Summary
Burns are a major health problem worldwide, with high mortality and morbidity in addition to causing changes in the quality of life of burn patients. Utilizing antioxidant therapeutic strategies depending on new mechanisms involved in the pathogenesis of burns-related "oxidative stress" may be considered a promising step in burns management. This study involved 180 burn patients of varying age and either sex and with varying burns percentages. The patients were subdivided into six groups (A, B, C, D, E, and F); each group thus included 30 patients. Patients in groups B, C, D, E, and F were treated with antioxidants (vitamin E with vitamin C, zinc sulphate, allopurinol, melatonin, and N-acetylcysteine respectively) while group A was treated according to hospital policy, without any antioxidant; also, healthy subjects (group G) were involved in the study as a control group for comparison.In each group we examined serum malondialdehyde and serum glutathione levels, serum zinc and copper levels, liver function, renal function, mortality rate, and healing time, using standard methods. It was found that the administration of antioxidants to burn patients produced significant improvement in the parameters studied compared with group A (no antioxidant given). This study clearly shows the importance of the therapeutic targeting of oxidative stress in the treatment of burns. It is important to consider antioxidant a most effective weapon that must be added to the arsenal available in the combating of burn complications.
Keywords: ANTIOXIDANTS, TREATMENT, BURN LESIONS
Abstract
Les brûlures constituent un important problème de santé dans tout le monde et sont la cause d'une mortalité et d'une morbidité élevée, comme aussi de diverses modifications dans la qualité de vie des patients brûlés. L'emploi de certaines stratégies thérapeutiques oxydatives dérivées des nouveaux mécanismes intéressés à la pathogénie du «stress oxydatif» des brûlures peut être considéré un progrès important dans la gestion de brûlures. Cette étude s'occupe de 180 patients brûlés des deux sexes et d'âge variable atteints de brûlures de pourcentage variable. Les patients ont été subdivisés en six groupes, nominés A, B, C, D, E et F; chaque groupe était composée par conséquence de 30 patients. Les patients inclus dans les groupes B, C, D, E et F ont été traités avec des antioxydants (vitamine E avec vitamine C, sulfate de zinc, allopurinol, mélatonine et N-acétylcysteine, respectivement), tandis que le groupe A été traité selon les normes de l'hôpital sans aucun antioxydant; aussi, des sujets sains (groupe G) ont été inclus comme groupe témoin pour pouvoir faire des comparaisons. Dans chaque groupe nous avons examiné les niveaux de malondialdéhyde sérique et de glutathione sérique, les niveaux de zinc sérique et de cuivre sérique, la fonction hépatique, la fonction rénale, le taux de mortalité et les temps de guérison, utilisant des méthodes standard. Nous avons trouvé que l'administration d'antioxydants aux patients brûlés améliorait en manière significative les paramètres étudiés par rapport au groupe A (aucun antioxydant administré). Cette étude démontre en manière claire l'importance dans la thérapie des brûlures de prendre le stress oxydatif comme cible thérapeutique. Il faut considérer l'antioxydant comme une arme extrêmement efficace que nous devons ajouter à l'arsenal que nous possédons dans la lutte contre les complications des brûlures.
Introduction
Burns are one of the most devastating conditions encountered in medicine. Burn injury can be considered an assault on all aspects of the patients, from the physical to the psychological.
Despite advances in burn care techniques, there is still a tendency towards therapeutic failure in patients who sustain burns, especially in a large percentage of the total body surface area (TBSA); the modification of medical treatment protocols and the search for new mechanisms involved in the pathogenesis of burns may be helpful in the successful treatment of burn patients.1
Burns are a common traumatic injury that results both in local tissue damage and in a systemic mediator-induced response; there is evidence of both local and systemic oxidant changes manifested by increased free radical activity and lipid peroxidation. At the same time burn injury causes a remarkable decrease in total antioxidant status and a reduction in antioxidant scavenging capacity when compared with control.2 The presence of free radicals in concentrations that overwhelm natural radical blocking or scavenging mechanisms results in oxidative stress, and targeting of this condition with antioxidants may be considered a promising step for improving the outcome in burns.
The major source of free radicals or reactive oxygen species (ROS) in burns trauma is the xanthine oxidase (XO) enzyme, which plays an important role in ischaemia reperfusion injury, producing superoxide anion and hydrogen peroxide, the deleterious ROS that overwhelms the scavenging capacity of endogenous enzymes.3 The other major source is the adherent and activated neutrophils that produce a burst of free radicals represented mainly by superoxide anion.4
Various antioxidants are used to antagonize ROS: vitamin E, the lipid soluble chain-breaking antioxidant;5 vitamin C, which in addition to its antioxidant effect serves to recycle vitamin E;6 zinc sulphate, which has acute and chronic antioxidant effects;7 allopurinol, the well-known XO inhibitor;8 melatonin, the pineal gland product that has powerful antioxidant effects through its direct scavenging effect and stimulation of antioxidant enzymes;9 and finally N-acetylcysteine, which historically was used as a mucolytic agent - this agent is rapidly metabolized to cysteine, which is a direct precursor in the synthesis of intracellular glutathione (GSH), the natural antioxidant.10 Interference by such antioxidants in the treatment of burns may have an improving effect on burn outcomes.
Patients and methods
This study was carried out in 180 burn patients of either sex (age range, 20-45 years) with varying burns percentages (15-40% TBSA), estimated by the rule of nine.
The patients were allocated to six groups and treated as indicated below:
Group A (30 patients). This group was already present in the burns unit and treated according to hospital policy, by which antioxidants are not given
Group B (30 patients), treated with vitamin E 400 mg capsule and vitamin C 500 mg tablet daily
Group C (30 patients), treated with 75 mg/day zinc sulphate capsule
Group D (30 patients), treated with 100 mg/day allopurinol tablet
Group E (30 patients), treated with 3 mg/day melatonin capsule, taken at night
Group F (30 patients), treated with 500 mg/day Nacetylcysteine capsule
Group G (30 healthy subjects) of same age range as the patients selected to act as controls for comparative purposes
All patients treated took their antioxidants in addition to other drugs prescribed according to the hospital drug policy.
Blood samples were collected from all subjects by venepuncture. Ten millilitres were taken on admission to the burns unit within the first 24 h post-burn and before starting drug treatment, which was considered to be zero time, and then on days 3,7, and 14 and on discharge day, in order to check any changes in the parameters studied.
Serum malondialdehyde (MDA) levels were measured according to the standard method of Stocks and Dormandy,11 as modified by Gilbert et al.12 Serum glutathione levels were measured by the method of Godin et al.; 13 serum zinc and copper levels were determined by the method of Taylor and Bryant;14 the activities of the liver enzymes glutamate-pyruvate transaminase (SGPT) and glutamate oxaloacetate transaminase (SGOT) were calculated calorimetrically according to the method of Reitman and Frankel;15 alkaline phosphatase was measured spectrophotometrically according to the method of Kind and King;16 and, in addition, serum creatinine,17 blood urea,18 mortality rate, and healing time were determined according to standard methods.
Statistical analyses were performed, and Student's ttest and the ANOVA test were used to examine the degree of significance. A p value of less than 0.05 was considered significant; the results were expressed as mean ± standard deviation (SD).
Results
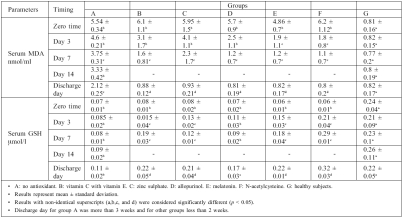
The results in Table I show that serum MDA levels were significantly increased (p > 0.05) at zero time in burn patients when compared with control; in group A (no antioxidant used), serum MDA levels were non-significantly decreased on days 3,7, and 14 compared with zero time, while on discharge day serum MDA levels were significantly decreased by 61.37% compared with zero time.
However, this result was still significantly higher than control (group G). Results in groups B, C, and D showed a
Table I. Effects of drug treatment on serum malondialdehyde (MDA) and serum glutathione (GSH) in burn patients.
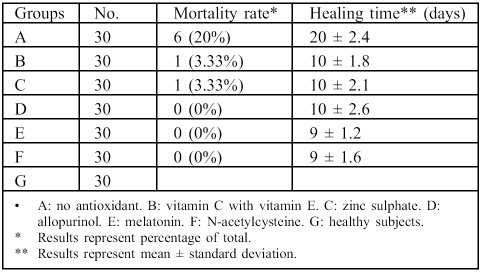
Table III. Effects of drug treatment on mortality rate and healing time in burn patients.
significant reduction in serum MDA levels on day 7; in contrast, groups E and F showed a significant reduction in serum MDA levels on day 3 compared with zero time. In addition, in all groups treated with antioxidant, serum MDA levels were significantly reduced on discharge day compared with zero time and group A (no antioxidant).
With regard to serum glutathione, Table I shows that serum GSH levels were significantly reduced in burn patients at zero time compared with healthy subjects; in group A serum GSH levels remained significantly lower than control until discharge day, while in antioxidant-treated groups serum GSH levels were significantly increased on discharge day compared with zero time.
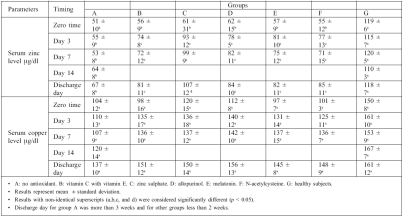
Concerning serum zinc and copper levels, results at zero time show that serum zinc levels in all groups were significantly lower than control (Table II). Treatment with antioxidants caused a significant increase in serum zinc levels in all antioxidant-treated groups compared with zero time. However, serum copper levels were non-significantly lower than control and increased gradually until discharge day, when the value of serum copper came within the normal range, as compared with control.
Table II. Effects of drug treatment on serum zinc levels and serum copper levels in burn patients.
The activity of liver enzymes showed a significant increase in all burn patients at zero time, while enzyme activity gradually returned to normal levels on discharge day in group A; in the antioxidant-treated group, liver enzyme activity returned to normal levels on day 3 post-burn, the same results profile obtained for serum creatinine and blood urea.
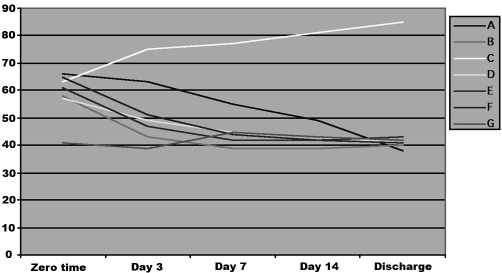
The exception was the alkaline phosphatase enzyme (Fig. 1), whose activity increased continuously until the day of discharge in group C only, where zinc sulphate was administered.
Fig. 1. Effects of drug treatment on serum alkaline phosphatase activity in burn patients.
Table III shows that the mortality rate fell from 20% in group A to 3.33% in groups B and C, while in groups D, E, and F the mortality rate was 0%. Table III also shows that in group A the healing time, represented as mean ± SD, was 20 ± 2.4 days, which was reduced by 50% in groups B, C, and D, while in groups E and F it was respectively reduced to 9 ± 1.2 and 9 ± 1.6 days.
Discussion
Thermal injury of the skin is an oxidation process, associated with biological and metabolic alterations; thermal injury generates free radicals from various cellular populations through many pathways; and the modulation of generated free radical activity with antioxidants seems to be an important part in pharmacological treatment of burns.19
In burn patients alterations in the antioxidant micronutrient status and in the endogenous antioxidant defence against the deleterious effects of free radicals seem crucial, as pointed out by recent studies.20 Data reported by Cetinkale et al.21 demonstrated that in addition to loss of plasma antioxidants like vitamin E and sulphydryl groups in patients with burns, vitamin C diminished early post-burn. Recently, Traber et al.22 suggested that a-tocopherol should be administered to burn patients to prevent vitamin E depletion and to protect against oxidative stress from burn injury.
While vitamin E effectively scavenges free radicals within the cells, vitamin C would serve to scavenge free radicals within the extracellular space.23 There is substantial experimental and clinical evidence indicating an interacting dependence of vitamin E and C in antioxidant defence.24
It has been shown that trace elements play an important role in oxygen metabolism and therefore in the formation of free radicals. Zinc is considered an antioxidant owing to its ability to protect the cell from the effects of oxidative damage through its interaction with cellular thiols, preventing their oxidative inactivation and competing with metal ions that produce ROS.25 In the case of allopurinol, it is clear that circulating XO activity has been detected during skin burn, and it represents the major source of free radicals in the serum of burn patients.26 Allopurinol, and its principal metabolite, oxypurinol, inhibits the enzyme XO, which catalyses the sequential oxidation of hypoxanthine to xanthine and xanthine to uric acid,27 suggesting the beneficial action of allopurinol in ischaemia reperfusion injury, as occurs in burns.28
It has been reported that the pineal hormone melatonin may function as a powerful antioxidant, functioning as a scavenger of hydroxyl, peroxyl, and superoxide radicals,29
in addition to hydrogen peroxide30 and peroxynitrite - the ugly free radical.31 Furthermore, melatonin has the ability to stimulate the activity of antioxidant enzymes like superoxide dismutase, catalase, GSH-Px, and GSH-R;32 this may explain the strong indication of melatonin as a rational antioxidant therapy in the treatment of burns - the rich free radical environment.
Concerning the antioxidant effect of N-acetylcysteine (NAC), it has been shown that NAC acts as a source of sulphydryl groups and facilitates GSH synthesis; it is also a direct scavenger for ROS.33
It is clear that the data obtained in this study regarding the reduction of MDA serum levels and the elevation of serum GSH levels - the natural antioxidant - in burn patients are compatible with the other above-mentioned results.
The results show that the use of antioxidants in the treatment of burn patients increased serum zinc level on day 3 post-burn. The reason behind this may be the attenuation by the use of antioxidants of vascular permeability, which leads to immediate and continuous loss of substances ranging from water to macromolecules.34 The results also show that the administration of 75 mg zinc sulphate (group C) to burn patients significantly increased serum zinc levels on day 3 post-burn and on discharge day compared with other groups in this study. This result is also compatible with that obtained by Li et al.,35 who reported that the addition of zinc to the diet of burn patients rapidly raised the level of zinc in the serum.
Increased liver enzyme activity reflects cellular damage due to burns but according to many authors enzyme activity becomes normal before patients are discharged.36
The same observation was made when investigating blood urea and serum creatinine in burn patients.37 In addition, data have shown that an increased concentration of the lipid peroxidation product MDA in the early post-burn period may affect the liver and kidney, resulting in the release of enzymes into blood stream, and that such damage may be ameliorated by antioxidants, which cause the return of enzymes to normal levels38 - this is exactly what happened in this study, and the same thing exactly occurred as regards blood urea and serum creatinine.
The exception was in group C, where zinc sulphate was administered: alkaline phosphatase activity significantly increased on day 3 and on discharge day. This is because alkaline phosphatase is a zinc-dependent enzyme that is sensitive to dietary levels.39
It has been found that oxidative stress plays an important role both in the activation of the inflammatory response and in tissue damage resulting in the systemic inflammatory response syndrome (SIRS), the leading cause of the multiple organ dysfunction syndrome. Several antioxidant substances have been proposed as a treatment for SIRS, and it has been shown that antioxidants, given postburn, restored antioxidant defences and prevented mortality.40
The results obtained in this study are compatible.
Free radicals and their scavenging systems are known to play a very important role in the normal and delayed healing of certain types of wounds.41 The magnitude of the generation of free radicals and their disposal mechanisms are known to be altered in burn patients, and some kind of correlation exists between altered free radical cascades and delayed wound healing.20
In addition, Rasik and Shukla42 showed that low levels of antioxidants accompanied by raised levels of markers of free radical damage played a significant role in delaying wound healing and that ROS produced in response to cutaneous injury impeded the healing process by causing damage to cellular membranes, DNA, proteins, and lipids; it was also shown that antioxidants promoted wound healing.43 It was additionally reported that the administration of antioxidants enhanced the repair and healing process in cutaneous tissue; silymarin, the well-known antioxidant, was shown to be a useful agent for improving skin tissue regeneration and cutaneous healing.44 Aljawad and Al-Ani45 reported that some medicinal plants were able to accelerate the burn lesion healing rate - an effect related to these plants' containing antioxidant agents. All this evidence strongly supports results obtained in our study.
Conclusion
In conclusion, the results obtained in this study clearly show the strong involvement of oxidative stress in the pathogenesis of thermally injured patients. They also show that the prognosis of burn patients is negatively affected by oxidative stress. This study also clearly shows the beneficial effect of the antioxidants used in the study itself, as indicated by the improvement of burn outcome in general.
Finally, our study shows that it is highly recommendable to add antioxidants to the list of treatments for burn patients order to combat morbidity and mortality in burn patients more effectively.
References
- 1.Latah B., Babu M. The involvement of free radicals in burn injury:A review. Burns. 2001;27:309–17. doi: 10.1016/s0305-4179(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 2.Kao C.C., Garner W.L. Acute burns. Plast.Reconstr.Surg. 2000;105:2482–93. [PubMed] [Google Scholar]
- 3.Granger D.N., Rutili G., McCord J.M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–29. [PubMed] [Google Scholar]
- 4.Horton J.W., White D.J. Role of xanthine oxidase and leukocytes in post-burn cardiac dysfunction. J.Am.Coll.Surg. 1995;181:129–37. [PubMed] [Google Scholar]
- 5.Flohe R.B., Traber M.G. Vitamin E.Function and metabolism. FASEB J. 1999;13:1145–55. [PubMed] [Google Scholar]
- 6.Levine M. New concepts in the biology and biochemistry of ascorbic acid. N.Engl.J.Med. 1986;314:892–902. doi: 10.1056/NEJM198604033141407. [DOI] [PubMed] [Google Scholar]
- 7.Powell S.R. The antioxidant properties of zinc. J.Nutr. 2000;130:1447S–54S. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- 8.Pacher P., Nivorozhkin A., Szabo C. Therapeutic effects of xanthine oxidase inhibitors.Renaissance half a century after the discovery of allopurinol. Pharmacol.Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiter R.J., Tan D.X., Osuna C., Gitto E. Actions of melatonin in the reduction of oxidative stress. J.Biomed.Sci. 2000;7:444–58. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 10.Grinberg L., Fibach E., Amer J. N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. FreeRadic.Biol.Med. 2005;38:136–45. doi: 10.1016/j.freeradbiomed.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Stocks J., Dormandy T.L. The auto-oxidation of human red cell lipids induced by hydrogen peroxide. Br.J.Haematol. 1971;20:95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert H.S., Stemp D.D., Roth E.F. A method to correct errors caused by generation of interfering compounds during lipid per oxidation. Ann.Biochem. 1984;173:282–6. doi: 10.1016/0003-2697(84)90086-1. [DOI] [PubMed] [Google Scholar]
- 13.Godin D.V., Wahaieb S.A., Garnet M.E. Antioxidant enzyme alteration in experimental and clinical diabetes. Mol.Cell.Biochem. 1988;84:223–31. doi: 10.1007/BF00421057. [DOI] [PubMed] [Google Scholar]
- 14.Taylor A., Bryant T.N. Comparison of procedures for determination of copper and zinc in serum by atomic absorption spectroscopy. Clin.Chim.Acta. 1981;110:83–90. doi: 10.1016/0009-8981(81)90304-1. [DOI] [PubMed] [Google Scholar]
- 15.Reitman S., Frankel S. GOT/GPT procedures. Am.J.Clin.Path. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 16.Kind R.R.N., King E.J. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J.Clin.Path. 1954;7:322–26. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henry J.B. W.B.Saunders. Philadelphia: 1984. Clinical diagnosis and management (17th ed) [Google Scholar]
- 18.Fawcett J.K., Scott J.E. Determination of urea in blood or serum. J.Clin.Path. 1960;13:156–9. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arturson G. Pathophysiology of the burn wound and pharmacological treatment.R Hermans Lecture. Burns. 1996;22:255–74. doi: 10.1016/0305-4179(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 20.Horton J.W. Free radicals and lipid peroxidation mediated injury in burn trauma.The role of antioxidant therapy. Toxicology. 2003;189:75–88. doi: 10.1016/s0300-483x(03)00154-9. [DOI] [PubMed] [Google Scholar]
- 21.Cetinkale O., Demir M., Sayman H.B., Ayan F., Onsel D. Effects of allopurinol, ibuprofen, and cyclosporine A on local microcirculatory disturbances due to burn injuries. Burns. 1997;23:43–9. doi: 10.1016/s0305-4179(96)00079-4. [DOI] [PubMed] [Google Scholar]
- 22.Traber M.G., Shimoda K., Murakami K., Leonard S.W., Enkhbaatar P., Traber L.D., Traber D.L. Burn and smoke inhalation injury in sheep depletes vitamin E.Kinetic studies using deuterated tocopherols. Free Radic.Biol.Med. 2007;42:1421–9. doi: 10.1016/j.freeradbiomed.2007.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinnell S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J.Am.Acad.Dermatol. 2003;48:1–19. doi: 10.1067/mjd.2003.16. [DOI] [PubMed] [Google Scholar]
- 24.Huang H.Y., Appel L.J., Croft K.D., Miller E.R., Mori T.A., Puddey I.B. Effects of vitamin C and vitamin E on in vivo lipid peroxidation.Results of a randomized controlled trial. Am.J.Clin.Nutr. 2002;76:549–55. doi: 10.1093/ajcn/76.3.549. [DOI] [PubMed] [Google Scholar]
- 25.Delima R., Trinder D., Olynyk J.K. Potential protective effects of zinc in iron overload. Liver Inter. 2007;27:4–5. doi: 10.1111/j.1478-3231.2006.01428.x. [DOI] [PubMed] [Google Scholar]
- 26.Burton L.K., Velasco S.E., Patt A., Terada L.S., Repine J.E. Xanthine oxidase contributes to lung leak in rats subjected to skin burn. Inflammation. 1995;19:31–8. doi: 10.1007/BF01534378. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto K., Eger B.T., Nishino T., Kondo S., Pai E.F., Nishino T. An extremely potent inhibitor of xanthine oxidoreductase. J.Biol.Chem. 2003;278:1848–55. doi: 10.1074/jbc.M208307200. [DOI] [PubMed] [Google Scholar]
- 28.Das D.K., Engelman R.M., Clement R., Otani H., Prasad M.R., Rao P.S. Role of xanthine oxidase inhibitor as free radical scavenger A novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem.Biophys.Res.Commun. 1987;148:314–9. doi: 10.1016/0006-291x(87)91112-0. [DOI] [PubMed] [Google Scholar]
- 29.Zang L.Y., Cosma G., Gardner H., Vallyathan V. Scavenging of reactive oxygen species by melatonin. Biochim.Biophys.Acta. 1998;1425:469–77. doi: 10.1016/s0304-4165(98)00099-3. [DOI] [PubMed] [Google Scholar]
- 30.Tan D.X., Manchester L.C., Reiter R.J., Plummer B.F., Lieson J., Weintraub S.T., Qi W. Melatonin directly scavenges hydrogen peroxide.A potentially new metabolic pathway of melatonin biotransformation. Free Radic.Biol.Med. 2000;29:1177–85. doi: 10.1016/s0891-5849(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 31.Eli G., Salvatore C., Basilia Z., Andrew S.L., Csaba S. Melatonin is a scavenger of peroxynitrite. Life Sci. 1997;60:PL169–PL174. doi: 10.1016/s0024-3205(97)00008-8. [DOI] [PubMed] [Google Scholar]
- 32.Karbowink M., Reiter R. Antioxidant effects of melatonin in protection against cellular damage caused by ionizing radiation. PSEBM. 2000;225:9–22. doi: 10.1177/153537020022500102. [DOI] [PubMed] [Google Scholar]
- 33.Benrahmoune M., Therond P., Abedinzadeh Z. The reaction of superoxide radical with N-acetylcysteine. Free.Radic.Biol.Med. 2000;29:775–82. doi: 10.1016/s0891-5849(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 34.Latha B., Ramakrishnan M., Jayaraman V. The efficacy of trypsin Chymotrypsin preparation in the reduction of oxidative damage during burn injury. Burns. 1998;24:532–8. doi: 10.1016/s0305-4179(98)00066-7. [DOI] [PubMed] [Google Scholar]
- 35.Li L., Guo Z., Zhao L. Effects of supplemented Zn on levels of Zn in serum, growth hormone and hydroxyproline. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi. 1998;14:425–8. [PubMed] [Google Scholar]
- 36.Chiarelli A., Casadei A., Pornaro E., Siliprandi L., Mazzoleni F. Alanine and aspartate aminotransferase serum levels in burned patients A long-term study. J.Trauma. 1987;27:790–4. doi: 10.1097/00005373-198707000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Vanholder R., Vanden B.J., Vogelaers D., Colardyn F. Renal function in burns. Acta.Anaesthesiol.Belg. 1987;38:367–71. [PubMed] [Google Scholar]
- 38.Kumar R., Seth R.K., Sekhon M.S., Bhargava J.S. Serum lipid peroxide and other enzyme levels of patients suffering from thermal injury. Burns. 1995;21:96–7. doi: 10.1016/0305-4179(95)92131-u. [DOI] [PubMed] [Google Scholar]
- 39.Davies S. Assessment of zinc status. Int.Clin.Nutr. 1984;4:122–9. [Google Scholar]
- 40.La Londe, C. Nayak, U. Hennigan, J. Demling, R.H. Excessive liver oxidant stress causes mortality in response to burn injury combined with endotoxin and is prevented with antioxidants. J.Burn Care Rehabil. 1997;18:187–92. doi: 10.1097/00004630-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Shukla A., Rasik A.M., Patanaik G.K. Depletion of reduced glutathione, ascorbic acid, vitamin E, and antioxidant defense enzymes in healing cutaneous wound. Free Radic.Res. 1997;26:93–101. doi: 10.3109/10715769709097788. [DOI] [PubMed] [Google Scholar]
- 42.Rasik A.M., Shukla A. Antioxidant status in delayed healing type of wounds. Int.J.Exp.Path. 2000;81:257–63. doi: 10.1046/j.1365-2613.2000.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta A., Singh R.L., Raghubir R. Antioxidant status during cutaneous wound healing in immunocompromised rats. Mol.Cell.Biochem. 2002;241:1–7. doi: 10.1023/a:1020804916733. [DOI] [PubMed] [Google Scholar]
- 44.Svobodova A., Walterova D., Psotova J. Influence of silymarin and its flavonolignans on H2O2-induced oxidative stress in human keratinocytes and mouse fibroblasts. Burns. 2006;32:973–9. doi: 10.1016/j.burns.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Al-Jawad F.H., Al-Ani N. A study of the effect of some medicinal plants in healing of thermally induced burn lesion. Iraqi J.Med.Science (in press) 2006 [Google Scholar]