Summary
Thermal injury is known to induce alterations in the immune system, but the precise mechanisms have yet to be elucidated. It has been shown that thermal injury in more than 20% of the total body surface area (TBSA) leads to disturbances in the cortisol metabolism and the equilibrium of the hypothalamic-pituitary-adrenal axis. We investigated the temporal relationship between serum cortisol levels, C-reactive protein, and immunoglobulin levels in the post-burn period. Twenty-one adult burn patients (mean age, 52 ± 17 yrs) were included in the study (TBSA, 10-80%); nine developed sepsis and five died. The nonseptic group consisted of twelve patients. Thirty healthy blood donors served as controls. Our results suggest that increased cortisol and decreased immunoglobulin levels could be related to severe sepsis and clinical outcome.
Keywords: CORTISOL, IMMUNOGLOBULIN, C-REACTIVE PROTEIN, BURN PATIENTS
Abstract
Les lésions thermiques provoquent des altérations bien connues dans le système immunitaire mais les mécanismes précis responsables de ces altérations restent à élucider. Il a été démontré que les lésions thermiques dans plus de 20% de la surface corporelle totale causent des troubles dans le métabolisme du cortisol et l'équilibre de l'axe hypothalamique-pituitaire-surrénal.Nous avons étudié le rapport temporel entre les niveaux du cortisol sérique, la protéine C réactive et les niveaux d'immunoglobuline dans la période immédiate post-brûlure. Notre étude comprenait 21 patients adultes brûlés (âge moyen, 52 ± 17 ans; surface corporelle totale brûlée, 10-80%), avec neuf cas de septicité et cinq décès. Le groupe non-septique était composé de 12 patients.Nous avons utilisé trente donateurs de sang sains comme cas témoins. Sur la base de nos résultats nous avons formulé l'hypothèse qu'il existe un rapport entre, d'une part, l'incrément des niveaux de cortisol et la diminution des niveaux d'immunoglobuline et, de l'autre, la septicité grave et le résultat clinique final.
Introduction
Numerous studies have shown that increased burn size leads to increased mortality among burn patients. It has been hypothesized that burn size determines the extent of the hypermetabolic response.1It has not been definitely established whether this mortality is due to inflammation, hypermetabolism, or other contributory physiological factors.
Increased cortisol levels have been shown to reflect the severity of injury in traumatic insults. Significant increases in cortisol and catecholamines are seen following major burns and are thought to initiate the cascade of events leading to the hypermetabolic response, with its ensuing catabolic state.
The large initial rise in cortisol has been shown to lead to increased resting energy expenditure and short-term bone loss and to reduce T-helper lymphocyte proliferation, with a subsequent reduction in the patient’s ability to combat infection.2
The purpose of the present study was to investigate the cortisol level in burn patients and its correlation with the clinical outcome.
Materials and methods
Patients
All the patients were admitted to the Plastic Surgery and Burns Operating Unit, Azienda di Rilievo Nazionale di Alto Rilievo, Civic Hospital, Palermo, Italy.
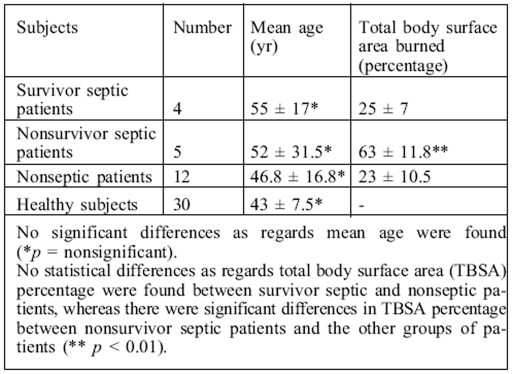
A series of serum samples were obtained from 21 burn patients (14 males, 7 females; total body surface area [TBSA] burned, 10-80%; mean age, 52 ± 17 yr) and 30 healthy subjects (19 males, 11 females; mean age, 43 ± 7.5 yr). Patient samples were collected at 1, 3, 7, 10, 14, and 20 days and serum was separated from whole blood by centrifugation for measurement of cortisol, immunoglobulin, and C-reactive protein.
Informed consent for serum analysis was obtained from the patients and the healthy subjects.
Cortisol determination
Venous blood was collected in sampling tubes and immediately centrifuged. Cortisol serum levels were measured by radioimmunoassay (RADIM, Italy), following the manufacturer’s instructions.
The minimum detectable doses of cortisol were > 5 µg/dl.
C-reactive protein and immunoglobulin determination
C-reactive protein (CRP) and immunoglobulin were determined by standard procedures using an automated haematology analyser (Sysmex XT 1800 L).
Statistical analy
Data were expressed as mean ± SD and Student’s ttest was used to compare responses in the different groups; p < 0.05 was chosen for rejection of the null hypothesis.
Results
Patient characteristics
The burn patients' clinical and pathological characteristics are summarized in Table I, together with those of the healthy subjects.
Table I. Clinical characteristics of burn patients and healthy subjects.
Patients were classified as septic on the basis of one of the following criteria (signs and symptoms):
positive blood or tissue culture for bacteria and fungi
hyperthermia (> 38 °C)
altered mental status
haemodynamic instability, usually requiring vasopressors
Nine out of the 21 patients in the study revealed signs of sepsis after a mean period of 3 ± 1 days.
Cortisol determination
In this study, serial changes in circulating cortisol levels in burn patients were determined by radioimmunological assay.
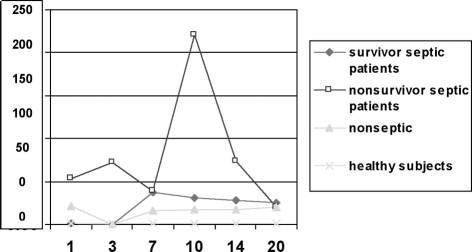
The results shown in Fig. 1 demonstrate that at all points of our study increases in serum cortisol could be detected in the nonsurvivor septic patients, while serum cortisol remained low in septic survivors and in nonseptic patients with values comparable to the normal range.
Fig. 1. Serum total cortisol was determined on admission and 3, 7, 10, 14, and 20 days after burn injury. Cortisol was higher in nonsurvivor septic patients than other groups. In particular, a peak level was observed on day 10 post-burn only in nonsurvivor septic patients (220 ± 115 µg/dl).
Immunoglobulin and determination of C-reactive protein
Immunoglobulin and CRP were determined by standard procedures, using an automated haematology analyser.Serum IgG concentrations were low during the initial post-burn period in survivor and nonsurvivor septic patients and returned to normal and above-normal levels in the late post-burn period in both groups. In particular, major differences were observed at day 3, when we found 400 ± 80 mg/dl levels in survivors and a mean value of 252 ± 125 mg/dl in nonsurvivor septic patients. In nonseptic patients, the IgG levels at day 3 were 759 ± 211 mg/dl.
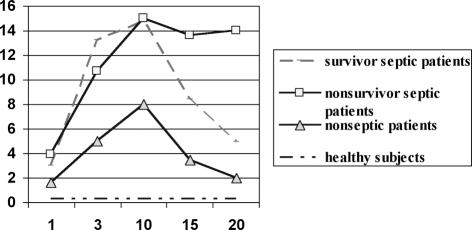
The results shown in Fig. 2 show that CRP levels were higher in septic than in nonseptic patients (p < 0.05), but until day 15 day CRP values did not distinguish survivor from nonsurvivor septic patients.
Fig. 2. C-reactive protein (CRP) levels (mg/dl) in the groups studied at days indicated. CRP levels were higher in septic patients than in nonseptic patients, although by day 15 CRP values no longer discriminated between survivor and nonsurvivor septic patients.
Discussion
A burn wound is perhaps the most intense stress that a human body can suffer.
Severe burns result in major metabolic, physiological, and immunological changes.2,3,4 An increase has been reported in cortisol levels in the early stage of burns treatment.This increase in cortisol, as also the increase in circulating catecholamines following a massive burn, initiates a cascade of events leading to the hypermetabolic response with its ensuing catabolic state.5
Immune dysfunction has been suggested as a contributory factor involved in the development of sepsis after thermal injury. Defects in innate and acquired immunity have been reported among burn victims, e.g. depressed Th1 and increased Th2 cytokine production, macrophage dysfunction, altered natural killer and T-cell activities, and a depressed cytotoxic response.6 We recently demonstrated that burn victims who developed sepsis exhibited a reduction both in circulating monocytoid dendritic cells and in plasmacytoid dendritic cells at all time points in our study.7,8
We investigated the temporal relationship between serum immunoglobulin and cortisol levels in the post-burn period over a period of 20 days. Serum cortisol remained low in septic survivors and nonseptic patients, while increased serum cortisol was detected in nonsurvivor septic patients throughout the study.
Subsequently the levels in septic survivors and nonseptic patients were very similar and comparable to the normal range. This result is consistent with those of other studies, suggesting that cortisol levels increase in proportion to the severity of sepsis.9,10
To determine the effect of burn injury on humoral immunity we measured serum immunoglobulin and found that humoral immunity was altered, as shown by changes in serum immunoglobulin levels - one day post-burn, serum immunoglobulin levels were lower among septic cases than among nonseptic cases and remained so until day 10 (p < 0.001).
In the septic group, in septic survivor patients, concentrations of immunoglobulin gradually increased up to normal limits by day 14, while in nonsurvivors the levels were reduced until day 20. In a murine model the decrease in immunoglobulin levels was probably due to increased clearance from the circulation,11 whereas in burn patients the decrease could be related to a reduction in the number of B cells.12
Conclusion
In conclusion, our results suggest that high cortisol levels are predictors of the severity of sepsis and clinical outcome.Also, an analysis of immunoglobulin levels suggests an intricate interaction between the immune system and the hypothalamic-pituitary-adrenal axis that leads to a hypermetabolic response and impaired immune defence, the precise mechanisms of which have yet to be elucidated.
Acknowledgments
We gratefully acknowledge the assistance of Mr Antonio Armetta for his graphics work.
References
- 1.Pileri D., Accardo-Palumbo A., D'Amelio L. Concentrations of cytokines IL-6 and IL-10 in plasma of burn patients: Their relationship to sepsis and outcome. Ann. Burns and Fire Disasters. 2008;21:182–185. [PMC free article] [PubMed] [Google Scholar]
- 2.Palmieri T.L., Levine S., Schoenfeld-Warden N. Hypothalamic-pituitary-adrenal axis response to substained stress after major burn injury in children. J. Burn Care Res. 2006;27:742–748. doi: 10.1097/01.BCR.0000238098.43888.07. [DOI] [PubMed] [Google Scholar]
- 3.Norbury M.B., Herndon M.D., Ludwik K. Urinary cortisol and catecholamine excretion following burn injury in children. J. Clin. Endocrin. Metab. 2008;93:1270–1275. doi: 10.1210/jc.2006-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bariar L.M., Bal A., Hasan A. Serum levels of immunoglobulins in thermal burns. J. Indian Med. Assoc. 1996;94:133–134. [PubMed] [Google Scholar]
- 5.Heideman M., Bengtsson A. The immunological response to thermal injury. World J. Surg. 1992;16:53–56. doi: 10.1007/BF02067115. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs P.C., Groger A., Bozkurt A. Cortisol in severely burned patients: Investigations on disturbance of the hypothalamic-pituitary-adrenal axis. Shock. 2007;28:662–667. [PubMed] [Google Scholar]
- 7.Pileri D., Accardo-Palumbo A., D’Amelio L. Concentrations of cytokines IL-6 and IL-10 in plasma of burn patients: Their relationship to sepsis and outcome. Ann. Burns and Fire Disasters. 2008;21:182–185. [PMC free article] [PubMed] [Google Scholar]
- 8.D’Arpa N., Accardo-Palumbo A., Amato G. Decrease of circulating dendritic cells in burn patients. Ann. Burns and Fire Disasters. 2007;20:199–202. [PMC free article] [PubMed] [Google Scholar]
- 9.D’Arpa N., Accardo-Palumbo A., Amato G. Circulating dendritic cells following burn. Burns, in press, accepted May 2008. 2008 doi: 10.1016/j.burns.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Christ-Crain M., Stoltz D., Jutla S. Free and total cortisol levels as predictors of severity and outcome in communityacquired pneumonia. Am. J. Respir. Care Med. 2007;176:913–920. doi: 10.1164/rccm.200702-307OC. [DOI] [PubMed] [Google Scholar]
- 11.Torpy D.J., Ho J.T. Value of free cortisol measurement in systemic infection. Horm. Metab. Res. 2007;39:439–444. doi: 10.1055/s-2007-980200. [DOI] [PubMed] [Google Scholar]
- 12.Gadd M.A., Hansbrough J.F., Soderbeg C.S. Antibody formation after murine injury. J. Surg. Res. 1988;44:649–657. doi: 10.1016/0022-4804(88)90096-0. [DOI] [PubMed] [Google Scholar]
- 13.Kagan R.J., Bratescu A., Jonasson O. The relationship between the percentage of circulating B cells, corticosteroid levels, and other immunologic parameters in thermally injured patients. J. Trauma. 1989;29:208–213. doi: 10.1097/00005373-198902000-00010. [DOI] [PubMed] [Google Scholar]