Summary
We experimentally studied the effects of antithrombin III (AT III) on bacterial translocation (BT) and intestinal morphology in the early period of burn injury. For this aim, 30 male albino rats were used. A sham burn group (group 1, no. 10) was exposed to 21 °C water. A burn group (group 2, no. 10) and a burn + AT III group (group 3, no. 10) were exposed to 95 °C water for 10 sec, producing full-thickness burn in 30% of the total body surface area. In group 3 the rats received 250 U/kg AT III via the right jugular vein, 15 min before burn injury. One ml 0.9% NaCl was given as a placebo in group 1 and in two rats by the same route. All group 3 rats were sacrificed on day 2 post-burn using an overdose anaesthetic. Cultures of the mesenteric lymph nodes, liver, spleen, blood, and caecal contents were performed. Histopathological examinations, including polymorph nuclear leukocyte (PNL) infiltration and villus morphologies, were qualitatively evaluated on the resected distal ileal segment. The incidence of BT was 1/10 (10%) in group 1, 7/10 (70%) in group 2, and 1/10 (10%) in group 3. A significant increase in BT incidence was observed in group 2 compared with groups 1 and 3 (p = 0.02), while a significant decrease in BT incidence was found in group 3 rats with AT III treatment. Although the PNL infiltration rate was reduced by AT III treatment, a significant decrease was not found compared with group 2 (50% and 90%, respectively). On the other hand, the villus degeneration rate was significantly reduced by AT III treatment compared with group 2 (30% and 90%, respectively). These results suggest that the incidence of BT was enhanced by the burn injury. AT III decreased the incidence of BT in the early period of burn injury. We conclude that AT III can be effectively used to protect from intestinal mucosal injury and to prevent bacterial translocation, especially in early post-burn period.
Keywords: ANTITHROMBIN III, AT III, PREVENTS, BACTERIAL, TRANSLOCATION, BURN, INJURY, EARLY
Abstract
Les Auteurs ont conduit une étude expérimentale des effets de l'antithrombine III (AT III) sur la translocation bactérienne (TB) et la morphologie intestinale pendant la période précoce des brûlures. Pour ce but ils ont utilisé 30 rats mâles albinos. Un groupe simulé de brûlures (groupe 1, no. 10) a été exposé à de l'eau à 21 °C. Un groupe de rats brûlés (groupe 2, no. 10) et un groupe de rats brûlés + AT III (groupe 3, no. 10) ont été exposés à de l'eau à 95 °C pour 10 sec pour produire des brûlures à toute épaisseur dans 30% de la surface totale corporelle. Les rats du groupe 3 ont reçu 250 U/kg AT III à travers la veine droite jugulaire 15 min avant la lésion par brûlure. Un ml 0,9% NaCl a été administré comme placebo dans le premier groupe et dans deux rats par la même voie. Tous les rats du troisième groupe ont été sacrifiés le deuxième jour après la brûlure moyennant une overdose d'anesthésie. Des cultures des ganglions lymphatiques mésentériques, du foie, de la rate, du sang et du contenu cæcal ont été effectuées. Les examens histopathologiques, y inclus l'infiltration des leucocytes polymorphes nucléaires (LPN) et les morphologies des villosités ont été qualitativement évalués sur le segment distal iléal sectionné. La fréquence de la TB était 1/10 (10%) dans le premier groupe, 7/10 (70%) dans le deuxième et 1/10 (10%) dans le troisième. Un incrément significatif de la fréquence de la TB a été noté dans le deuxième groupe par rapport aux groupes 1 et 3 (p = 0,02), tandis qu'un incrément significatif de la TB a été observé dans les rats du troisième groupe traités avec l'AT III. Bien que le taux d'infiltration des LPN ait été réduit par le traitement avec l'AT III, une diminution significative n'a pas été observée par rapport au deuxième groupe (50% et 90%, respectivement). Par contre, le taux de dégénération des villosités a été réduit en manière significative par le traitement avec l'AT III par rapport au deuxième groupe (30% et 90%, respectivement). Ces résultats suggèrent que la fréquence de la TB a été incrémentée par la brûlure. L'AT III a diminué la fréquence de TB dans la phase précoce de la lésion. Les Auteurs concluent que l'AT III peut être utilisé avec efficacité pour protéger contre les lésions mucosales intestinales et pour prévenir la translocation bactérienne, particulièrement dans la phase précoce des brûlures.
Introduction
It is well established that burn injury promotes bacterial translocation (BT) from the gastrointestinal tract to the mesenteric lymph nodes (MLNs) and beyond.1-3 Mucosal barrier deficiency, changes in gut microflora, and impaired immune defence mechanisms are reported to be the main causes of BT in burn injury.4-10 In particular, disruption of the mucosal barrier function due to intestinal ischaemia is accepted as the main cause of increased BT incidence and endotoxaemia in the early period of burn injury.2,4,7-11 Conversely, studies have demonstrated that attempts to prevent intestinal ischaemia reduce BT and endotoxaemia.2,8,9,11
It was recently demonstrated that antithrombin III (AT III), which is a natural anticoagulant, prevents intestinal mucosal damage due to ischaemia-reperfusion injury.12 Clinical studies have indicated that severely burned patients frequently present alterations of the coagulation system.13-15 A decrease in the AT III serum level is important among these.13,16-18 However, the effects of AT III on BT in burn injury have not previously been investigated.
The present study evaluates the effects of AT III on BT and intestinal morphology in the early period of burn injury.
Materials and methods
Thirty male albino rats (weight, 150-200 g) were used in this study. All animals were obtained from the Surgical Research Centre of Pamukkale University Faculty of Medicine with approval. The rats, housed five per cage in an animal room maintained at 22 °C with a 12-h light period, were fed the same amount of a laboratory pelleted diet. They were randomly assigned to three groups: 1. sham burn group (group 1, no. 10); 2. burn group (group 2, no. 10); and 3. burn + AT III group (group 3, no. 10).
The rats were anaesthetized with an intramuscular injection of 50 mg/kg ketamine hydrochloride and 10 mg/kg xylazine. Total body surface area (TBSA) was calculated using the formula described by Horst et al.19 The backs of all the rats were shaved to allow direct skin contact with the hot water, determined at 30%. The right jugular vein of all the rats was exposed by dissection. One ml 0.9% NaCl was given as a placebo in group 1 and 2 rats via right jugular vein injection using a 26-gauge catheter. Group 3 rats received 250 units/kg AT III (Kybernin P, human antithrombin III, pasturized, Behringwerke, Marburg, Germany, via Farmatek, ïzmir) by the same route 15 min before burn injury. Group 1 rats were exposed to 21 °C water for control purposes. Group 2 and 3 rats were exposed to 95 °C hot water for 10 sec to produce a full-thickness burn injury in 30% of TBSA, according to the standard animal burns model described by Walker and Mason.20 All rats were resuscitated with 25 ml/kg 0.9% NaCl via subcutaneous injection.
All three groups of rats were sacrificed on day 2 postburn by an overdose anaesthetic. Laparotomy was performed and a 0.5 ml blood sample was drawn from the inferior vena cava for blood culture. MLNs, spleen, liver, and caecal content were removed and homogenized for quantitative cultures, after determination of the weight of the samples. A 10-cm distal ileal segment from the ileocaecal valve was resected for pathological examination. The bacteria per g tissue/ml were estimated 24-48 h later as colony-forming units. The presence of 100 or more bacteria per g tissue/ml was accepted as the criterion for translocation. Histopathological specimens were evaluated blind by a pathologist. On histopathological examination, the neutrophil infiltration rate and villus morphologies were evaluated qualitatively.
Data were reported as the mean ± standard error of mean (SEM) where appropriate. The incidence of BT and alterations in villus morphologies were compared by the chisquare with continuous correction and Fisher's exact test. The number of translocating bacteria and the caecal population of bacteria was compared using the Kruskal-Wallis analysis of variance and the Mann-Whitney U tests. Differences were considered significant when p was less than 0.05
Results
The incidence of BT was 1/10 (10%) in group 1, 7/10 (70%) in group 2, and 1/10 (10%) in group 3 (Table I). The results for translocation were not significant between groups 1 and 3 (p > 0.05), while a significant increase in BT incidence was observed in group 2 compared with groups 1 and 3 (p < 0.02). These data show that treatment with AT III significantly decreased the incidence of BT.
Table I. Incidence and sites of bacterial translocation.
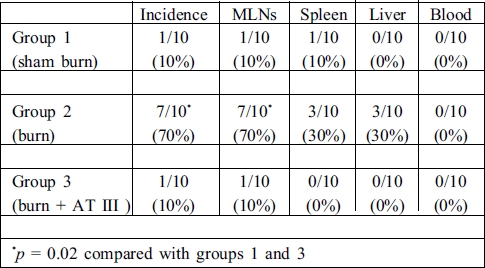
The main site of translocation was only to MLNs (7/10, 70%) in the burned rats. Although the incidence of BT was 30% for spleen and liver in group 2, this was non-significant compared with groups 1 and 3 (p > 0.05). No microorganism was isolated from the blood samples. Escherichia coli was the major micro-organism isolated in the cultures. In addition to E. coli, Proteus mirabilis and Klebsiella sp. were isolated from MLNs in only one rat in group 2.
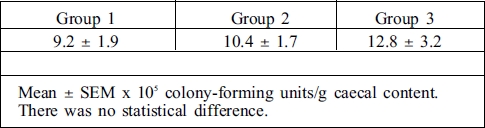
There was no difference between groups 1, 2, and 3 with regard to caecal cultures (p > 0.05) (Table II).
Table II. Quantitative results of caecal content.
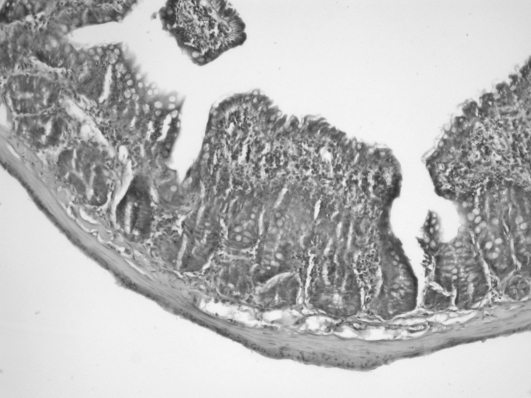
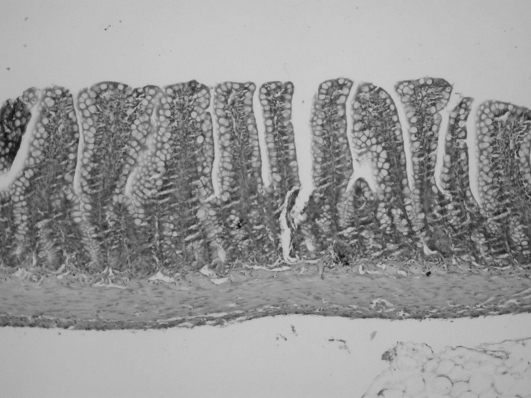
Histopathological examinations revealed that polymorph nuclear leukocyte (PNL) infiltration was promoted by burn injury. The rate of PNL infiltration was 0/10 (0%) in group 1, 9/10 (90%) in group 2, and 5/10 (50%) in group 3. Although PNL infiltration rate was reduced by treatment with AT III, a significant decrease was not found compared with the control group (Table III). Conversely, in the burn + AT III group of rats, villus morphologies were massively prevented by the administration of AT III, while a significant degeneration was found in burned rats (Fig. 1) (Table III).
Table III. Polymorph nuclear leukocyte (PNL) infiltration and villus degeneration rate.
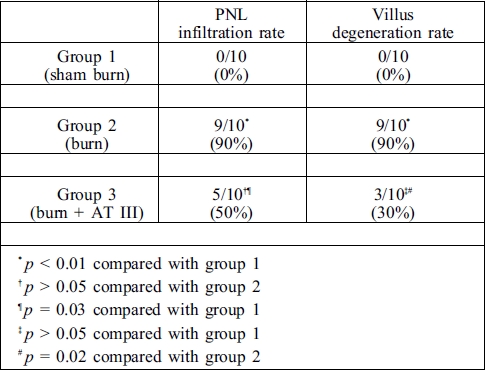
Fig. 1. Ileal mucosal structure 24 h after 30% TBSA burn injury. Fragmentation, degeneration, and atrophy of the mucosal villi are apparent when compared to Fig. 2.
Fig. 2. Normal ileal mucosal structure.
Discussion
Bacterial translocation has been defined as the passage of viable or non-viable bacteria and their products, including endotoxin, across an anatomically intact intestinal barrier to the MLNs and beyond.21,22 BT is caused and enhanced by haemorrhagic shock, burn injury, intestinal obstruction, total parenteral nutrition, trauma, or pancreatitis. 1,22-27 It is well established that burn injury promotes BT. Studies have demonstrated that mesenteric blood flow is decreased by thermal injury.2,4,7-11 This decrease in mesenteric blood flow is most significant during first 8 h after thermal injury, and returns to normal levels within 24 h.2,4,8-11 The alterations in the mesenteric blood flow cause intestinal ischaemia-reperfusion injury. Thus, mucosal barrier dysfunction occurs owing to splanchnic ischaemia and reperfusion injury. It has been implicated as the major cause of post-burn BT, which significantly increases in the first 24 h.2,4,7-11 Although O'Brien et al. reported that early adequate fluid resuscitation decreased BT incidence, based on studies that monitored mesenteric blood flow in burn injury, this splanchnic ischaemia occurs despite rigorous fluid resuscitation. 2,4,8-11-28 It has been reported that thromboxane A2 may be a major cause of a decrease in mesenteric blood flow in the early post-burn period.2-9 Conversely, the administration of OKY-046 (a specific thromboxane synthetase inhibitor), allopurinol (a xanthine oxidase inhibitor), and enalapril (an angiotensin-converting enzyme inhibitor) reduces the decrease in mesenteric blood flow and early BT in burn injury.2,8,9,11 Normally, this process is transient, and in uncomplicated burn injury the intestinal mucosa generally heals, with gut barrier functions being repaired within four days.7 For this reason, BT does not occur or increase four days post-burn in uncomplicated burn injury. However, burn wound sepsis, antibiotherapy, immune deficiency, and the use of H2-receptor blockers may cause prolonged BT.1,5-729
It is well established that neutrophils play a critical role in ischaemia-reperfusion injury.12,30 Thrombin is a terminal serine protease that plays important role in the coagulation system.31 It has also been implicated in inflammation, wound healing, and angiogenesis. Studies have demonstrated that thrombin enhances endothelial P-selectin expression and endothelial platelet-activating factor production. It also induces rapid ICAM-1 expression.31, All these effects cause firm leukocyte adhesion. Ostrovsky et al. concluded that thrombin played a critical role in reperfusion induced leukocyte recruitment (rolling and adhesion) as also in increased microvascular permeability alterations.32 For these reasons, blocking thrombin activity may decrease reperfusion-induced tissue dysfunction.
AT III is an µ2-globulin that inactivates serine proteases, including thrombin, factor Xa, factor IXa, factor XIa, and kallikrein.12,32-34 AT III affects heparin-like glycosaminoglycans (GAGs) on the endothelial cell surface.33,34 The interaction of GAGs is very important for the occurrence of AT III's anti-inflammatory effects. AT III inactivates factor XIIa, kallikrein, and thrombin, which play an important role in the activation of neutrophil infiltration and the neutrophil-induced inflammatory response.32-34 It has also been demonstrated that this interaction promotes the release of prostocyclin I2 (PGI2) from the endothelial cell in vitro and in vivo .33-35 PGI2 is a strong vasodilator. Also, PGI2 inhibits leukocyte activation by inhibiting TNF-µ production from monocytes, increasing intracellular cyclic AMP, and inhibiting neutrophil adhesion to the endothelial cell surfaces.33-35 All of these effects of AT III may prevent tissue injury by neutrophil-induced ischaemiareperfusion injury.32-34 Uchiba et al. demonstrated that intravenous administration of AT III (250 U/kg) significantly inhibited pulmonary neutrophil accumulation and subsequent pulmonary vascular injury.33 This effect of AT III was dose-dependent - 250 U/kg was found to be an effective dose in rats.32,33 Recently, Özden et al. reported that the administration of AT III before intestinal ischaemia prevented mucosal damage.12 They postulated that this effect was related to inhibition of neutrophil infiltration.12 Although in their study heparin and AT III were used in combination, Uchiba et al. demonstrated that the combined use of heparin and AT III did not prevent pulmonary vascular injury.34 They suggested that heparin bound AT III in the circulation, preventing AT III from interacting with GAGs on the endothelial cell surfaces.34
Heparin is an endogenous GAG used in the treatment of burn injury.36 Zapata-Sirvent et al. reported that heparin reduced BT incidence in burn injury.37 They demonstrated that heparin treatment after burn injury decreased BT measured 24 h postburn. Also, in their study, small intestine wet weight and mucosal thickness were increased by heparin treatment.37 They concluded that the administration of heparin prevented gastrointestinal mucosal damage in the early period of burn injury by similar effects to those of AT III, which was generally decreased and required substitution treatment. However, its effects on BT have not been investigated in burn injury.
In our present study, we evaluated the effects of AT III on BT and intestinal mucosal morphology in the early period of burn injury. Our results show that burn injury promoted BT in the early period. On the basis of histopathological studies, we found that mucosal barrier damage occurred owing to burn injury while the intestinal ecological equilibrium was normal. These data suggest that the increase of BT incidence is related to mucosal barrier dysfunction in the early burn period. We also found that AT III decreased the incidence of BT in burn injury, but significantly prevented mucosal villus morphology and integration. Although the PNL infiltration rate was reduced by AT III administration, no significant difference was found compared with the burn injury group. On the basis of these findings, it may be that AT III prevents mucosal barrier damage by stimulating PGI2 production and reducing neutrophil infiltration. Although we were unable to determine the PGI2 levels of serum in this study, we conclude that AT III reduced BT in the early post-burn period by stimulating the production of PGI2. Thus, AT III prevented mesenteric ischaemia and ischaemia-reperfusion injury in thermal injury
Conclusion
In conclusion, in our study AT III reduced the incidence of bacterial translocation incidence in burn injury, especially in the early period. This effect of AT III may be related not only to a reduction in PNL infiltration but also to stimulation of PGI2 production. AT III prevented mucosal damage due to mesenteric ischaemia in the postburn period. For these reasons, we conclude that the administration of AT III in burn injury effectively prevented bacterial translocation and reduced mortality.
References
- 1.Herek O., Kara I.G., Kaleli I. Effects of antibiotics and Saccharomyces boulardii on bacterial translocation in burn injury. Surg. Today. 2004;34:256–260. doi: 10.1007/s00595-003-2677-1. [DOI] [PubMed] [Google Scholar]
- 2.Herndon D.N., Zeigler S.T. Bacterial translocation after thermal injury. Crit. Care Med. 1993;21:S50–S54. doi: 10.1097/00003246-199302001-00010. [DOI] [PubMed] [Google Scholar]
- 3.Maejima K., Deitch E.A., Berg R.D. Bacterial translocation from gastrointestinal tracts of rats receiving thermal injury. Infect. Immun. 1984;43:6–10. doi: 10.1128/iai.43.1.6-10.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron P., Traber L.D., Traber D.L., Nguyen T., Hollyoak M., Heggers J.P., Herndon D.N. Gut failure and translocation following burn and sepsis. J. Surg. Res. 1994;57:197–204. doi: 10.1006/jsre.1994.1131. [DOI] [PubMed] [Google Scholar]
- 5.Herek O., Öztürk H., Özyurt M., Albay A., Çetinkurflun S. Effects of treatment with immunoglobulin on bacterial translocation in burn wound infection. Annals of Burns and Fire Disasters. 2000;13:13–17. [Google Scholar]
- 6.Jones W.G. II, Jones J.P., Richardson R.P., Fahey T.J. III, Calvano S.E., Antonacci A. et alL. Pathophysiologic glucocorticoid elevations promote bacterial translocation after thermal injury. Infect. Immun. 1990;58:3257–3261. doi: 10.1128/iai.58.10.3257-3261.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones W.G. II, Minei J.P., Barber A.E., Rayburn J.L., Fahey T.J. III, Shires G.T. III et all. Bacterial translocation and intestinal atrophy after thermal injury and burn wound sepsis. Ann. Surg. 1990;211:399–405. doi: 10.1097/00000658-199004000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones W.G. II, Barber A.E., Minei J.P., Fahey T.J. III, Shires G.T. III, Shires G.T. Differential pathophysiology of bacterial translocation after thermal injury and sepsis. Ann. Surg. 1991;214:24–30. doi: 10.1097/00000658-199107000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokyay R., Loick H.M., Traber D.L., Heggers J.P., Herndon D.N. Effects of thromboxane synthetase inhibition on post-burn mesenteric vascular resistance and the rate of bacterial translocation in a chronic porcine model. Surg. Gynecol. Obstet. 1992;174:125–132. [PubMed] [Google Scholar]
- 10.Tokyay R., Zeigler S.T., Traber D.L., Stothert J.C., Loick H.M., Heggers J.P., Herndon D.N. Post-burn gastrointestinal vasoconstriction increases bacterial and endotoxin translocation. J. Appl. Physiol. 1993;74:1521–1257. doi: 10.1152/jappl.1993.74.4.1521. [DOI] [PubMed] [Google Scholar]
- 11.Jones W.G. II, Minei J.P., Barber A.E., Fahey T.J. III, Shires G.T. III, Shires G.T. Splanchnic vasoconstriction and bacterial translocation after thermal injury. Am. J. Physiol. 1991;261:H1190–H1196. doi: 10.1152/ajpheart.1991.261.4.H1190. [DOI] [PubMed] [Google Scholar]
- 12.Özden A., Tetik C., Bilgihan A., Calli N., Bostanci B., Yis Ö., Duzcan E. Antithrombin III prevents 60 min warm intestinal ischaemia reperfusion injury in rats. Res. Exp. Med. 1999;198:237–246. doi: 10.1007/s004330050107. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Avello A., Lorente J.A., Cesar-Perez J., Garcia-Frade L.J., Alvarado R., Arevalo J.M. et all. Degree of hypercoagulability and hyperfibrinolysis is related to organ failure and prognosis after burn trauma. Thrombosis Research. 1998;89:59–64. doi: 10.1016/s0049-3848(97)00291-0. [DOI] [PubMed] [Google Scholar]
- 14.Kowal-Vern A., Gamelli R.L., Walenga J.M., Hoppensteadt D., Sharp-Pucci M., Schumacher H.R. The effect of burn wound size on haemostasis: A correlation of the haemostatic changes to the clinical state. J. Trauma. 1992;32:50–57. doi: 10.1097/00005373-199207000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Neely A.N., Warden G.D., Rieman M., Friedberg D.L., Holder I.A. Components of the increased circulating proteolytic activity in paediatric burn patients. J. Trauma. 1992;33:807–812. doi: 10.1097/00005373-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Danielsson P., Nilsson L., Nettelblad H., Sjöberg F. Is there a need for antithrombin III substitution early after burn injury? Burns. 1997;23:300–305. doi: 10.1016/s0305-4179(96)00135-0. [DOI] [PubMed] [Google Scholar]
- 17.Kowal-Vern A., McGill V., Walenga J.M., Gamelli R.L. Antithrombin III concentrate in the acute phase of thermal injury. Burns. 2000;26:97–101. doi: 10.1016/s0305-4179(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 18.Kowal-Vern A., Walenga J.M., McGill V., Gamelli R.L. The impact of antithrombin (H) concentrate infusions on pulmonary function in acute phase of thermal injury. Burns. 2001;27:52–60. doi: 10.1016/s0305-4179(00)00057-7. [DOI] [PubMed] [Google Scholar]
- 19.Horst K., Mend L.B., Lafayette B., Benedict F.G. The metabolism of the albino rat during prolonged fasting at two different environmental temperatures. J. Nutr. 1930;3:177–200. [Google Scholar]
- 20.Walker H.L., Mason A.D. A standard animal burn. J. Trauma. 1968;8:1049–1054. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Berg R.D., Garglinton A.W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect. Immun. 1979;23:403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steffen E.K., Berg R.D. Relationship between caecal population levels of indigenous bacteria and translocation to the mesenteric lymph nodes. Infect. Immun. 1983;39:1252–1259. doi: 10.1128/iai.39.3.1252-1259.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alverdy J.C., Aoys E., Moss G.S. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery. 1988;104:185–190. [PubMed] [Google Scholar]
- 24.Çolak T., Ipek T., Paksoy M., Polat E., Uygun N., Kayaba¸sı B. The effects of cefephim, G-CSF, and sucralfate on bacterial translocation in experimentally induced acute pancreatitis. Surg. Today. 2001;31:502–506. doi: 10.1007/s005950170109. [DOI] [PubMed] [Google Scholar]
- 25.Deitch E.A., Bridges W.M., Ma J.W., Ma L., Berg R.D. Specian R.D.: Obstructed intestine as a reservoir for systemic infection. Am. J. Surg. 1990;159:394–401. doi: 10.1016/s0002-9610(05)81280-2. [DOI] [PubMed] [Google Scholar]
- 26.Salman F.T., Buyruk M.N., Gurler N., Celik A. The effect of surgical trauma on the bacterial translocation from the gut. J. Pediatr. Surg. 1992;27:802–804. doi: 10.1016/0022-3468(92)90368-h. [DOI] [PubMed] [Google Scholar]
- 27.Topaloˇglu Ü.,, Yılmazcan A., Güloˇglu R., Ta¸scıoˇglu O., Müftüoˇglu T., Ünalmı¸ser S. Hypertonic saline prevents early bacterial translocation in haemorrhagic shock. Surg. Today. 1999;29:47–50. doi: 10.1007/BF02482969. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien R., Murdoch J., Kuehn R., Marshall J.C. The effect of albumin or crystalloid resuscitation on bacterial translocation and endotoxin absorption following experimental burn injury. J. Surg. Res. 1992;52:161–166. doi: 10.1016/0022-4804(92)90299-f. [DOI] [PubMed] [Google Scholar]
- 29.Avanoˇglu A., Herek Ö., Ulman ï., Ergün O., Tünger A., Alkanat M., Erdener A. Effects of H2 receptor blocking agents on bacterial translocation in burn injury. Eur. J. Pediatr. Surg. 1997;7:1–4. [PubMed] [Google Scholar]
- 30.Grisham M.B., Hernandez L.A., Granger D.N. Xanthine oxidase and neutrophil infiltration in intestinal ischaemia. Am. J. Physiol. 1986;251:G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerman G.A., Zimmerman T.M., Prescott S.M. Thrombin stimulates the adherence of neutrophils to human endothelial cell in vitro. J. Clin. Invest. 1985;76:2235–2246. doi: 10.1172/JCI112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostrovsky J., Woodman R.C., Payne P., Teoh D., Kubes P. Antithrombin III prevents and rapidly reverses leukocyte recruitment in ischaemia/reperfusion. Circulation. 1997;96:2302–2310. doi: 10.1161/01.cir.96.7.2302. [DOI] [PubMed] [Google Scholar]
- 33.Okojima K., Okojima M. The anti-inflammatory properties of antithrombin III: New therapeutic implications. Semin. Thromb. Hemost. 1998;24:27–32. doi: 10.1055/s-2007-995820. [DOI] [PubMed] [Google Scholar]
- 34.Uchiba M., Okajima K. Antithrombin III (AT III) prevents LPSinduced pulmonary vascular injury: Novel biological activity of AT III. Semin. Thromb. Hemost. 1997;23:583–590. doi: 10.1055/s-2007-996140. [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi T., Umeda F., Inoguchi T., Nawata H. Antithrombin III stimulates prostocyclin production by cultured aortic endothelial cells. Biochem. Biophys. Res. Commun. 1989;163:1404–1411. doi: 10.1016/0006-291x(89)91135-2. [DOI] [PubMed] [Google Scholar]
- 36.Saliba M.J. Heparin in the treatment of burns: A review. Burns. 2001;27:349–356. doi: 10.1016/s0305-4179(00)00130-3. [DOI] [PubMed] [Google Scholar]
- 37.Zapata-Sirvent K.C., Hansbrough J.F., Greenleaf G.E., Grayson L.S., Wolf P. Reduction of bacterial translocation and intestinal structural alterations by heparin in a murine burn injury model. J. Trauma. 1994;36:1–6. doi: 10.1097/00005373-199401000-00001. [DOI] [PubMed] [Google Scholar]