Summary
This paper analyses the results of immunological examinations of 124 patients with thermal injury. We examined indices of peripheral blood such us the size of the population of circulating T-lymphocytes (CD3), T-helpers (CD4), T-suppressors (CD8 and CD25), natural cell killers (CD16), transmembrane protein of apoptotic activity (CD16), B-lymphocytes, phagocyte activity of lymphocytes, and the level of serum antibody of the basic classes (Ig G, A, M). It was discovered that the burn disease was accompanied by deeply marked secondary immune deficiency primarily caused by all components of the active suppression of the cellular link of the immune system and reduction of phagocyte activity of neutrophils.
Keywords: DISORDERS, IMMUNE SYSTEM, SEVERELY, BURNED PATIENTS
Abstract
Les Auteurs ont analysé les résultats des examens immunologiques de 124 patients atteints de lésions thermiques. Ils ont examiné les indices du sang périphérique, c'est-à-dire la mesure de la population de lymphocytes T circulants (CD3), les Thelpers (CD4), les T-suppresseurs (CD8 et CD25) (CD16), les lymphocytes NK (CD16), la protéine transmembranaire de l'activité apoptotique (CD16), les lymphocytes B, l'activité phagocytaire des lymphocytes, et le niveau des anticorps sériques des classes de base (Ig G, A, M). Les Auteurs ont trouvé que la brûlure est suivie d'une insuffisance immunitaire secondaire profondément marquée causée principalement par tous les composants de la suppression active du lien cellulaire du système immunitaire et par la réduction de l'activité phagocytaire des polynucléaires neutrophiles.
Introduction
In many burns centres, 80-85% of fatalities are due to severe thermal damage aggravated by the development of sepsis.1,2 Infection in burn patients is the result of abnormalities in the local skin barrier, changes in the normal flora, wound ischaemia, disturbed receipt of protection factors, and suppression of humoral and cellular immunity to the point of depression of the latter.3 Depression of the immune system caused by pyoinflammatory processes becomes more complicated in the course of the burn disease and has a difficult pathogenesis presenting separate mechanisms that do not always receive single-valued treatment.
Material and research methods
We studied the immunological parameters of peripheral blood: indicators of the population of circulating Tlymphocytes (CD3), T-helpers (CD4), T-suppressors (CD8 and CD25), natural cell killers (CD16), transmembrane protein apoptotic activity (CD95), B-lymphocytes, FAN, and the level of plasmatic antibodies of basic classes (Ig G, A, M) in 124 patients with thermal injury. The immunological research was conducted at the Scientific Research Institute of Immunology in the Republic of Uzbekistan. The results were compared with standard immune system indicators, which were indicated in the Institute's special immunogram forms. Because of the lack of descriptive statistical data concerning the samples from healthy donors from whom standard indicators were received (sample volume, average quadratic deviation, etc.), the reliability of the difference in the results of the immunological studies of burn patients was estimated by the standard mathematical method "Comparison of selective average from hypothetical general average normal totality". For the calculation of the critical point of bilateral critical area level a = 0.05, and therefore F(U) = 0.475 was chosen. Also, using the Laplace Table, the U-crit value = 1.96 was defined. The observable value U-emp on each indicator was calculated separately using the formula:
U = (X-a0 )/y(X) = (X-a0)/√n/y
where X = average value of indicator in 124 burn patients
a0 = normative indicator
n = number of patients (124)
y = average quadratic deviation of indicators in burn patients
Deviation from normal result was considered reliable in cases of U-emp > U-crit.
Results and discussion
The immunograms of patients with thermal injury indicated a tendency for the number of leukocytes to increase on average to 1000 cells in 1 μl (Table I), as the most likely reaction at the onset of wound infection and the developing inflammatory response. There is a considerable lack of cellular immunity in patients with thermal injury, which is associated with a decrease in lymphocyte population in both absolute and percentage terms. A decrease in lymphocytes was found in 95 out of the 124 burn patients (76.6%). As is known, lymphocytes - the main cells of the immune system - possess a unique property, i.e. the ability to distinguish antigens that make it possible to initiate an immune response. In other words, the observation in the burn disease of expressed lymphocytopenia definitely points to inferiority of the immunological status.
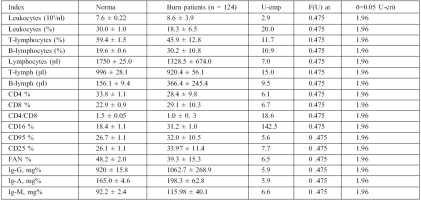
Table I. Initial indices of immunity in patients with thermal trauma, M ± y.
The pathogenesis of similar pathological changes explains why various stressful events (serious trauma, burns, massive bleeding, surgical operations, sports overload, mental trauma, etc.) cause the same reaction in the organism, expressed as increased development of ACTH and corticosteroids, in particular. This therefore leads to atrophy of the thymus.3 The patient's subsequent condition is considerably aggravated by the onset of sepsis and multiorgan failure, which are the causes of massive apoptosis of the thymus as also of the spleen, lymph nodes, and lymphoid tissue in the gastrointestinal tract.4 Here it is primarily the T link of immunity that is damaged.5
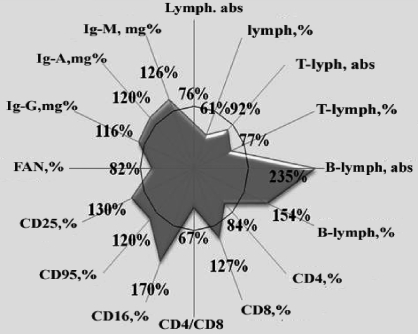
We also noted the presence of marked insufficiency of T-lymphocytes and a wide disproportion in their main immunoregulator subpopulations (Fig. 1).
Fig. 1. Indices of immune system in burn disease and normal indices admitted for 100%.
The different relative and absolute parameters of cellular immunity reflected true immune insufficiency in the system. In particular, the number of T-lymphocytes decreased in burn patients: the absolute quantity decreased on average to 8%, relatively to 23%. The concentration of T-helpers (CD4) decreased considerably (to 16%) in the main immunoregulator populations of T-lymphocytes, which by producing interleukins in association with macrophages lead to complete and intact network interaction of the cells of the immune system. In 72.6% of the burn patients we detected a reduced concentration of Thelpers.2 This marked insufficiency of the basic population of T-lymphocytes indicates defective development of the immune response in the organism of the patients examined.
The activation of the expression of CD8-receptors recorded on this background at 27% and the reduction of the immuno-regulatory index (CD4/CD8) to 33% also indicate depression of the cellular link of immunity. As is known, the molecule of CD8 is produced in T-lymphocytes (almost exclusively in suppressors/killers) and is involved in the transfer of suppressor signals. Numerous data in the literature testify to the inhibitory influence of CD8-cells on the blastogenesis of lymphocytes, as also on the production and activity of cytotoxic lymphocytes and natural cell-killers. The onset of clinical septicotoxaemia, despite the presence of an adequate number of cytotoxic lymphocytes, may be caused by the suppressing action of CD8-lymphocytes.6
These assumptions have recently been confirmed by new investigations, which indicated that the increased susceptibility of thermal trauma victims to pathogenic infections is mainly due to hyperactivity of the monocyte/macrophage system against a background of T-lymphocyte functional insufficiency. As is known, inflammatory cytokines are mainly produced by macrophages and monocytes, playing a dominant role at the beginning of the inflammatory reaction.7,8
It is also known that there is a certain interrelation between the activity of natural killer cells and the organism's resistance to pyogenic infections. The biological role of these cells is defined by their selective effector functions, i.e. their ability to lyse sensitive micro-organism targets. Thus, the basic stage of killing results from damage to cellular membranes with proteolytic enzyme azurophil granules. The action mechanism of natural killers follows the principles of immune control. Natural cellular cytotoxity was observed in healthy individuals. In the burn patients that we investigated, the number of natural killers (CD16) increased on average up to 70% of its first level to more than 31.2 ± 1.7%, ranging widely from 7 to 65 % and considerably different from standard indicators (18.4 ± 1.1%). A raised expression of a 16-receptor was noted in 85.5% of the CD patients.
According to published data, changes in the quantities and functions of natural killers during infectious-inflammatory processes are ambiguous, as their activity depends on a variety of factors - first of all, interleukins, interferons, b-endorphin, and viral and bacterial products. Generally speaking, it is possible to assume a potentiation of the effector function of CD16-lymphocytes in those pathologies, whose development is accompanied by increased production of interferons and IL-1. It was also noticed that potentiation of the expression of CD16 cytotoxic lymphocytes, along with activation of CD8, is characteristic in manifest forms of infectious diseases. In the last few years there has been more information about the development of immunological tolerance to pathogenic infections and tumours, caused by the activity of natural killers. It is possible to assume that the high concentration of receptor CD16 that we found clearly reduced the system of wound infection immunity.
Data concerning the duration of infectious diseases depending on the level of lymphocyte apoptotic activity have been collected. Apoptosis is a fundamental biological process that is necessary for the removal from the organism of damaged, old, and infected cells. This process, together with the interferon system and the immune system, is related to important protective systems in the organism. The existence was shown of a relationship between disorders in the regulation of the process of apoptosis and the development of oncological, inflammatory, autoimmune, and other diseases, together with decreased efficiency of immunological control. Numerous research findings have shown new mechanisms for the suppression of the immunological control of apoptosis and the death of CD95 (Fas/APO-1) receptors.
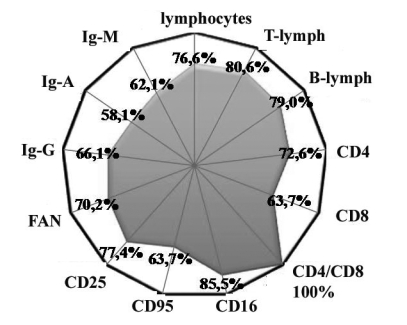
This circumstance induced us to study the maintenance of CB95 receptors in the blood of patients with burn disease. It was found that thermal trauma caused a mean increase (average, 20%) in the apoptotic activity of lymphocytes in the blood (Fig. 1). This kind of change was observed in 63.7% of the patients (Fig. 2). It seems that the development of activated apoptosis of the lymphocytes was the main cause of the decrease in the number of general pool lymphocytes, T-lymphocytes, and T-helpers (CD4).
Fig. 2. Percentage of positive tests - weight of patients with deviation from normal.
Another component of active immunosupression is the regulator CD4-T-lymphocytes. These express activation CD25 antigens and are therefore named CD4 + CD25 + regulator T-cells.9 Our research showed a considerable increase - on average 30% - in the maintenance of CD25 antigens, which mainly inhibited activity of CD3-lymphocytes (T-cells). Similar immunogram changes were found in 77.4 % of the patients examined.
Phagocytosis is one of the necessary reactions, providing both natural resistance by the organism and representation of the antigen for development of the specific immune response. This is a multiphase process, including haemotaxis (movement in the direction of the object of phagocytosis), capture of the object with subsequent formation of phagosomes, and merging of phagosomes and lysosomes with formation of phagolysosomes and photolytic degradation of the absorbed object. Disorders at different stages of phagocytosis lead to the development of numerous pathological conditions, including some septic complications of burn disease.10 In 70.2% of the patients we examined, a decrease in the phagocytic activity of neutrophils was noticed that was also clearly reflected in the mean indices, registering 39.3 ± 1.4% (norm, 48.2 ± 2.0%).
It is known that the final stage of the immune response is the production of immunoglobulins, which are synthesized by activated B-lymphocytes, transformed to plasmatic cells. A number of researchers have shown that production of sufficient antibodies to burns is necessary not only for neutralization of bacterial toxins but also for support of effective opsonization and stimulation of complement activity, as also for lysis of pathogenic microflora.11
Proceeding from the significant suppression we found of the T-systems of immunity, we expected to observe an insufficiency and humoral link. However, as it appeared, the absolute content of B-lymphocytes in the burn patients examined increased to more than double (235%) on average, and their relative figure increased 1.5 times (154 %). These changes were found in 79% of the patients (Figs.1,2). Simultaneously with the increase of concentration of B-cells, we also noted the content of antibodies - IgG, IgA, and IgM - respectively, 16%, 20%, and 26% in the blood.
The same changes in the humoral link of immunity were observed by Chen et al.,12 who explained the increased quantity of B-lymphocytes as a response to endotoxicosis expressed by bacterial toxins in wound infection. Shortly before Chen et al., Rapaport and Bachvaroff13 published findings showing that severe thermal trauma promoted an increase in B-lymphocytes and immunoglobulins. They noted a direct proportional dependence of these changes on the depth and prevalence of the burn wound. It was found in experimental mice that the effect began 1 h post-burn and persisted for two weeks, gradually disappearing by day 21. Rapaport and Bachvaroff could not explain the mechanism stimulating the production of antibodies; however, they assumed that the burn wound was the source of a certain factor, or factors, capable of strengthening humoral immunity. They also specifically stated that these factors were most unlikely to be endotoxins.
Ambiguity in the interpretation of causes and uncertainty of opinion concerning the mechanisms of the increased concentration of B-lymphocytes and immunoglobulins prevent us from asserting that thermal trauma is accompanied by activation of the humoral link of immunity. Also, most publications devoted to the immune system in burn disease speak of the oppression of basic subpopulations of T-lymphocytes, B-cells, and antibody production. We are therefore more inclined to believe that the substantial increase of number of B-lymphocytes that we observed and the concentration of basic classes of antibodies reflect more likely the severity of the thermal insult (as the experiments of Rapaport and Bachvaroff testify) than activation of humoral immunity.
Conclusions
The burn disease is thus accompanied by a sharply expressed secondary immunodeficiency, mainly caused by strengthening of all components of active suppression of a cellular link of immunity and by a decrease in the phagocytic activity of neutrophils. The results justify the performance of effective immune correction therapy in patients presenting a thermal trauma.
References
- 1.Alekseev A.A., Yakovlev V.P., Fedorov V.D. Infection in burned patients: Problems of pathogenesis, prophylactics and treatment. Surgery. 1999;6:4–9. [PubMed] [Google Scholar]
- 2.Arens R., Baars P.A., Jak M. Cutting edge: CD95 maintains effector T cell homeostasis in chronic immune activation. J. Immunol. 2005;174:5915–5920. doi: 10.4049/jimmunol.174.10.5915. [DOI] [PubMed] [Google Scholar]
- 3.Sarkisov D.S., Kaem R.I. Morphologic disorders in organs at burned disease and in its pathogenesis. Surgery. 1980;3:8–12. [Google Scholar]
- 4.Bandyopadhyay G., De A., Laudansky K. Negative signals contribute to T-cell anergy in trauma patients. Crit. Care Med. 2007;35:794–801. doi: 10.1097/01.CCM.0000256847.61085.A5. [DOI] [PubMed] [Google Scholar]
- 5.Tinsley K.W., Grayson M.H., Swanson P.E. et al. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J. Immunol. 2003;171:909–914. doi: 10.4049/jimmunol.171.2.909. [DOI] [PubMed] [Google Scholar]
- 6.Enoh V.T., Lin S.N., Lin C.Y. et al. Mice depleted of alphabeta but not gammadelta T cells are resistant to mortality caused by cecal ligation and puncture. Shock. 2007;27:507–519. doi: 10.1097/SHK.0b013e31802b5d9f. [DOI] [PubMed] [Google Scholar]
- 7.Samonte V.A., Goto M., Ravindranath T.M. et al. Exacerbation of intestinal permeability in rats after a two-hit injury: Burn and Enterococcus faecalis infection. Crit. Care Med. 2004;32:2267–2273. doi: 10.1097/01.ccm.0000145579.66001.05. [DOI] [PubMed] [Google Scholar]
- 8.Al-Ghoul W.M., Khan M., Fazal N., Sayeed M.M. Mechanisms of post-burn intestinal barrier dysfunction in the rat: Role of epithelial cell renewal, E-cadherin and neutrophil extravasation. Crit. Care Med. 2004;32:1730–1739. doi: 10.1097/01.ccm.0000132896.62368.01. [DOI] [PubMed] [Google Scholar]
- 9.Murphy T.J., Ni Choileain N., Zang Y. CD4+ CD25+ regulatory T cells control innate immune reactivity after injury. J. Immunol. 2005;174:2957–2963. doi: 10.4049/jimmunol.174.5.2957. [DOI] [PubMed] [Google Scholar]
- 10.Stoilova Y.D., Haidushkal I.A., Murdjeval M.A. et al. Immunological and microbiological investigations of patients with burn injuries. Folia Med. 2007;49:49–58. [PubMed] [Google Scholar]
- 11.Barrington R., Zhang M., Fischer M., Carroll M.C. The role of complement in inflammation and adaptive immunity. Immunol. Rev. 2001;180:5–15. doi: 10.1034/j.1600-065x.2001.1800101.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y.H., Hancock R.E., Mishell R.I. Mitogenic effects of purified outer membrane proteins from Pseudomonas aeruginosa. Infect. Immun. 1980;28:178–184. doi: 10.1128/iai.28.1.178-184.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapaport F.T., Bachvaroff R.J. Kinetics of humoral responsiveness in severe thermal injury. Ann. Surg. 1976;184:51–59. doi: 10.1097/00000658-197607000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]