Summary
Objectives. Superoxide dismutase, acting as a scavenger of oxygen free radicals, has shown mixed results in increasing survival from burn wounds. We previously demonstrated that human recombinant copper-zinc superoxide dismutase could increase the survival of failing ischaemic flaps in a rat model. Because of the similar pathophysiology of tissue ischaemia in flaps and intermediate zone burns, we conducted a later study employing two groups of rats with standardized intermediate burns, to ascertain whether or not human recombinant copper-zinc superoxide dismutase could increase intermediate burn zone survival in rats. The results showed that post-burn human recombinant copper-zinc superoxide dismutase failed to improve intermediate burn zone survival. We decided to undertake a new study to ascertain whether there was a protective effect of human recombinant copper-zinc superoxide dismutase in intermediate burns. Methods. This controlled study employed two groups of rats, one of which received prophylactic treatment with human recombinant copper-zinc superoxide dismutase before the induction of standardized intermediate burns. Results. The results showed that pre-burn human recombinant copper-zinc superoxide dismutase also failed to improve intermediate burn zone survival. Conclusions. Further studies are needed to fully understand the effect of oxygen free radicals in burn wound pathophysiology and to determine whether human recombinant copper-zinc superoxide dismutase has a place in the clinical management of burns.
Keywords: PROTECTIVE, EFFECT, RECOMBINANT, COPPER-ZINC, SUPEROXIDE, DISMUTASE, HR-CuZnSOD, SURVIVAL, RATS
Abstract
Buts. La superoxyde dismutase, qui agit comme scavenger des radicaux libres de l'oxygène, a eu des résultats contradictoires pour ce qui concerne l'amélioration de la survie en cas de brûlures. Nous avons déjà démontré que la superoxyde dismutase recombinante humaine cuivre-zinc augmentait la survie des lambeaux ischémiques non réussis dans un modèle qui utilisait des rats. En considération de la pathophysiologie similaire de l'ischémie tissulaire dans les lambeaux et dans les zones intermédiaires des brûlures, nous avons effectué une nouvelle étude qui utilisait deux groupes de rats exposés à des brûlures intermédiaires standardisés dans le but d'établir si la superoxyde dismutase recombinante humaine cuivre-zinc pouvait augmenter la survie de la zone intermédiaire de la brûlure chez les rats. Les résultats ont indiqué que la superoxyde dismutase recombinante humaine cuivre-zinc post-brûlure n'était pas capable d'améliorer la survie de la zone intermédiaire de la brûlure. Nous avons décidé d'effectuer une autre étude pour établir s'il existait un effet protecteur de la survie de la zone intermédiaire de la brûlure intermédiaire. Méthodes. Dans cette étude controlée nous avons employé deux groupes de rats, dont l'un a reçu un traitement prophylactique avec la superoxyde dismutase recombinante humaine cuivre-zinc avant d'infliger des brûlures intermédiaires standardisées. Résultats. Les résultats ont démontré que la superoxyde dismutase recombinante humaine cuivre-zinc administrée aussi avant la brûlure n'est pas réussie à améliorer la survie de la zone intermédiaire de la brûlure. Conclusions. Il faut effectuer de nouvelles études pour bien comprendre l'effet des radicaux libres de l'oxygène dans la pathophysiologies des lésions causées par les brûlures et pour déterminer si la superoxyde dismutase recombinante humaine cuivre-zinc peut jouer un rôle dans la gestion cliniques des brûlures.
Introduction
Thermal burn injuries are a major health care issue and any therapeutic mode that could benefit burn patients would benefit society in general. The aim of all burn treatment regimens is to increase survival of the intermediate burn zone, thus decreasing morbidity, mortality, and the need for surgery and hospitalization.
Superoxide dismutase, acting as a scavenger of oxygen free radicals (OFRs), has shown mixed results in increasing survival in experimental burn wounds. 1, 2Complex local and systemic events are responsible for intermediate burn zone viability. Among them are the levels of inflammation, ischaemia, protein coagulation, and OFR formation. These pathophysiological changes are similar to those seen in the ischaemic cutaneous flap. The enzyme human recombinant copper-zinc superoxide dismutase (Hr-CuZnSOD), with its ability to neutralize superoxide radicals, has been shown to be effective in increasing ischaemic flap survival. 3Owing to the similarities between failing flaps and intermediate zone burns, we conducted our original study to evaluate whether or not CuZnSOD, which causes increased survival of ischaemic flaps, could also increase intermediate zone burn survival. When our original work, in which Hr-CuZnSOD was given to burned rats post-injury, failed to show any beneficial effect, we decided to repeat the experiment, but to change the protocol. In our present study the Hr-CuZnSOD was given prophylactically since Hr-CuZnSOD has been shown to improve ischaemic flap survival, when given pre-injury.
Materials and methods
Twenty male Sprague-Dawley rats (average weight, 250 ± 20 g), were randomly divided into two experimental groups:
CuZnSOD treatment group (n = 10);
control group (n = 10).
The Hr-CuZnSOD treatment group received an intravenous injection of 20 mg/kg of Hr-CuZnSOD 20 min before burn infliction. The control group received sham injections of saline.
Each rat was anaesthetized using an intraperitoneal injection of pentobarbital (40 mg/kg). The back of each rat was shaved. A brass comb measuring 20 x 20 x 55 mm was used to inflict the burns. The comb had four rectangular contact sectors measuring 10 x 20 mm each, in line, with a 5-mm separation between each sector ( Fig. 1 ).
Fig. 1. Brass comb with four contact sectors measuring 10 x 20 mm set at intervals of 5 mm.
The brass comb was equilibrated in boiling water, briefly blotted, and then placed without pressure for 30 sec on one side of the rat’s back. After re-equilibration of the brass comb in boiling water, a second identical comb burn was made on the other side. This created a standard injury with four areas of full thickness burn on each side of the rat’s back, corresponding to the comb’s four prongs. Each area measured 10 x 20 mm and between and around these full-thickness burn zones, intermediate burns were created, 4i.e. in the area studied in the experiment.
All the animals were allowed to recover spontaneously and were housed in separate cages. They were given water and food ad lib. and cared for according to the animal experimental guidelines of our medical centre. The total burn area was evaluated on days 1, 3, and 7. The areas were measured on days 1 and 3 by manual immobilization of the animals without anaesthesia and simple visual evaluation of the various zones of survival. The evaluation on day 7 was done under general anaesthesia by intraperitoneal injection of pentobarbital (40 mg/kg); an intravenous injection of fluorescein (15 mg/kg) was then performed to obtain fluorescence of viable tissue. In all evaluations of the various zones of the burn wound, the zones were copied onto a clear plastic sheet. The burn areas were copied onto paper of known weight, cut out, and weighed on an analytical scale to give the burn area. Student’s t-test was used to determine if there was a statistical difference between the groups. All the experimental animals were humanely sacrificed at the end of the experiment.
Results
The burn area paper-weight value of the treated group was 85 ± 3.1 mg. The burn area paper-weight value for the control group was 87 ± 2.4 mg. Statistical analysis of the results failed to show any significant difference between the treated and the control groups. There was no significant difference in the rats’ well-being or any change in morbidity or mortality.
Discussion
Human skin burns can be divided into three distinct zones:
coagulative zone - immediate tissue necrosis due to the thermal injury;
intermediate zone - damaged tissue, with potential for survival;
erythematous zone - minor degree of thermal injury zone that nearly always heals uneventfully.
Since the burn coagulation area will invariably slough, burn treatment protocols aim to increase the survival of the intermediate burn area. OFRs are likely to be involved in the inflammatory process following thermal injury. They are among the metabolites that participate in a number of pathophysiological processes, such as an increase in capillary permeability, promoting the formation of oedema. Complex events affect the viability of the intermediate burn zone. Among these are inflammation, ischaemia, protein coagulation, and OFR formation. These changes are similar to those seen in the failing cutaneous flap. It therefore seemed logical to us that any therapeutic mode that could increase ischemic flap survival should also benefit the intermediate burn zone.
The pathophysiological reactions determining random pattern flap survival that are similar to those seen in intermediate burn area survival include:
a decrease in perfusion and ischemia;
the specific and non-specific inflammatory reaction;
OFR formation.
Ischaemia
Tissue ischaemia is a combination of systemic and local reactions. The systemic reaction causes an increase in vascular permeability. This “third spacing” decreases the effective vascular volume and decreases perfusion. Systemic cardiac depressant factors may further decrease cardiac output, thus decreasing perfusion of non-vital organs such as the skin. Local reactions, e.g. vasoconstriction, coagulation (due to low flow and tissue damage), oedema (due to increased local vascular permeability), and vascular plugging (due to inflammatory infiltrate), can accentuate local ischaemia.
Inflammatory reaction
The extensive damage caused by the burn stimulates the immune system, with possibly devastating effects. Polymorphonuclears (PMNs) and macrophages may damage potentially viable tissue by OFR formation. 5, 6The inflammatory infiltrate may cause plugging of blood vessels, causing ischaemia. Other cytokines and echinoid products such as thromboxane A2 and prostaglandin F2a may stimulate vasoconstriction and coagulation and further increase ischaemia.
Oxygen free radical formation
OFRs have the ability to peroxidize cell membranes, neutralize enzymes containing sulphydryl groups, depolymerize carbohydrates, and hydroxilate nucleic acids. All of these reactions, either alone or in combination, can cause cell death. 7, 8In our previous work we showed that Hr-CuZnSOD, a free oxygen radical scavenger, had a beneficial effect on failing random pattern flaps in rats.
After a burn, several reactions can generate OFRs:
inflammation - PMNs and macrophages arriving at the burn site;
reperfusion injury - ischaemic organs would switch to anaerobic metabolism. When resuscitation begins, OFRs may be formed;
injured cells – these can generate OFRs;
free metal ions - released from damaged cells and red blood cells (Fe), these form OFRs, via the Haber-Weiss and Fenton reactions:9
Equation.
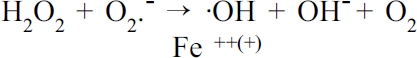
However, we once again failed to find any beneficial effect. We entertained several possibilities for this lack of improvement in wound survival. Hr-CuZnSOD has been shown to improve ischaemic flap survival when given pre-injury. 3This form of SOD, rather than the manganese-SOD, significantly increases as the body’s natural response to tissue injury by burns. 1, 2Theoretically, Hr-CuZnSOD, given post-burn as in a clinical situation, should improve intermediate burn zone survival by diminishing the devastating effects caused by the superoxide radicals created by all the pathophysiological mechanisms listed above.
Although in one study using only intraperitoneal Hr-CuZnSOD and in another using topical liposome-encapsulated Hr-CuZnSOD1 there was an improvement in intermediate burn area survival, we were unable to demonstrate a similar effect in our previous study.
We felt that this lack of success might be due to the timing of the administration of the drug. In our previous study we gave the medication post-burn in order to simulate the clinical treatment of burns. In our present experiment the drug was given prophylactically pre-burn.
Possible reasons for the failure of the treatment were:
dosage - the drug may not have reached effective levels at the burn site owing to haemodynamic compromise or local tissue events;
mode of administration - purely intraperitoneal Hr-CuZnSOD and topical liposome-encapsulated Hr-CuZnSOD may possibly be a better mode of administration than giving the drug in a combination of intravenous and intraperitoneal administration. Hr-CuZnSOD has a short biological half life, a relatively high molecular weight (33 kDa), and hydrophilic capacity, which might be disadvantageous when given intravenously;
the length of time for which the medication was administered was shorter than needed;
Hr-CuZnSOD may itself form new free radicals, the reason being that when SOD dismutases superoxide, it generates peroxide, which could further generate hydroxyl ions - both of which are potent free radicals that could theoretically accelerate tissue damage in the burn;
OFR production may play only a small part in intermediate burn survival.
Conclusion
This study failed to show any beneficial effect in intermediate burn zone survival when Hr-CuZnSOD was administered prophylactically in experimental burns in rats. It is known that OFRs play an important role in the pathophysiology of burn wounds, and we therefore recommend further studies in order to fully understand the effect of OFRs in burn wound pathophysiology and to determine if Hr-CuZnSOD has a place in the clinical management of burns.
References
- 1.Vorauer-Uhl K., Furnschlief E., Wagner A., et al. Topically applied liposome-encapsulated superoxide dismutase reduces post-burn wound size and edema formation. Eur. J. Pharm. Sci. 2001;14:63–7. doi: 10.1016/s0928-0987(01)00149-x. [DOI] [PubMed] [Google Scholar]
- 2.Tan Q., Ma W.X., Wang L., et al. Can superoxide dismutase prevent post-burn dermal ischemia? Burns. 1997;23:228–31. doi: 10.1016/s0305-4179(96)00109-x. [DOI] [PubMed] [Google Scholar]
- 3.Shalom A., Nimrod A., Parizade B., et al. Effect of human recombinant superoxide dismutase on random pattern flap in rats. Sixty-sixth Annual Scientific Meeting ASPRS-PSEF-ASMS, San Francisco, USA, September. 1997 [Google Scholar]
- 4.Eadie P.A., Williams R., Dickson W.A. Thirty-five years of paediatric scalds: Are lessons being learned? Br. J. Plast. Surg. 1995;48:103–5. doi: 10.1016/0007-1226(95)90105-1. [DOI] [PubMed] [Google Scholar]
- 5.Gabig T.G., Babior B.M. Oxygen-dependent microbial killing by neutrophils. In: Oberley L.W., editor. “Superoxide Dismutase”. Vol. 2. CRC Press; Boca Raton, Florida: 1985. pp. 1–13. [Google Scholar]
- 6.Boveris A., Cadenas E. Production of superoxide radicals and hydrogen peroxide in mitochondria. In: Oberley L.W., editor. “Superoxide Dismutase”. Vol. 2. CRC Press; Boca Raton, Florida: 1985. pp. 15–30. [Google Scholar]
- 7.Frank L. Oxygen toxicity in eukaryotes. In: Oberley L.W., editor. “Superoxide Dismutase”. Vol. 3. CRC Press; Boca Raton, Florida: 1985. pp. 1–43. [Google Scholar]
- 8.Halliwell B., Gutteridge J.M.C. The role of transition metals in superoxide-mediated toxicity. In: Oberley L.W., editor. “Superoxide Dismutase”. Vol. 3. CRC Press; Boca Raton, Florida: 1985. pp. 45–82. [Google Scholar]
- 9.Oberley L.W. Superoxide dismutase and cancer. In: Oberley L.W., editor. “Superoxide Dismutase”. Vol. 2. CRC Press; Boca Raton, Florida: 1985. pp. 127–65. [Google Scholar]



