Summary
The purpose of the current study was to determine the optimal treatment duration of high-voltage pulsed galvanic current (HVPC) in treating chronic pressure ulcers. Sixty volunteers suffering from chronic pressure ulcers participated in the study for a treatment period of five weeks. They were divided randomly and equally into four groups (three treatment groups and one control group). Patients in the treatment group (G1, G2, G3) received HVPC respectively for 45, 60, and 120 min seven days a week, while patients in the control group received sham HVPC (45 min, seven days a week). The wound surface area (WSA) was used to measure outcomes before starting the study and after three and five weeks' treatment. It was found that there was a significant reduction in WSA in G2 (60 min) and G3 (120 min) compared to G1 (45 min) and the control group (sham HVPC). There was no significant difference between G2 and G3. Application of HVPC for 60 and for 120 min, seven days a week, therefore proved to be the optimal duration for enhancing chronic dermal ulcer healing.
Keywords: HIGH-VOLTAGE, GALVANIC, TREATMENT DURATION, PRESSURE, ULCERS
Abstract
Les Auteurs de cette étude se sont proposés de déterminer la durée optimale du traitement avec le courant galvanique à pulsation de tension élevée (CPTÉ) pour traiter les ulcères de décubitus. Soixante volontaires qui souffraient d'ulcères de décubitus chroniques ont participé à l'étude pour une période de traitement de cinq semaines. Ils ont été divisés au hasard et en manière égale en quatre groupes (trois groupes de traitement et un de contrôle). Les patients assignés au groupe de traitement (G1, G2, G3) ont reçu le CPTÉ pour 45, 60 et 120 min respectivement sept jours par semaine; les patients du groupe de contrôle ont reçu le CPTÉ simulé pour 45 min, sept jours par semaine. L'extension superficielle de la lésion (ESL) a été utilisée pour évaluer les résultats avant de commencer l'étude et après trois et cinq semaines de traitement. Les Auteurs ont trouvé que l'ESL présentait une réduction significative dans les groupes G2 (60 min) et G3 (120 min) par rapport au groupe G1 (45 min) et au groupe de contrôle (CPTÉ simulé). Ils n'ont trouvé aucune différence significative entre G2 et G3. Il est donc possible de conclure que l'application de CPTÉ pour 60 et 120 min, sept jours pour semaine, représente la durée optimale pour améliorer la guérison des ulcères dermiques chroniques.
Introduction
Wound healing is an extremely complex and dynamic tissue process. Scientific enquiry into the many facts of wound healing is far from complete and our knowledge base is continually being enriched by new input both from the clinician at the patient’s bedside and from the researcher’s bench.
A pressure ulcer is defined as “a maceration of skin and/or deeper tissues due to unrelieved pressure, shear force(s), and/or frictional force(s)”.2 The development of pressure ulcers is a problem that threatens people’s everyday activities. There are many precipitating factors for ulcer formation: intrinsic factors include sensory, autonomic, and motor impairment, obesity, malnourishment, and diabetes, while extrinsic factors include unrelieved pressure, friction, direct trauma, and inadequate skin hygiene.
Conservative (non-surgical) management of established pressure sores requires control of the causal factors, e.g. removal of pressure, avoidance of skin maceration, correction of nutritional deficiencies, removal of necrotic tissue, control of infection, and the encouragement of soft tissue repair.4,5 A plethora of methods exist in medical practice for accelerating granulation and re-epithelialization in chronic wounds.
The role of electrotherapeutic treatment is not new within the realms of physiotherapy, and there is a long history of electrical, electromagnetic, and electrophysical applications that have been employed to relieve pain, promote tissue repair, and assist in the restoration of normal function.
It was reported that a 12-h period of tourniquet-induced ischaemia prevented gangrene in the leg of control dogs.9 It was also found that a maximum effect on DNA and protein synthesis in cultured human fibroblasts, using a high-voltage pulsed galvanic current (HVPC) intensity of 50 to 75 V, stimulated a frequency of 100 pulses per second and a negative electrode polarity. Maximal bactericidal effects were found using HVPC with an intensity of 250 V at the cathode for a treatment period of 2 h.
However, it was found that a 30-min application of HVPC produced no bactericidal effect at any intensity and it was hypothesized that a longer treatment time than 30 min might be required to produce an in vitro bactericidal effect with HVPC.11 It was also found that a 1-h hour application of HVPC produced a significant increase in the healing rate of pelvic ulcer in patients with spinal cord injury.6 Another finding was that an application of HVPC for 45 min three times a week for four weeks significantly increased the chronic leg ulcer healing rate.
This study aimed to compare the effect of different HVPC treatment times on chronic dermal ulcer healing.
Material and methods
Patient population
Sixty patients with 60 wounds in four investigation sites participated in the study. Subjects with an indolent pressure ulcer of grade II (Yarkony-Kirk classification)13 were randomly assigned to three treatment groups and one control group.
Treatment groups (15 wounds in each group)
Group I: received HVPC for 45 min seven days a week.
Group II: received HVPC for 60 min seven days a week.
Group III: received HVPC for 120 min seven days a week.
Control group (15 wounds)
Received sham HVPC for 45 min seven days a week, in addition to conventional wound therapy (wet dressing and whirlpool therapy four or five times a week).
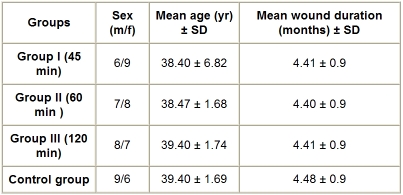
All wounds were debrided before admission to the study (wound size, 4-10 cm. Table I provides a general description of the study population (treatment groups and control group). The subjects in the study had stage II chronic pressure ulcers. Their ages ranged from 30 to 50 yr. There were no sex restrictions for participants in the study. The patients participated in the study for five weeks, because we believed that the same measurable effect on healing would occur in that amount of time. Patients were excluded from the study if they had a cardiac pacemaker, peripheral vascular diseases disposing them to thrombosis, or active osteomyelitis and if they were pregnant or receiving long-term radiation therapy, steroid therapy, or chemotherapy. Following the initial evaluation to determine whether the wound and the patients met the selection criteria, each patient signed an informed consent form.
Table I. Patients’ general characteristics.
Equipment
A small, portable high-voltage monophase twin-pulsed generator was used in the study. The unit parameters were carefully set at a frequency of 120 Hz, an interphase interval of 50 µsec, and a voltage just below that capable of producing a visible muscle contraction (100-175 V).
Study procedure
HVPC treatment protocol (treatment phase)
Patients in the treatment groups received 45, 60, and 120 min of HVPC applied to the ulcer site once daily seven days per week. A piece of heavy-duty aluminium foil, slightly wet and larger than the perimeter of the ulcer, was attached with an alligator clip to the negative lead of the HVPC unit. The foil electrode was placed over the ulcer on top of saline-soaked gauze. A sandbag or elastic wrap was used if needed to hold the wound electrode in place. The dispersive electrode was strapped over the patient’s medial thigh with wet gauze placed between the electrode and the patient’s skin. The active electrode was of negative polarity for the first three days of HVPC application, while the dispersive electrode was positive. After this 3-day period, positive polarity was in the active electrode and negative polarity was in the dispersive electrode. Positive polarity was maintained in the active electrode until the wound healed or a healing plateau was noted. If such a plateau was reached, the protocol of negative polarity in the wound site for a 3-day period was restarted.
Control group (treatment phase)
Patients in the control group had electrodes applied in the same manner as patients in the treatment groups, except that voltage was maintained at zero.
Wound healing assessment phase
The wound surface area (WSA) was measured by tracing the wound perimeter, as reported by Kloth and Feedar.14 This measurement was conducted as we now describe.
A sterilized transparency film was placed over the ulcer. The ulcer perimeter was traced by using the film-tipped transparency marker. Each ulcer was traced three times to establish measurement reliability. After tracing, the side of the transparency film facing the ulcer was cleaned with a piece of cotton and alcohol. Carbon paper was placed over the 1-mm-squared metric graph paper. The traced transparency film was placed over the carbon paper with white paper in between and the tracing was transcribed onto the metric graph paper. WSA was calculated by counting the number of square millimetres on the metric graph within the wound tracing. The mean value of the three trials was calculated and taken to be the WSA. WSA measurements were taken at zero time (“pre”), week 3 (“post 1”), and week 5 (“post II”).
Data analysis. A paired t-test was conducted to compare the wound areas initially and after 3 and 5 weeks of treatment. An unpaired t-test was conducted to compare the treatment groups (GI, GII, GIII) with the control group.
Results
Results of treatment group
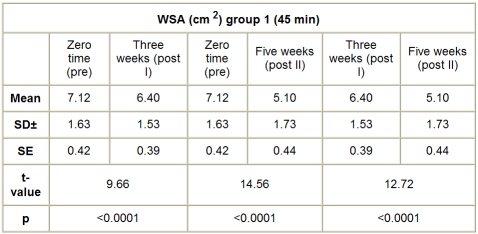
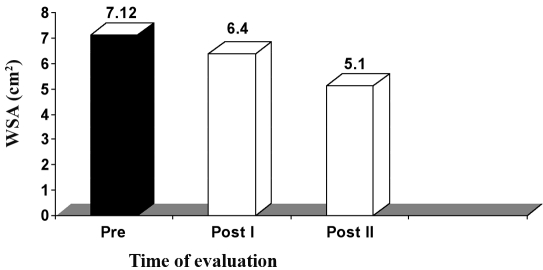
A. Group I (HVPC for 45 min). The mean value and SD of WSA in this group, before application of the treatment (pre), was 7.12 ± 1.63 cm2, while the mean values of WSA after application of HVPC for 45 min, measured after three weeks (post I) and five weeks (post II), were respectively 6.40 ± 1.53 and 5.10 ± 1.73 cm2. There was a significant decrease in WSA measured after three weeks (post I) and five weeks (post II) after application of HVPC for 45 min compared to the initial measurement (before application of the treatment) (p < 0.001), as shown Table II and Fig. 1.
Table II. WSA in group I (HVPC for 45 min) at zero time (pre), after three weeks (post I), and after five weeks (post II).
Fig. 1. Mean WSA values in group I (HVPC for 45 min) at zero time (pre), after three weeks (post I), and after five weeks (post II).
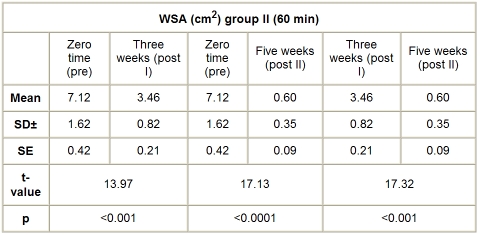
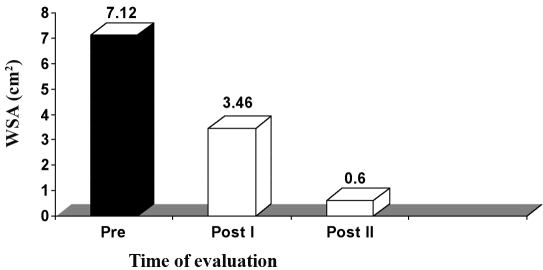
B. Group II (HVPC for 60 min). The mean value and SD of WSA in this group, before application of the treatment, was 7.12 ± 1.62 cm2, while the mean values of WSA, after application of HVPC for 60 min, measured after three weeks (post I) and five weeks (post II), were respectively 3.46 ± 0.82 and 0.60 ± 0.35 cm2. There was a significant decrease in WSA measured after three (post I) and five weeks (post II) after application of HVPC for 60 min compared to the initial measurement (p < 0.001), as shown in Table III and Fig. 2.
Table III. WSA in group II (HVPC for 60 min) at zero time (pre), after three weeks (post 3), and after five weeks (post II).
Fig. 2. Mean WSA values in group II (HVPC for 60 min) at zero time (pre), after three weeks (post I), and after five weeks (post II).
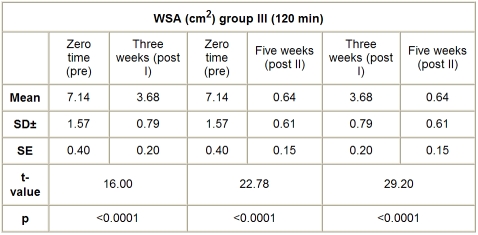
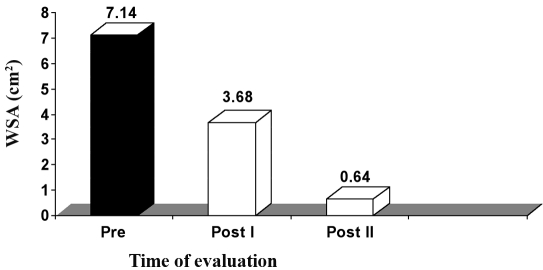
C. Group III (HVPC for 120 min). The mean value and SD of WSA in this group, before application of the treatment, was 7.14 ± 1.57 cm2, while the mean values of WSA, after application of HVPC for 120 min, measured after three weeks (post I) and five weeks (post II), were respectively 3.68 ± 0.79 and 0.64 ± 0.61 cm2. There was a significant decrease in the WSA measured after three (post I) and five weeks (post II) weeks after application of HVPC for 120 min compared to the initial measurement (p < 0.001), as shown in Table IV and Fig. 3.
Table IV. WSA in group III (HVPC for 120 min) at zero time (pre), after three weeks (post I), and after five weeks (post II).
Fig. 3. Mean WSA values in group III (HVPC for 120 min) at zero time (pre), after three weeks (post I), and after five weeks (post II).
Results of control group
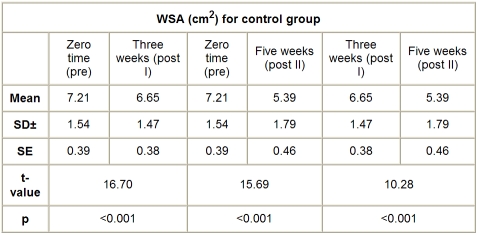
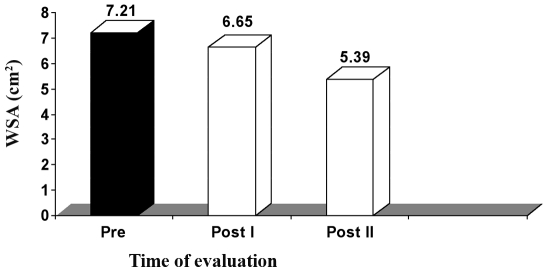
The mean value of WSA in the control group, before application of the treatment, was 7.21 ± 1.54 cm2, while the mean values of WSA after application of sham HVPC for 45 min, seven days a week, and conventional wound therapy (wet dressing, whirlpool therapy four or five times a week), measured after three weeks (post I) and five weeks (post II), were respectively 6.65 ± 1.47 and 5.39 ± 1.79 cm2. There was a significant reduction in WSA measured three weeks (post I) and five weeks (post 2) after application of sham HVPC, compared to the initial measurement (p < 0.001), as shown in Table V and Fig. 4.
Table V. WSA in control group at zero time (pre), after three weeks (post I), and after five weeks (post II).
Fig. 4. Mean WSA values in control group at zero time (pre), after three weeks (post I), and after five weeks (post II).
Comparison and analysis of mean values of WSA in treatment groups and control groups before application of treatment (zero time) and after three and five weeks’ treatment
A. Before application of treatment (zero time)
There were no significant differences in WSA between the control group and the treatment groups (GI, GII, and GIII) (p > 0.05).
B. After three weeks’ treatment
During this period of measurement, there was a significant reduction in WSA in the three treatment groups compared to the control group (p < 0.001). There was a significant reduction in WSA in GII compared to GI (p < 0.001); a significant reduction in WSA in GIII compared to GI (p < 0.001); and a significant reduction in WSA in GII compared to GIII (p < 0.001).
C. After five weeks’ treatment
During this period of measurement, there was a significant reduction in WSA in the three treatment groups compared to the control group (p < 0.001). There was a significant reduction in WSA in GII compared to GI (p < 0.001) and also in WSA in GIII compared to GI (p < 0.001), but no significant reduction in WSA in GIII compared to GII (p > 0.05).
Discussion
This study was designed to compare the effect of different HVPC application times on the acceleration of pressure ulcer healing. No significant differences were found to exist between the treatment groups and the control group that might be expected to affect treatment outcome.
Since all the treatment groups received identical ulcer management except for the duration of application, any differences in healing between the three treatment groups can be attributed to the duration of HVPC application.
It was found that after the third week of HVPC application there was a significant reduction in WSA measured after 60 min of application that was greater than that reported after 120 min of application, while after five weeks’ application of HVPC there was no significant difference in WSA measured after 60 and 120 min of application.
The results of this study are consistent with those of other studies showing that the application of HVPC for 60 min enhances the rate and extent of healing in chronic wounds.
A similar finding was reported by Kloth and Feedar,14 who stated that the HVPC treatment time that satisfactorily enhanced tissue healing did not exceed 60 min per day for five to seven days a week. This treatment time contrasts with the 20 to 45 hours of electrical stimulation treatment per week reported in other studies.
A treatment time of three to seven hours per week, as reported by Kloth and Feedar,14 and of seven hours per week, as reported in this study, therefore appears to be beneficial.
We were not surprised to find there was a significant reduction in WSA in the control group measured after three and five weeks because all of these wounds received an intensive amount of additional care, including the maintenance of a moist wound microenvironment as part of the sham treatment.
There is growing evidence that exogenous electrical currents can augment the healing process in dermal ulcers, perhaps by mimicking the body’s own bioelectrical signals. Convincing evidence exists that the electrically augmented healing of a delayed ulcer is best facilitated by HVPC stimulation for 60 min seven days per week. Additional studies are needed to identify the mechanism involved in the promotion of wound healing with HVPC and to determine the stimulus variables that most effectively accelerate tissue repair.
It is well established that all cells are electrically active, not just those in excitable tissue. The cell membrane has a membrane potential that averages 70 mV, and this electrical cell membrane activity is critical to normal cell functions. The level of electrical activity of the cell membrane influences the cell’s general activity. If the membrane is electrically quiescent, the cell downregulates and its functional capacity diminishes. Conversely, with increased levels of electrical activity, upregulation occurs and the general cell activity levels increase.14
By influencing cell membrane activity levels, it is possible to adjust the excitement level in the cell. This can be achieved with a variety of exogenous energy sources.
Conclusion
The results of our own and previous studies lead us to suggest that wound closure is enhanced by HVPC, if the duration of application is appropriate. Our study confirms the efficacy of HVPC given for wound healing via epithelial closure, and our results indicate that the application of HVPC at a dosage and in the manner of this study is a safe and effective way to treat stage II chronic dermal ulcer.
WSA significantly decreased after HVPC for three weeks and for five weeks. However, there was no significant difference between the application of HVPC for 60 and for 120 min.
It can be concluded that the application time of high-voltage pulsed galvanic current for 60-120 min seven days a week is the optimal duration for the enhancement of chronic dermal ulcer healing.
References
- 1.Kumar S., Wong P.F., Leaper D.J. What is new in wound healing? Turk. J.Med.Sci. 2004;34:147–160. [Google Scholar]
- 2.Crenshaw R.P., Vistnes L.M. A decade of pressure sore research 1977-1987. J.Rehabil.Res.Dev. 1989;26:63–74. [PubMed] [Google Scholar]
- 3.Nussbaum E.L., Biemann I., Mustard B. Comparison of ultrasound/ultraviolet-C and laser for treatment of pressure ulcer in patients with spinal cord injury. Phys.Ther. 1994;74:812–25. doi: 10.1093/ptj/74.9.812. [DOI] [PubMed] [Google Scholar]
- 4.Skylar C.G. Pressure ulcer management in the neurologically impaired patient. J.Neuroscience Nursing. 1985;17:30–6. doi: 10.1097/01376517-198502000-00007. [DOI] [PubMed] [Google Scholar]
- 5.De Lisa J.A., Mikulic M.A. Pressure ulcer: What to do if preventive management fails. Postgraduate Med. 1985;77:209–20. doi: 10.1080/00325481.1985.11698993. [DOI] [PubMed] [Google Scholar]
- 6.Griffin J.W., Tooms R.E., Mendius R.A., Clifft J.K., vander Zwaag R., el-Zeky F. Efficacy of high-voltage pulsed current for healing of pressure ulcer in patients with spinal cord injury. Phys.Ther. 1991;71:433–44. doi: 10.1093/ptj/71.6.433. [DOI] [PubMed] [Google Scholar]
- 7.Watson T. Current concepts in electrotherapy. Haemophilia. 2002;8:413–8. doi: 10.1046/j.1365-2516.2002.00613.x. [DOI] [PubMed] [Google Scholar]
- 8.Watson T. The role of electrotherapy in contemporary physiotherapy practice. Man.Ther. 2002;5:132–41. doi: 10.1054/math.2000.0363. [DOI] [PubMed] [Google Scholar]
- 9.Young H.G. Electrical impulse therapy aids wound healing. Modern Veterinary Practice. 1966;47:60–2. [Google Scholar]
- 10.Bourguignon G.J., Bourguignon L.Y.W. Electrical stimulation of protein and DNA synthesis in human fibroblasts. FASEBJ. 1987;1:398–402. doi: 10.1096/fasebj.1.5.3678699. [DOI] [PubMed] [Google Scholar]
- 11.Guffey J.S., Asmussen M.D. In vitro bactericidal effects of high-voltage pulsed current versus direct current against Staphylococcus aureus. Clinical Electrophysiology. 1989;1:5–9. [Google Scholar]
- 12.Houghton P.E., Kincaid C.B., Lovell M., Campbell K.E., Keast D.H., Woodbury M.G., Harris K.A. Effect of electrical stimulation on chronic leg ulcer size and appearance. Phys.Ther. 2003;83:17–28. [PubMed] [Google Scholar]
- 13.Yarkony G.M., Kirk P.M., Carlson C., Roth E.J., Lovell L., Heineman A., King R., Lee M.Y., Betts H.B. Classification of pressure ulcer. Archives Dermatology. 1990;126:1218–9. [PubMed] [Google Scholar]
- 14.Kloth L.C., Feedar J.A. Acceleration of wound healing with high-voltage monophasic pulsed current. Phys.Ther. 1988;68:503–8. doi: 10.1093/ptj/68.4.503. [DOI] [PubMed] [Google Scholar]
- 15.Charman R.A. Bioelectricity and electrotherapy toward a new paradigm Part 1, The electrical cell. Physiotherapy. 1990;76:503–8. [Google Scholar]