Summary
The clinical significance of Pseudomonas aeruginosa and Escherichia coli is a strong factor for regular monitoring of their sensitivity to both established and novel antimicrobial compounds. Human isolates of these organisms were collected from different pathological sources and tested for their sensitivity to gentamicin - an established aminoglycoside antibiotic - and to honey, a natural product that is generating renewed interest for its therapeutic application. In an agar-cup diffusion method, three undiluted different samples of honey and their 1:2 to 1:6 aq. dilutions showed activity on 100% and 96.4% respectively of Pseudomonas aeruginosa isolates compared with 95.4% of Escherichia coli using either of the undiluted or 1:2 aq. dilutions of the honey samples. Gentamicin used in concentrations of 8.0 and 4.0 µg/ml varied in its activity against both organisms but was generally lower than the antibacterial activity of each undiluted honey and its 1:2 aq. dilution. In the event of therapeutic failure with gentamicin or any other related antibiotics, honey offers a suitable and better alternative in managing infected burn wounds and other forms of infected wounds as well as prophylaxis in trauma wounds.
Keywords: ESCHERICHIA COLI, PSEUDOMONAS AERUGINOSA, COMPARATIVE, BACTERIAL, ANTIBACTERIAL, HONEY, GENTAMICIN, ACTIVITY
Abstract
La signification clinique de Pseudomonas aeruginosa et Escherichia coli constitue un facteur important pour la monitorisation régulière de leur sensibilité vers les composés antimicrobiens utilisés depuis longtemps et aussi vers les composés nouveaux. Des isolés humains de ces organismes ont été collectionnés de diverses sources pathologiques et testés pour leur sensibilité à la gentamicine - un antibiotique aminoglycoside bien connu - et au miel, un produit naturel qui a eu un regain d'intérêt en considération de son application thérapeutique. Les Auteurs, utilisant une méthode de diffusion «agar-cup», ont trouvé que trois différents échantillons non dilués de miel et leurs dilutions aq. 1:2 à 1:6 exerçaient une activité sur 100% et 96,4% respectivement des isolés de Pseudomonas aeruginosa par rapport à 95,4% d'Escherichia coli utilisant les isolés non dilués ou les dilutions aq. 1:2 des échantillons du miel. L'activité de la gentamicine utilisée à concentrations de 8,0 et 4,0 µg/ml contre tous les deux organismes variait, mais généralement elle était inférieure à l'activité antibactérienne de chaque miel non dilué et à sa dilution aq. 1:2. Dans les cas d'insuccès thérapeutique avec la gentamicine ou avec d'autres antibiotiques similaires, le miel offre une alternative appropriée et meilleure dans la gestion des brûlures infectées et d'autres formes de lésions infectées, comme aussi dans la prophylaxie des lésions dues aux traumatismes.
Introduction
Pseudomonas aeruginosa owes its clinical significance to the fact that it is an aetiology of a good number of infections such as septic burns and wounds, conjunctivitis, endocarditis, meningitis, and urinary tract infections. Notably, it serves as a reference species in antimicrobial susceptibility testing on account of its notorious resistance to most antimicrobial compounds.1
Similarly, Escherichia coli, though normally a gut commensal, has attracted clinical significance owing to the recognition of several strains of diarrhoeagenic E. coli with distinct virulent factors. Collier et al.2 identified these strains as enteropathogenic, enterotoxigenic, enteroinvasive, verocytotoxin-producing, enteroaggregative, and diffusively adherent E. coli (EPEC, ETEC, EIEC, VTEC, Egg EC, and DAEC, respectively).
Gentamicin is a standard antibiotic noted for its activity against Gram-negative bacteria, especially in combination with vancomycin or a penicillin.3 At a concentration of 4.0 µg/ml, it exerted pronounced activity against P. aeruginosa 41 NCTC 6750 (unpublished work4). Similarly, honey has been associated with antibacterial and antifungal activity.5 Specifically, P. aeruginosa and E. coli were among the three laboratory isolates that had their growth inhibited by honey.6 Ibrahim7 and Jeddar et al.8 reported bactericidal activity of honey on Salmonella spp. and Shigella spp. as also enteropathogens such as E. coli, Vibrio cholerae and other Gram-negative and Gram-positive bacteria.
Comparative studies have however identified honey as a more effective remedy than some antimicrobial compounds. This was the situation found between honey and silver sulphadiazine and between honey and certain antibiotics. 9-12 This study reports the antibacterial activity of honey from three different sources and of gentamicin on isolates of P. aeruginosa and E. coli from different pathological sources.
Materials and methods
Bacteriology
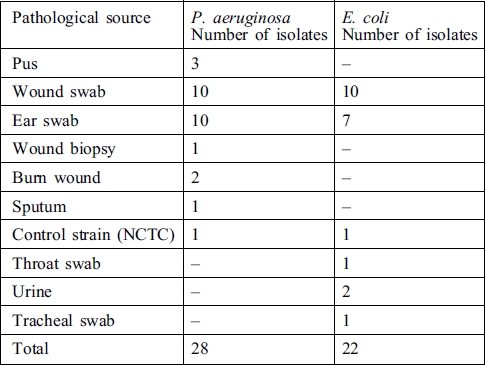
A total number of fifty isolates of P. aeruginosa and E. coli from various pathological sources (Table I) were collected on sterile nutrient agar (OXOID) slants from the Routine Section of the Medical Microbiology, Laboratory, University College Hospital, Ibadan, Nigeria. The pseudomonal isolates were purified on cetrimide agar and the escherichial isolates on eosin methylene blue agar. Both sets of isolates were confirmed by conventional tests12 and then preserved on fresh nutrient agar slants in a refrigerator at 4 °C.
Table I. Pathological sources of P. aeruginosa and E. coli.
Honey
Honey was obtained from three pure natural honey collection centres (A, B, and C) in Ibadan and Abeokuta, South West Nigeria. Each stock was used undiluted and also as fresh aq. dilutions of 1:2, 1:4, and 1:6 against the respective bacterial isolates tested.
Gentamicin
Gentamicin sulphate (BP), a product of Medreich, India, was obtained in ampoule vials (2 ml) from a local pharmacy store. The antibiotic was used in 4 and 8 ug/ml (aq.) dilutions alongside honey against every bacterial isolate.
Sensitivity test
The agar-cup diffusion method 6,13 was employed to obtain the susceptibility pattern of the bacterial isolates against each undiluted honey and its fresh aq. dilutions and of 4 and 8 µg/ml of gentamicin. Considerations for the sensitivity and resistance of bacteria were based on the extent of the presence or absence of zones of growth inhibition.13
Results
Samples of honey from sources A, B, and C, as also gentamicin in 4.0 and 8.0 µg/ml dilutions, exhibited varying levels of antibacterial activity against the bacterial cultures tested as indicated by zones of growth inhibition (Table II). Undiluted honey from each source produced the strongest activity, followed by 1.2 and 1.4 dilutions in decreasing order. Only one isolate of E. coli - and none of P. aeruginosa - was inhibited by a 1:6 dilution. Percentage ranking shows that 100% of all the pseudomonal isolates, including the control strain (NCTC culture), were sensitive to every undiluted honey sample, followed respectively by 96.4%, 67.85%, and 64.28% sensitivity to 1:2 aq. dilution of honeys A, B, and C. Against E. coli isolates, 95.45% of all the cultures, including the control stain, were sensitive to every undiluted honey sample, with only one strain totally resistant. The 1:2 aq. dilution of honey A gave the same result but yielded respectively 86.36% and 77.27% with honeys B and C (Table III).
Table II. Sample results of sensitivity test on honey and gentamicin against E. coli and P. aeruginosa.
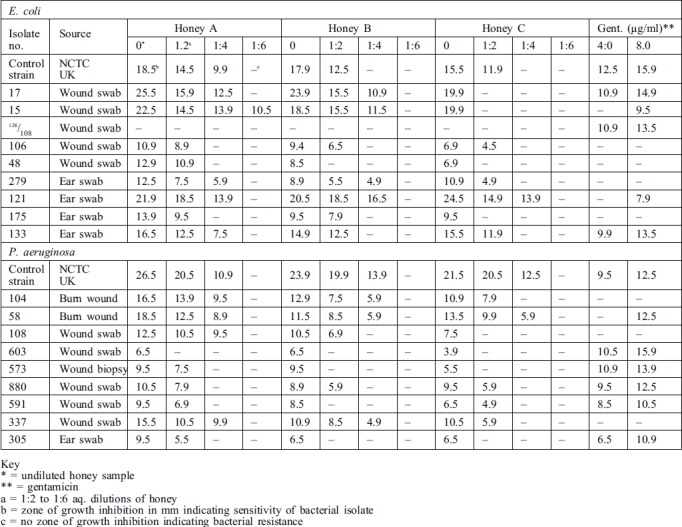
Table III. Relative percentage sensitivity of E. coli and P. aeruginosa to honey and gentamicin.
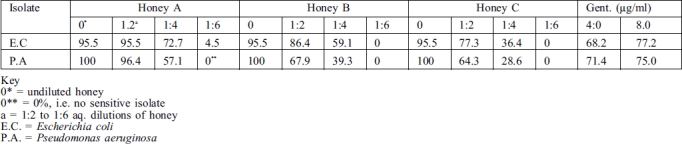
With gentamicin the 4.0 and 8.0 µg/ml tested recorded respectively 71.4% and 75.0% of sensitivity among P. aeruginosa isolates. Against E. coli the values were respectively 68.1% and 77.2%. Remarkably, one particular strain of E. coli (No. 126/108) that was sensitive to both 4.0 and 8.0 µg/ml of gentamicin was totally resistant to every undiluted honey and the aq. dilutions. Also, two isolates of P. aeruginosa from burns were inhibited by the three honey samples, compared with only one of them by gentamicin.
Discussion and conclusions
P. aeruginosa and E. coli are Gram-negative aerobic rods and can constitute environmental contaminants both of burn wounds and of other trauma, through dressing fluids or other sources, thereby causing sepsis. Specifically, the repeated occurrence of Pseudomonas spp. as pathogens in burns14,15 and other forms of trauma16 has been recognized as evidence of chronic or acute infections.17
In a marked departure from the intuitive use of honey as an effective remedy,18 various reports have associated the effectiveness of honey with its high antimicrobial activity,7,8 which has been attributed to osmotic effect, acidity, hydrogen peroxide, phytochemical factors, and seven tetracycline derivatives.19,20 The high antimicrobial activity found support in this study against the two Gram-negative bacteria tested along with the control strains. This was evident in the percentage levels of bacterial sensitivity - as high as 100% for P. aeruginosa and 96.4% for E. coli.
Remarkably, for the first time the strong activity of undiluted honey from each of the three sources of P. aeruginosa contrasted sharply with the strong activity of 1:2 aq. dilution of honey against E. coli.
Also of interest is the finding that the activity of gentamicin, both 4.0 and 8.0 µg/ml, was found to be virtually lower than that of undiluted honey or any of its aq. dilutions. This result supports earlier reports on honey and silver sulphadiazine in the treatment of burn wounds,9 as also on honey and some antibiotics.10,11 The variations recorded in the antibacterial activity of the types of honey tested were consistent with the reports of Jeddar8 and Molan20 and have been attributed to delayed levels of hydrogen peroxide/thermal stability of the glucose oxidase enzyme, non-peroxide factors, and the plant/floral source.21,22 There is therefore a need for a microbiological assay of every honey sample in order to determine its "inhibin number"21 before it can be used as an antimicrobial agent. With appropriate standardization and with its lack of toxicity and allergy,23 coupled with the encouraging results of this and other studies, the therapeutic application of honey in septic burn wounds and other forms of trauma could effectively complement standard antibiotics with beneficial healing effects.
References
- 1.Geddes A.M. In: "Ciprofloxacin Product Monograph". New Zealand: First Printing ADIS Press; 1986. Antibiotics and drug therapy in hospital. pp. 14–18. [Google Scholar]
- 2.Collier L., Albert B., Max S.Bacterial infections In: "Microbiology and Microbial Infections" 9thNew York: Oxford University Press Inc.1998513–575. [Google Scholar]
- 3.Kilka L.J., Goodman J.N. Antibiotic Interactions. J. American Medical Association. 248:1309–1309. [Google Scholar]
- 4.Adeleke O.E., Odelola H.A. Observations on the effect of different concentrations of gentamicin and amikacin on Pseudomonas aeruginosa. unpublished work. [Google Scholar]
- 5.Tysett C., de Rautlin de la Roy Y. Assays on the study of osmophilic yeasts, organisms causing fermentation of honey collected in France. Faculty of Pharmacology, University of Nancy Bull. 1993;134:1–26. [Google Scholar]
- 6.Allen K.I., Radwan S., Reid G.M. Antimicrobial potential of honey on some microbial isolates. J. Medical and Pharmaceutical Sciences. 2000;2:75–79. [Google Scholar]
- 7.Ibrahim A.S. Antibacterial action of honey. Bull. Islam Med. 1985;1:363–365. [Google Scholar]
- 8.Jeddar A., Kharsany A., Ramsaroop U.G., Bhamjee A., Hafejee I.E., Moosa A. The antimicrobial action of honey. South Afr. Med. J. 1985;67:257–258. [PubMed] [Google Scholar]
- 9.Molan P. "The Curative Property of Honey: The Nature of the Antibacterial Activity and the Bee World". New Zealand: Waikato University Press; 2000. pp. 10–15. [Google Scholar]
- 10.Subrahmanyam M., Shahapure A.G., Nagame N.S. et al. Effects of topical application of honey on burn wound healing. Annals of Burns and Fire Disasters. 2001;14:3–5. [Google Scholar]
- 11.Stokes E.S., Ridway G.I., Wren G.M."Clinical Microbiology" 7th1993London: Arnold; 20–30. [Google Scholar]
- 12.Cowan S.T."Cowan and Steel's Manual for the Identification of Medical Bacteria" 2th1974London: Cambridge University Press; 1–30. [Google Scholar]
- 13.Singleton P."Bacteria in Biology, Biotechnology and Medicine" 4th1999New York: John Wiley & Sons Ltd; 333–338. [Google Scholar]
- 14.Artz C.P., Moncrief J.A. "The Treatment of Burns". Philadelphia: W.B. Saunders Co.; 1999. pp. 585–585. [Google Scholar]
- 15.Teplitz C. The pathology of burn and fundamentals of burn wound sepsis. In: Artz C.P., Moncrief J.A., Pruitt B.A., editors. In: "Burns: A Team Approach". Philadelphia: W.B. Saunders Co.; 1979. pp. 45–94. [Google Scholar]
- 16.Heggers J.P., Barners S.T., Robson M.C. et al. Microbial flora of orthopaedic war wounds. Milit. Med. 1969;134:602–602. [PubMed] [Google Scholar]
- 17.Heggers J., Linares H.A., Edgar P. et al. Treatment of infections in burns. In: Herndon D.N., editor. Treatment of infections in burns. Philadelphia: W.B. Saunders Co.; 1996. pp. 98–135. [Google Scholar]
- 18.Efem S.E. Recent advances in the management of Fournier's gangrene: Preliminary observations. Surgery. 1993;113:200–204. [PubMed] [Google Scholar]
- 19.Radwan S., El-Essawy A., Sarhan M.M. Experimental evidence for the occurrence in honey of specific substances active against micro-organisms. Zentral. Mikrobiol. 1984;139:249–255. [PubMed] [Google Scholar]
- 20.Molan P.C., Smith I.M., Reid G.M. A comparison of the antibacterial activities of some New Zealand honeys. J. Agric. Res. 1998;27:252–256. [Google Scholar]
- 21.Willix D.J., Molan P.C., Harfoot C.G. A comparison of the sensitivity of wound-infecting species of bacteria to the antibacterial activity of manuka honey and other honeys. J. Appl. Bacteriol. 1999;73:388–394. doi: 10.1111/j.1365-2672.1992.tb04993.x. [DOI] [PubMed] [Google Scholar]
- 22.Moudoi M.A., Pandila-Zakour O.I., Worobo R.W. Antimicrobial activity of honey against food pathogens and food spoilage micro-organisms. Department of Food Science and Technology, Cornell University, NYSAES, s.d. 1:61–71. [Google Scholar]
- 23.Subrahamanyam M., Shahapure A.G., Nagame N.S. et al. Free radical control - The mechanism of the action of honey in burns. Annals of Burns and Fire Disasters. 2003;16:135–137. [Google Scholar]


