol. 1996;24:243–55. [Google Scholar]
- 5.Servin F. Remifentanil: From pharmacological properties to clinical practice. Adv. Exp. Med. Biol. 2003;523:245–60. doi: 10.1007/978-1-4419-9192-8_22. [DOI] [PubMed] [Google Scholar]
- 6.Joly V., Chauvin M. Pharmacologie et utilisation clinique du rémifentanil. Cah. Anesthesiol. 2003;51:491–6. [Google Scholar]
- 7.Torres L.M., Calderon E., Velazquez A. Remifentanil. Indications in anesthesia. Rev. Esp. Anestesiol. Reanim. 1999;46:75–80. [PubMed] [Google Scholar]
- 8.Gold M.I., Watkins W.D., Sung Y.F., Yarmush J., Chung F., Uy N.T., Maurer W., Clarke M.Y., Jamerson B.D. Remifentanil versus remifentanil/midazolam for ambulatory surgery during monitored anesthesia care. Anesthesiology. 1998;88:1124–6. doi: 10.1097/00000542-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Medina H.J., Galvin E.M., Dirckx M., Banwarie P., Ubben J.F., Ziljsta F.J., Klein J., Verbrugge S.J. Remifentanil as a single drug for extracorporeal shock wave lithotripsy: A comparison of infusion doses in terms of analgesic potency and side effects. Anesth. Analg. 2005;101:365–70. doi: 10.1213/01.ANE.0000159379.54705.84. [DOI] [PubMed] [Google Scholar]
- 10.Puchner W., Egger P., Puhringer F., Lockinger A., Obwegeser J., Gombotsz H. Evaluation of remifentanil as single drug for awake fiberoptic intubation. Acta Anaesthesiol Scand. 2002;46:350–4. doi: 10.1034/j.1399-6576.2002.460403.x. [DOI] [PubMed] [Google Scholar]
- 11.Moerman A.T., Foubert L.A., Herregods L.L., Struys M.M., De Wolf D.J., De Looze D.A., De Vos M.M., Mortier E.P. Propofol versus remifentanil for monitored anaesthesia care during colonoscopy. Eur. J. Anaesthesiol. 2003;20:461–6. doi: 10.1017/s0265021503000723. [DOI] [PubMed] [Google Scholar]
- 12.Calvo J., Stafin C., Pizzuto R., Conil C., Meites G., Samii K., Virenque C. Utilisation du rémifentanil en sédation vigile. Ann. Fr. Anesth. Réanim. 2000;19:695–6. doi: 10.1016/s0750-7658(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 13.Latarjet J. La douleur du brûlé. Pathol. Biol. 2002;50:127–33. doi: 10.1016/s0369-8114(01)00277-2. [DOI] [PubMed] [Google Scholar]
- 14.Latarjet J., Robert A., Foyatier J.L. Analgesia for bedside burn dressing by intravenous alfentanyl in adults. Communication 25th ABA Meeting Cincinnati March. 1993 [Google Scholar]