Summary
Patients suffering from considerable cutaneous loss must be treated under strict aseptic conditions and with positive pressure ventilation, which is available in burns units. The differentiation of severe bullous skin diseases remains a challenge for the clinician. We report seven cases of severe bullous skin diseases in a paediatric age group treated in a burns unit with the cooperation of a paediatric intensive care unit (King Saud Hospital, Unizah, Kingdom of Saudia Arabia) between 2001 and 2005. Toxic epidermal necrolysis (Lyell's syndrome) was encountered in five cases, staphylococcal scalded-skin syndrome in one, and generalized drug eruption in one. The mortality rate was 14% (one of the seven patients). Most of the children presented with about 35% desquamation and 45% intact bullous formation (70-80% total body surface area involvement). The majority presented after the sudden onset of high fever, signs of systemic toxicity, and intense mucocutaneous exfoliation. The diagnosis was confirmed by skin biopsy and culture swabs. All the patients were managed as for mixed second-degree burns with regard to fluid calculation and hydrotherapy. We used the closed technique for dressing. The results are presented, and the literature was searched for similar cases reported in other parts of the world. The importance of diagnosis and appropriate treatment of the condition is emphasized.
Keywords: SEVERE, BULLOUS, SKIN, DISEASES, ANALYSIS, SEVEN, CHILDREN, MANAGED, BURNS, UNIT
Abstract
Les patients atteints d'importantes pertes cutanées doivent être traités en strictes conditions aseptiques et avec la ventilation à pression positive, qui est disponible dans les unités des brûlés. La différentiation des maladies cutanées bulleuses sévères reste problématique pour le clinicien. Les Auteurs décrivent sept cas de maladies cutanées bulleuses sévères dans un groupe de patients en âge pédiatrique traités dans une unité des brûlures en coopération avec une unité pédiatrique de soins intensifs (Hôpital Roi Saud, Unizah, Royaume d'Arabie Saoudite) entre 2001 et 2005. La nécrolyse épidermique toxique (syndrome de Lyell) a été identifiée chez cinq patients, le syndrome de la peau ébouillantée staphylococcique chez un patient et l'éruption généralisée due aux médicaments chez un patient. Le taux de mortalité était 14% (un des sept patients). La plupart des petits patients présentaient environ 35% de desquamation et 45% de formation bulleuse intacte (intéressant 70-80% de la surface corporelle totale). Dans la plupart des cas la raison de l'hospitalisation était une brusque attaque de fièvre élevée, des signes de toxicité systémique ou une exfoliation mucocutanée intense. Le diagnostic a été confirmé par la biopsie cutanée et les écouvillons des cultures. La gestion des patients était identique à celle des patients atteints de brûlures de deuxième degré variable pour ce qui concerne le calcul des fluides et l'hydrothérapie. La technique fermée a été utilisée pour les pansements. Les Auteurs présentent leurs résultats, avec une recherche de la littérature pour des cas comparables décrits autre part dans le monde. Les Auteurs concluent en soulignant l'importance du diagnostic et du traitement approprié de la condition prise en considération.
Introduction
Over the last few years, understanding of the pathophysiology of severe bullous skin diseases has substantially increased. Toxic epidermal necrolysis (TEN), also known as Lyell's syndrome, is a rare but extremely serious nosological entity characterized by high fever, systemic toxicity symptoms, and extensive mucocutaneous exfoliation.1 It usually occurs in response to certain drugs.
The differential diagnosis of severe bullous skin disease commonly mentioned in the literature2-6 includes: erythema (exsudativum) multiforme (major) (EM); Stevens Johnson syndrome (SJS); TEN (Lyell's disease); and staphylococcal scalded-skin syndrome (SSSS). TEN was first made known in the medical literature through the works of Alan Lyell, whose first article was published in 1956 in the British Journal of Dermatology.1 In that article, the word "necrolysis" was proposed to describe the isolated necrosis of the epidermal layer of the skin and its detachment from the underlying dermis, where there are practically none of the inflammatory changes usually present in toxic erythematic conditions. SJS may also present as a dermatological emergency characterized by purpuric macules and targetoid lesions, full-thickness epidermal necrosis, although with lesser detachment of the cutaneous surface, and mucous membrane involvement. SJS and TEN may represent a spectrum of a single disease process. SJS and TEN are often drug-induced, but the pathophysiological mechanism is unknown. A number of theories have been proposed that may have implications for treatment. The existence of an immunological-based mechanism, however, is practically consensual, given the amount of clues in that direction. TEN can affect both infants and adults, but the onset is more frequent at extreme ages, i.e. before 5 years of age and after 64.2 It is more common in females, with a female/male ratio of 3:2 or even 2:1.3 No racial differences have been described. The incidence of TEN in the general population varies from 1 to 1.3 cases per million people per year.3 Internationally, the incidence of TEN in Sweden was reported to be 0.4 per million population per year, and a French group reported 1.2 cases per million population per year. A study in West Germany reported the respective incidences of TEN and SJS to be 0.93 and 1.1 cases per million population per year.
The cutaneous lesions are similar to those of extensive second-degree burns, i.e. massive hydroelectrolytic loss, breakdown of thermic regulation, increase in catabolism, and the abolition of cutaneous antimicrobial protection. Epidermal necrolysis may involve the whole body except for the scalp, which is usually unaffected. Denuded areas present an exudative, dark-red dermis.7 TEN patients differ from burn patients in the involvement of the oropharyngeal mucosa (which interferes with adequate rehydration and nutrition), the more diffuse character of skin lesions (impeding venous access), and stronger systemic expression.
The loosening of the epidermal layer results in the discharge of body fluids, proteins, and electrolytes which, if not replaced, will lead to haemodynamic changes with hypovolaemia and renal failure.8 Important prognostic factors include the percentage loss of body surface area (BSA), age, persistent neutropenia (defined as neutropenia lasting more than five days), hypoalbuminaemia (usually < 2.0 g/dl), and persistent azotaemia.3 According to the literature, only one patient out of 70 died when BSA involvement was less than 10%. In contrast, the mortality rate was 11% for patients with 10-30% BSA involvement and 35% for patients with BSA involvement exceeding 30%.6-9
We report seven cases of patients admitted to a burns unit with severe bullous skin diseases. We would like to draw the attention of dermatologists and paediatricians to these serious diseases and to how they can be managed.
Patients and methods
This study was performed in King Saud Hospital, Unizah, Kingdom of Saudi Arabia, between 2001 and 2005. All patients of paediatric age suffering from bullous skin disease affecting more than 10% BSA were admitted to a burns unit with the appropriate temperature (30-32 °C), humidity, positive pressure ventilation, and infrared lamps to prevent infection. The patients were evaluated according to their history of pre-existing diseases and medication, initial clinical diagnosis, suspected cause, histological findings, extent of skin involvement (total body surface area [TBSA]), mucosal involvement, definitive diagnosis, therapy, complications, and outcome. All patients were treated by the team of plastic surgeons from the burns unit, in collaboration with paediatric burns staff.
Patients were classified according to their history of infection or drug intake and the following clinical criteria: TBSA involved (extent of epidermal detachment) and the morphology and localization of the skin lesions. In addition, the presence and distribution of mucosal lesions were considered useful for including a disease within that spectrum but not for classifying it further.
Swab cultures and histopathological examination were performed in all patients to differentiate the skin disorders.
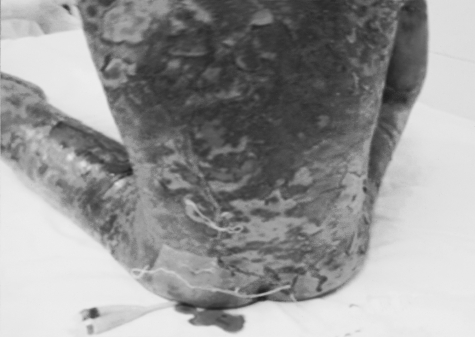
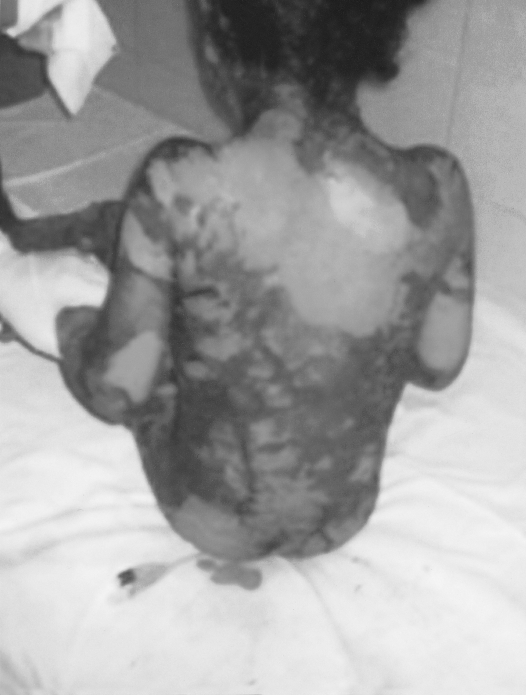
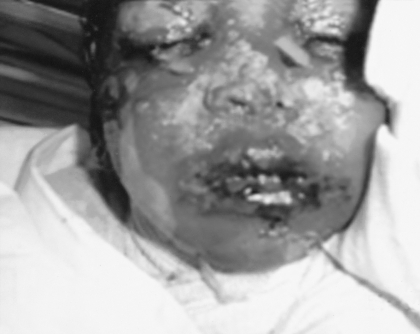
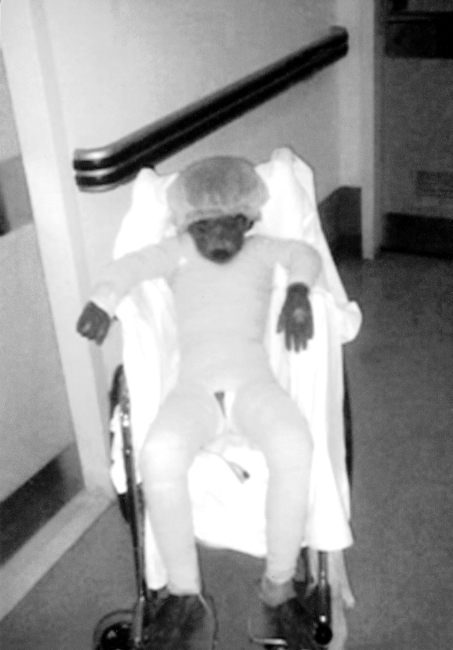
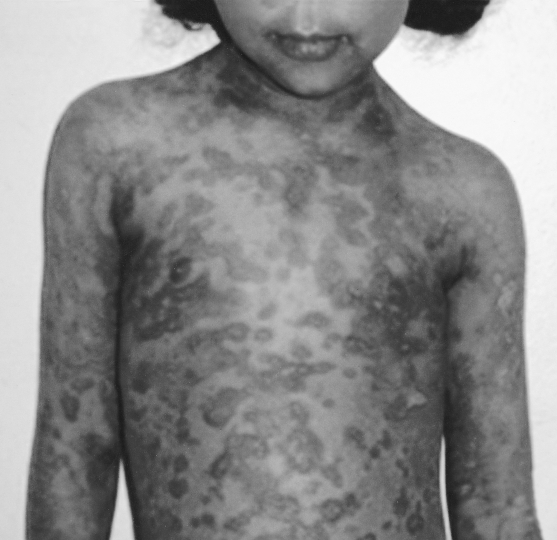
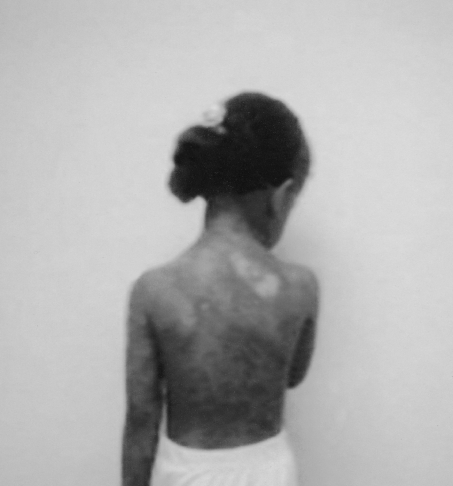
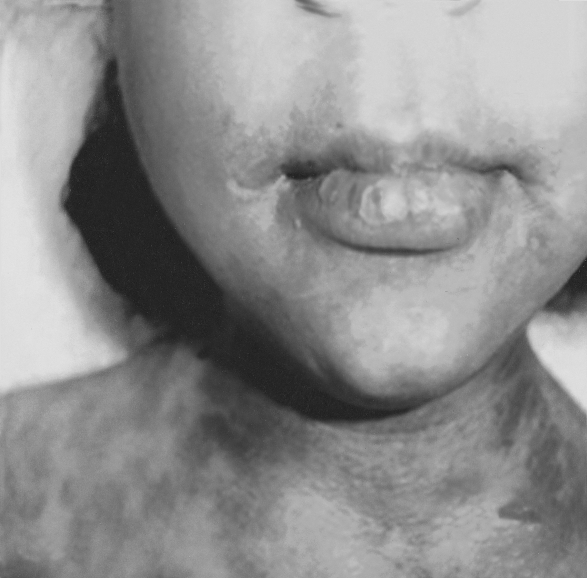
On examination, all the children (temperature range, 38-39.5 °C) looked very ill, toxic, and lethargic, with flaccid, large bullae over an erythematous base that coalesced in large, easily detachable plaques (over 3 cm wide) and with widespread areas of skin detachment over the face, limbs, abdomen, and back (Fig. 1-4). The skin rashes spread rapidly to cover approximately 30-80% TBSA of the body (15-35% desquamation and about 15-45% intact bullous formation). The oral cavity, nasal mucosa, genitalia, and eyes were involved in the five TEN patients but not in the other two patients. The lesions generally progressed in 3-4 days with exfoliation of the skin in some parts, where rubbing pressure over apparently intact skin areas caused the epidermis to peel away easily (Nikolsky's sign) (Fig. 5). Laboratory investigations revealed leukopenia; the WBC count was 2300 to 3800, with hypoalbumaemia in all patients. Cultures from cutaneous lesions, blood, urine, and the throat were negative, except in one patient in whom skin and blood cultures revealed methicillin resistant Staphylococcus aureus (MRSA). Skin biopsy showed necrosis of basal layer cells without massive inflammatory infiltration of the dermis, necrosis of keratinocytes involving all the epidermis with diffuse detachment of the epidermis at the level of the basal membrane, vacuolization of the basement membrane, the formation of subepithelial bullae, and necrosis of epidermal keratinocytes. Analysis of a detached skin section revealed only full-thickness epidermal necrosis in the five TEN patients and the generalized drug eruption patient, and an intraepithelial/intraepidermal cleavage plane in the granular layer in the patient diagnosed as having SSSS. Haemograms, biochemical tests, and coagulation tests were performed on a daily basis, coupled with regular collection of specimens for bacteriological analysis (swabs of affected areas, sputum, blood, and urine). Chest X-rays were done daily on the first seven days. The patients were managed in burns unit with i.v. fluids according to the standard formula applied in burns patients (Parkland formula). An indwelling silicon urinary catheter was inserted to monitor urine output (1 ml/kg/h was considered an acceptable minimum, as in thermal lesions) and to prevent post-healing urethral stricture. No prophylactic systemic antibiotics were given. Antibiotic therapy was administered only for infections proved by bacterial cultures or in cases of high clinical suspicion of airway infection or superinfection of the skin lesions. In the patient found to be suffering from SSSS, a combination of vancomycin and piperacillin was started immediately. Dexamethasone 0.5 mg/kg/day was given for 10 days to patients diagnosed as TEN, and total parenteral nutrition was started on day 4 using a peripheral catheter. The patients were also encouraged to use an oral support for their rehydration and nutrition, as well as ranitidine, immunoglobulin 400 mg/kg for four days, and antiseptic eye drops with oxytetracycline eye ointment. Oral gastrointestinal decontamination in the form of antifungal and metronidazole syrup was started on admission. Fever continued for three to five days, but new eruptions had ceased by day 5 in most cases. The patient with SSSS died on day 4 post-admission in spite of vigorous resuscitative measures. In the other patients, there were no chest or gastrointestinal complications, and the leukocyte count returned to normal by day7. Full oral feeding was resumed by day 10. Local antiseptic agents were applied after removal of crusts from oral and nasal desquamation. Conservative surgical treatment was selected and hydrotherapy was performed using chlorhexidine diluted in the hydrotherapy tank. Debridement of desquamated skin was effected, keeping the bullae intact, and routine skin cultures and sensitivities were obtained in all patients. Tulle gras was used over the desquamated areas and all the body was dressed with absorbable dressing and then bandaged (Fig. 6). Open dressings were used for the face with fucidine skin ointment after cleaning with saline and removing loose crusts. The first dressing was kept for five days to prevent more exfoliation. The second dressing was applied in the same manner and kept unchanged for one week. When we found signs of healing, an occlusive dressing was applied again and removed after one week, with complete healing, and kept exposed. Skin care was performed daily, using zinc oxide cream. An ocular antiseptic and/or antibiotic solution was administered to prevent accumulation of abrasive crusts over the cornea, and an ophthalmologist made a daily observation. Physiotherapy started immediately after application of the occlusive dressing in order to ascertain joint mobility. The surviving patients were followed up in our plastic surgery clinic for 3-6 months after discharge from hospital.
Fig. 1. Severe exfoliation of skin of back, buttocks, and thighs.
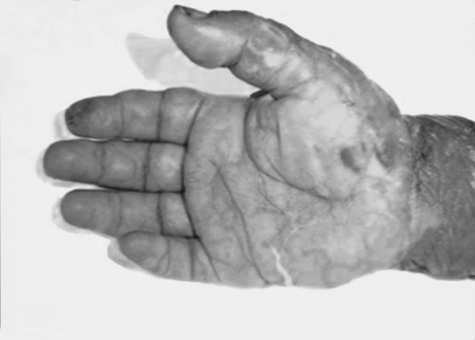
Fig. 4a. Bullous eruption of palm and skin exfoliation.
Fig. 4b. Extensive eruption on dorsum of hand.
Fig. 5. Examination by ophthalmologist leads to peeling of the skin (Nikolsky's sign).
Fig. 2. Back, buttocks, and thighs after removal of second dressing (10 days after admission when healing started).
Fig. 3. Severe exfoliation of face with involvement of skin, eyes, nose, and oral mucosa.
Results
During the study period, seven children with severe bullous skin disease were admitted to the burns unit, King Saud Hospital, Unizah, Kngdom of Saudi Arabia. Most of the patients were referred from outside institutions, none of which was a burns centre.
Analysis of the seven cases of severe bullous skin diseases in the burns unit. Six of the children were female and one male. The average age was 7 yr (range, 5 to 11 yr). The affected skin area, defined as the area of intact bullous and detached epidermis, ranged from 30 to 85% TBSA, with an average of 53%. The total length of stay in the burns unit ranged from 15 to 29 days, with an average of 19 days. Clinical criteria and histological examination indicated led to a diagnosis of TEN in five patients and of SSSS in one patient (involving 30% TBSA with no mucous membrane involvement after incision and drainage of a large gluteal abscess); the seventh patient presented the clinical appearance of a generalized drug eruption, a diagnosis which was confirmed by skin biopsy, the suspected causal agent being Cotrimoxazol.
Microbiological data. There was one positive blood and wound culture. The cultured organisms were mixed bacterial flora, including MRSA. In this patient, the wounds were debrided and cleaned with chlorhexidine solution and fucidine skin ointment. However, the patient died of severe sepsis with multi-organ failure on day 4 post-admission day (this patient presented to us five days after the onset of skin eruptions).
During regular follow-up visits at the out-patient department of the paediatric department and burns unit, the lesions showed a good evolution, although there were some sequelae. Most of the patients presented slight cutaneous hypochromia in the face and marked hypochromic maculae on the affected areas of the anterior trunk, dorsum, and limbs (Fig. 7-9). All the patients were advised to perform skin hydration with a fat cream and to use a sunscreen.
Fig. 6. Child after occlusive dressing.
Fig. 8. Skin healing with hyperpigmentation patches.
Fig. 7. Complete healing with hypopigmented patches.
Fig. 9. Healing of mucosa and skin hyperpigmentation.
Discussion
TEN (toxic epidermal necrolysis), or Lyell's syndrome, is a widespread life-threatening mucocutaneous disease. It may be idiopathic in origin or, most frequently, an idiosyncratic response to the administration of a certain medication or drug, irrespective of dosage.4 It is characterized by rash, bullae, and diffuse exfoliation of wide cutaneous surface areas, as in second-degree burns. Separation of the dermal-epidermal junction causes Nikolsky's sign and gives the skin the typical "wet dressing" appearance. In 1956 Lyell distinguished two entities in the description of TEN: SSSS (staphylococcal scalded-skin syndrome and what is today defined as TEN.1
TEN differs from SSSS because it is drug-related (no infectious causes have been demonstrated) and because not only the skin but also the mucosae are commonly involved. TEN is characterized by necrosis of all skin layers, with a serious deterioration of health conditions and high mortality (approximately 30%). Other problems of classification are due to the relationship between TEN and EM (erythema multiforme). EM and SJS (Stevens-Johnson syndrome) are two skin disorders that share some of the clinical features of TEN, making it sometimes confusing, or even controversial, to tell them apart. Chan et al.2 proposed some criteria to distinguish the three situations: EM would thus be characterized by the presence of localized lesions less than 3 cm wide; it may or may not present as targets and cover less than 20% of the body surface; and there may be an absence of mucosal involvement or the involvement of one single area, with minimum symptomatology. In SJS, the initial lesions, which may or may not be target-shaped, are also less than 3 cm wide but may coalesce and involve between 10 and 20% of the body surface; SJS always includes the involvement of two or more mucosal surfaces and fever may be high. In TEN, the affected area covers at least 20-30% of the body surface, including non-exposed areas, presenting as large bullae over an erythematous base that coalesces in large, easily detachable plaques (over 3 cm wide); fever is high and there is always mucosal involvement.10-13
Many factors are involved in the aetiology of TEN. Accumulated evidence suggests that, unlike humoral toxicity mediated by auto-antibodies or by the complement system, this is only a cell-mediated cytotoxicity situation,5-8 in which it has been possible to establish the presence of mononuclear cells adjacent to necrotic keratinocytes and a change in lymphocyte subsets of both peripheral blood and inflammatory cell infiltrates (with CD4+ T-lymphocytes in the upper dermis layer and CD8+ lymphocytes in the epidermis and the dermo-epidermal junction).9 Whatever its mode of action, this immunological response is certainly modulated by a number of factors, including a potential genetic predisposition. The possibility that a previous viral infection may trigger the whole process is an issue to be considered.10,11 Among the series of medications described as being capable of causing TEN, there are sulphonamides (the commonest trigger in adults,12 accounting for approximately one-third of all cases), antiepileptic drugs (particularly phenytoin, the most frequent agent among children),13 oral penicillins (ampicillin, amoxicillin), and non-steroidal anti-inflammatory agents with a prolonged half-life (especially those derived from pirazolone or oxicam).3,14 Other drugs reported less commonly in the literature range from paracetamol to various antibiotics, tuberculostatics,15 and vaccines.16 Surprisingly, the use of corticosteroids may also be associated with TEN.17 In our study the children received amoxicillin and paracetamol days before appearance of the rash in the five TEN patients and Cotrimoxazol in the child diagnosed as having a generalized drug eruption.
Immunohistological studies on cutaneous biopsies of patients affected by early TEN have shown a widespread dermal infiltrate in the dermal-epidermal junction, composed primarily of helper CD4 T-lymphocytes and CD8 T-cells, suggesting a cell-mediated reaction versus keratinocytes. It has been shown that in the fluid contained in the bullae of patients with TEN, T-lymphocytes predominate in the initial phases, while subsequently the cells of the monocyte-macrophagic line prevail10 and probably contribute to the progression of the necrosis through TNF production.18,19
TEN must be regarded as a multisystemic pathology. With regard to the respiratory apparatus, there may be erosions of the bronchial mucosa and respiratory insufficiency as a result of increased capillary permeability; pneumonia is also possible.20 Glomerulonephritis and tubular necrosis can occur, as also alteration of gastrointestinal mucosa permeability. There may be hepatic involvement, pancreatitis, alterations of glycaemia, and hypophosphoraemia, which can cause disturbances of consciousness. In many patients death occurs in the first or second week after onset, generally because of haemodynamic shock, intestinal haemorrhages, pulmonary oedema, renal failure, systemic infections, fluid loss, or diffuse intravascular coagulation.5,11 The mortality rate is about 30% (range, 20 70%).
The treatment of TEN includes protection of the cutaneous and mucosal surfaces involved, monitoring of the electrolytic balance, fluid replacement, nutritional support, and the prevention and treatment of infection. With regard to this last aspect, antibiotic prophylaxis is not recommended as a routine measure because of the risk of cross reactivity with the drug involved and the possibility of infection with resistant bacteria.11,21 We started antibiotics only if the culture results proved positive. The use of corticosteroids is a much debated question - we started dexamethasone on the second day of admission and continued for ten days. Some reports have described a dramatic improvement in TEN patients treated with corticosteroids.1 However, TEN can occur in patients subjected to long-term corticosteroid therapy: a study in Germany showed that 5% of patients with TEN had been on steroid therapy for at least a week before the clinical onset.22
Halebian et al.6 compared a first group of 15 patients affected by TEN or SJS and treated with high-dose corticosteroid therapy with a second group of patients not so treated after admission to a burns unit.6 The incidence of septic complications was not significantly different in the two groups, but increased mortality was observed in cases of sepsis.
The use of intravenous immunoglobulins (IVIG) has been reported in the literature. We gave IVIG 400 mg/kg for four days. The action mechanism of IVIG is not fully known: they probably inhibit the activity of the cytokines by reducing the expression of adhesion molecules and/or increasing the activity of T-suppressor cells. According to a recent study, IVIG prevent the apoptosis of keratinocytes mediated by a particular cell receptor called FAS (CD95).12,14 Also, the efficacy of IVIG can be explained by their powerful anti-inflammatory activity and by the protection they provide from possible infections due to common pathogen agents (sepsis is the principal cause of death in patients with TEN).
N-acetylcysteine, which is frequently used as a mucolytic agent and also in the treatment of paracetamol intoxication, shows effective activity when used in high doses in the treatment of patients with TEN. Its activity is thought to be related to the support of the anti-oxidant capability of cells, through the increase of intracellular levels of cysteine required for the production of glutathione (serving as a buffer to oxidant agents), and/or to the inhibition of the production of cytokines that mediate immunological reactions, such as TNF and interleukin-1 (IL 1), and of oxygen free radicals.10 A number of studies confirm the effectiveness of plasmapheresis in Lyell's syndrome.
Chaiademenos13 used plasmapheresis to treat seven patients affected by TEN in 30-80% of the skin surface involving mucous surfaces. In six patients the therapeutic scheme was used on alternate days, while the seventh patient was treated daily. None of the patients died and there were no side effects or complications in a follow-up lasting eight years.
Some researchers have proposed pentoxifylline - usually administered as a haemorrheological agent to reduce blood viscosity and improve flow conditions - as another drug to be considered for the treatment of TEN.9 In this particular case, its activity would be to interfere with the binding of T-lymphocytes to keratinocytes and also to inhibit the production of cytokines by macrophages and keratinocytes, including TNF, IL-1, and IL-6. Some researchers recommend the use of granulocyte colony-stimulating factors to overcome neutropenia associated with TEN, thus reducing the risk of sepsis. The literature also contains a reference to the treatment of such patients with a few sessions in a hyperbaric oxygen chamber, in view of this technique's capacity to enhance epidermal regeneration and dermal metabolism, associated with an antiseptic activity and a potential immunosuppressive effect; however, the effectiveness of this method has yet to be confirmed.4
Many controversies regarding topical therapy concern the application of zinc sulphate, up to the time of coverage with cryopreserved allografts,4,6 and the use of commercial preparations with polyethylene glycol, such as nitrofurazone, proved to be toxic when used for extensive burns. Perhaps the most widely used chemotherapeutic treatment in burns units is silver sulphadiazine, as it is easy to apply and has few side effects. It is not advisable in these cases, however, owing to its relationship with the onset of the illness.8,12 Some researchers recommend bandages soaked in silver nitrate 0.5%. These do not produce allergic reactions and do not inhibit epithelialization, but they can cause severe hyponatraemia if used extensively. The use of a cryopreserved allograft is closest to the ideal coverage available, as it reduces pain, hydroelectrolytic cutaneous losses, and bacterial multiplication, improving thermoregulation and promoting re-epithelialization.3-5 It is applied under general anaesthesia, avoiding endotracheal intubation so as not to increase damage to the upper airways, which are often involved in the syndrome, after operative debridement of slough-detached epithelium. If this biological dressing is unavailable, amnion or porcine cutaneous xenografts, or skin substitutes may be used.3,4,7,813,23
We overcame all these controversies by using a very simple, cheap technique, namely closed dressings with tulle gras and several layers of gauze, cotton, and crêpe bandages. These were changed every five to seven days at the same time as hydrotherapy. The advantages of this method are many: it is simple and can be performed in places lacking allograft facilities; there is no need of daily dressing, with exposure of affected areas and the risk of contamination, plus the risk of removing newly formed epithelium with frequent dressing; there is no need of anaesthesia but only sedation during dressing changes; and covering the whole body with bulky dressings prevents hypothermia and acts as a good protection against shearing movement of the skin with the bed (Nikolsky's sign) and the loss of more protecting skin. We obtained excellent results using this technique in such patients and advise its use in all TEN patients. Furthermore, burns units have specially trained personnel for topical care and provide intensive monitorization and the most aseptic environment.
Regarding prognosis, numerous clinical and laboratory factors are related to poor prognosis in TEN patients. These include the involvement of extensive skin areas, delay in withdrawing non-essential medication, extremes of age, a previously bad general condition, the intake of multiple drugs, the need for many blood transfusions, and the prolonged re-epithelialization time of affected areas (more than nine days). The results of laboratory tests show that persistent neutropenia is the condition most commonly related to higher mortality, revealing reduced ability to resist infectious agents. There is a very important logistic issue also associated with higher morbidity and mortality, namely delay (more than 48 h) in transferring patients to an intensive care unit with adequate conditions for their management, i.e. a burns unit.
Conclusion
Recommendation. The high mortality and morbidity associated with TEN make it necessary to familiarize all physicians with this syndrome, in particular those practising in health care centres and county and district hospitals, given the high risk arising from late diagnosis and therapy. In all patients presenting with erythematous or bullous eruptions associated with drug intake, this differential diagnosis should be excluded systematically and at an early point in time. Prompt referral to hospitals possessing a burns unit should be routine practice in suspect cases. The early suspension of all non-essential medication is crucial and this may alter the prognosis. The patients should be regarded as burn patients, and the new treatment procedures described here present promising signs and should be carefully considered and implemented, whenever possible, in patients with TEN.
References
- 1.Lyell A. Toxic epidermal necrolysis: An eruption resembling scalding of the skin. Br. J. Dermatol. 1956;68:355–361. doi: 10.1111/j.1365-2133.1956.tb12766.x. [DOI] [PubMed] [Google Scholar]
- 2.Chan H.L., Stern R.S., Arndt K.A. et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Arch. Dermatol. 1990;126:43–44. [PubMed] [Google Scholar]
- 3.McGee T., Munster A. Toxic epidermal necrolysis syndrome: Mortality rate reduced with early referral to regional burn center. Plast. Reconstr. Surg. 1998;102:1018–1022. doi: 10.1097/00006534-199809040-00014. [DOI] [PubMed] [Google Scholar]
- 4.Cabral L., Riobom F., Diogo C., Teles L., Cruzeiro C. Toxic epidermal necrolysis - Lyell's syndrome. Annals of Burns and Fire Disasters. 2004;17:90–102. [Google Scholar]
- 5.Yarbrough D.R. Experience with toxic epidermal necrolysis treated in a burn center. J. Burn Care Rehabil. 1996;17:30–33. doi: 10.1097/00004630-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Halebian P.H., Corder V.S., Madden M.R. et al. Improved burn center survival of patients with toxic epidermal necrolysis managed without corticosteroids. Ann. Surg. 1986;204:503–512. doi: 10.1097/00000658-198611000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Doval I., Lecleach L., Bocquet H., Otero X.L. et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: Does withdrawal of causative drugs decrease the risk of death? Arch. Dermatol. 2000;136:323–327. doi: 10.1001/archderm.136.3.323. [DOI] [PubMed] [Google Scholar]
- 8.Redondo P., De Felipe I., de la Peña A., Aramendia J.M., Vanaclocha V. Drug-induced hypersensitivity syndrome and toxic epidermal necrolysis. Treatment with N-acetylcysteine. Br. J. Dermatol. 1997;136:645–646. doi: 10.1111/j.1365-2133.1997.tb02175.x. [DOI] [PubMed] [Google Scholar]
- 9.Sanclemente G., de la Roche C.A., Escobar C.E., Falabella R. Pentoxifylline in toxic epidermal necrolysis and Stevens-Johnson syndrome. Int. J. Dermatol. 1999;38:878–879. [PubMed] [Google Scholar]
- 10.Brambilla G., Brucato F., Angrisano A., Palmieri G. Treatment of toxic epidermal necrolysis (TEN) Annals of Burns and Fire Disasters. 2002;15:17–21. [Google Scholar]
- 11.Paquet P., Jacob E., Damas P., Pierard G.E. Treatment of druginduced toxic epidermal necrolysis (Lyell's syndrome) with intravenous human immunoglobulins. Burns. 2001;6:652–655. doi: 10.1016/s0305-4179(01)00005-5. [DOI] [PubMed] [Google Scholar]
- 12.Tristani-Firouzi P., Petersen P.J., Saffle J.R., Morris S.E., Zone J.J. Treatment of toxic epidermal necrolysis with intravenous immunoglobulins in children. J. Am. Acad. Dermatol. 2002;47:548–552. doi: 10.1067/mjd.2002.127249. [DOI] [PubMed] [Google Scholar]
- 13.Chaidemenos G.C., Chrysomallis F., Sombolos K., Mourellou O. et al. Plasmapheresis in toxic epidermal necrolysis. Int. J. Dermatol. 1997;36:218–221. doi: 10.1046/j.1365-4362.1997.00192.x. [DOI] [PubMed] [Google Scholar]
- 14.Bachot N., Revuz J., Roujeau J.C. Intravenous immunoglobulin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis: A prospective non-comparative study showing no benefit on mortality or progression. Arch. Dermatol. 2003;139:33–36. doi: 10.1001/archderm.139.1.33. [DOI] [PubMed] [Google Scholar]
- 15.Cornish P., Mittmann N., Gomez M., Cartotto R.C., Fish J.S. Cost of medications in patients admitted to a burn center. Am. J. Clin. Dermatol. 2003;4:861–867. doi: 10.2165/00128071-200304120-00005. [DOI] [PubMed] [Google Scholar]
- 16.Narayanan V.S., Mamatha G.P., Ashok L., Rajashekar N. Stevens-Johnson syndrome due to I.V. ceftriaxone - a case report. Indian J. Dent. Res. 2003;14:220–223. [PubMed] [Google Scholar]
- 17.Bygum A., Gregersen J.W., Buus S.K. Acetaminophen-induced toxic epidermal necrolysis in a child. Pediatr. Dermatol. 2004;21:236–238. doi: 10.1111/j.0736-8046.2004.21309.x. [DOI] [PubMed] [Google Scholar]
- 18.Schmutz J.L., Barbaud A., Trechot P. Toxic epidermal necrolysis and celecoxib (Celebrex) Ann. Dermatol. Venereol. 2004;131:107–107. doi: 10.1016/s0151-9638(04)93559-4. [DOI] [PubMed] [Google Scholar]
- 19.Aguiar D., Pazo R., Duran I., Terrasa J., Arrivi A., Manzano H., Martin J., Rifa J. Toxic epidermal necrolysis in patients receiving anticonvulsants and cranial irradiation: A risk to consider. J. Neurooncol. 2004;66:345–345. doi: 10.1023/b:neon.0000014538.31561.bc. [DOI] [PubMed] [Google Scholar]
- 20.Spornraft-Ragaller P., Ragaller M., Meurer M. Toxic epidermal necrolysis induced by NSAID. J. Rheumatol. 2003;62:474–475. doi: 10.1007/s00393-003-0535-6. [DOI] [PubMed] [Google Scholar]
- 21.Kelemen J., Cioffi W., McManus W., Mason A., Pruitt B. Burn center care for patients with toxic epidermal necrolysis. J. Am. Coll. Surg. 1995;180:273–278. [PubMed] [Google Scholar]
- 22.Peters W., Zaidi J., Douglas L. Toxic epidermal necrolysis: A burn-centre challenge. Can. Med. Assoc. J. 1991;144:1477–1480. [PMC free article] [PubMed] [Google Scholar]
- 23.Atiyeh B.S., Kayle D.I., Nasser A.A. Burn-like syndromes. Annals of Burns and Fire Disasters. 1999;12:39–42. [Google Scholar]