Summary
Burns expose the deeper tissues of the skin or body to invasive microbes. Topical preparations for treating burn wounds, to be useful, should ideally have antibiotic power and promote healing. Silver compounds have been the mainstay of topical burn treatment for decades. However, most chemical substances retard wound healing. Several natural agents such as honey and moist exposed burn ointment (MEBO) are believed to protect wounds from infection and promote healing without causing any of the adverse effects of purified chemicals. In this study, we compared the wound healing properties of MEBO, a herbal preparation of Chinese origin, with silver sulphadiazine (SSD), a long-standing conventional burn dressing. Ten adult Sprague Dawley rats were divided into two groups. They were housed in separate cages and received partial-thickness burn wounds on their dorsal skin. They were then treated with MEBO and SSD. The wounds were inspected daily until day 8, when all the animals were sacrificed, perfused with normal saline, and had their wounds excised and prepared for histology. It was found that animals in both groups were well preserved. No clinical infections occurred. Wound healing was at an advanced stage by day 8 in all the animals. Clinical and histological examination showed that the two agents gave the animals comparable protection and healing possibilities. It is concluded that MEBO is a suitable and efficacious alternative to conventional silver-based topical therapies for treating partial-thickness burn wounds.
Keywords: COMPARATIVE STUDY, WOUND HEALING, MOIST EXPOSED BURN OINTMENT, MEBO, SILVER, SULPHADIAZINE
Abstract
Les brûlures exposent aux microbes invasifs les tissus plus profonds de la peau ou du corps. Idéalement, pour être utiles dans le traitement des brûlures, les préparations topiques doivent avoir des capacités antibiotiques et favoriser la guérison. Depuis des décennies les composés argentés constituent la base principale du traitement topique des brûlures. Cependant la plupart des substances chimiques retardent la guérison des lésions. On pense généralement que les agents naturels comme le miel et l'onguent pour les brûlures exposées humides (MEBO) peuvent protéger les lésions de l'infection et favoriser la guérison sans causer aucun des effets défavorables des substances chimiques purifiés. Dans cette étude nous avons comparé la capacité de guérison de MEBO (une préparation aux herbes d'origine chinoise) avec celle de la sulfadiazine argentée (SDA), un pansement conventionnel pour les brûlures utilisé depuis longtemps. Dix rats adultes Sprague Dawley, divisés en deux groupes et hébergés dans des cages séparées, ont reçu des brûlures sur la peau dorsale d'épaisseur. Ensuite ils ont été traités avec MEBO et SDA. Les lésions ont été examinées tous les jours jusqu'au jour 8, quand tous les animaux ont été sacrifiés, et perfusés solution saline normale; à ce point les lé sions ont été excisées et préparées pour l'examen histologique. Nous avons trouvé que les animaux dans tous les deux groupes étaient très bien conservés, sans aucune manifestation d'infection clinique. Dans tous les animaux la guérison des lésions était dans une phase avancée au huitième jour. Les résultats des analyses indiquaient que les deux agents donnaient aux animaux une protection et des possibilités de guérison comparables. Les Auteurs concluent que MEBO est une alternative appropriée et efficace aux thérapies conventionnelles à base d'argent dans le traitement des brûlures d'épaisseur variable.
Introduction
A burn is a coagulative necrosis of the skin and some times of deeper tissues caused by the dissipation of thermal energy into it. A variety of physical insults will cause burn wounds to the skin and deeper tissues. Most result from flames and hot liquids, but chemicals and electricity also cause thermal injury.1 In these last two cases, injury results from the conversion of chemical or electrical energy into heat, or thermal energy. Burns are assuming epidemic proportions in developing countries and certainly account for a significant share of emergencies and admissions in our hospitals every year.2 In the past few years in Nigeria, burn wounds from petroleum-related fires have become a cause of mass suffering and death.
Major burns - burns involving more than 20% of body surface area - cause physiological and pathological responses in most systems in the body. Significant changes occur in the immune, nervous, respiratory, and cardiovascular systems. One potentially fatal example of these is hypovolaemic shock resulting from plasma loss. However, in modern medical practice, vigorous resuscitation restores respiratory and haemodynamic stability in most cases of even severe thermal injuries. Where adequate facilities are available, most burn-related deaths are therefore due to wound infection.1,3 Devitalized materials in burned tissue serve as a nidus for bacterial proliferation. The delivery of systemically applied antibiotics is impaired by the occlusion of vessels in adjoining areas. Topical treatment of burn wounds is therefore of great importance both in preventing death from invasive infection and in determining other treatment outcomes, such as the occurrence of scar hypertrophy and keloids.3,4 Since the 1960s, silver compounds have been the mainstay of conventional topical treatment for burns. Silver compounds come in a variety of forms. Mafenide acetate, an 11% silver suspension, and silver sulphadiazine (SSD), a 1% suspension, are the leading members of this group.
Of the two, SSD penetrates eschar to a lesser extent and has less activity against Pseudomonas but causes less pain. Over a period of many years it has come to be a standard against which non-silver therapies are evaluated. The main advantage of silver compounds is their lethal power against a wide range of microbes, including even fungi.5 Several studies have shown that honey has achieved comparable results to those of SSD, at least in partial-thickness burns.6 MEBO is an oil-based, natural preparation widely used in Asia and the Middle East. It contains berberine oil and beta-sitosterol, a plant steroid. Oils soothe wounds, retain moisture, and relieve pain. Beta-sitosterol promotes epithelialization.7 Although several researchers have studied MEBO's wound healing properties, only a few controlled animal trials were found in the literature we re viewed. This study thus compares the efficacy of MEBO with that of SSD in partial-depth burn wounds in rats.
Matériel et méthodes
Sprague Dawley rats were obtained from the animal house of Lagos State University College of Medicine. MEBO, made by Gulf Pharmaceutical Industries, came from a donated stock. SSD was from LEK, Lubljana, Yugoslavia. Ten Sprague Dawley rats weighing 150-190 gm were divided into two groups of five rats each. They were acclimatized for two weeks in our laboratory in equal light and darkness periodicity, with liberal access to food and water. They were housed in separate hygienically maintained cages.
Induction of burn wounds
Partial-thickness burn wounds (2 by 2.5 cm) were cre ated on a shaved area of the animals' dorsal skin, modifying a method described by Smahel.8 Briefly, the animals were anaesthetized with intraperitoneal ketamine hydrochloride (40 mg/kg) and shaved. A steel plate 2 mm thick was heated in boiling water for 7 min and applied firmly to the skin for 12 sec.
Treatment with topical agents
Group 1 animals were treated with MEBO only, every 8 h.
Group 2 animals were treated with SSD only, every 12 h.
All wounds were exposed to air and inspected daily for evidence of clinical infection.
Vascular perfusion and wound excision
On day 8 post-burn, each animal was anaesthetized with intraperitoneal ketamine, and the hearts was exposed by bilateral division of the rib cage. The root of the aorta was cannulated with a 20G intravenous plastic cannula and the vascular space was perfused with normal saline. The wounds were then excised with a margin of healthy skin and as deep as the areolar tissue. The specimens were fixed in 10% formal saline for several days, stained in haematoxylin and eosin, and examined at various magnifications under a light microscope.
Results
The animals were generally well preserved and active, feeding within 2 h of the procedure. No clinical infection was recorded. By day 3, there was a hard dark-brown eschar over the burned areas. By day 8, the eschar had spontaneously detached, leaving pink tender skin in all but one animal. Clinical and histological tests showed that the two treatments produced comparable healing in the animals.
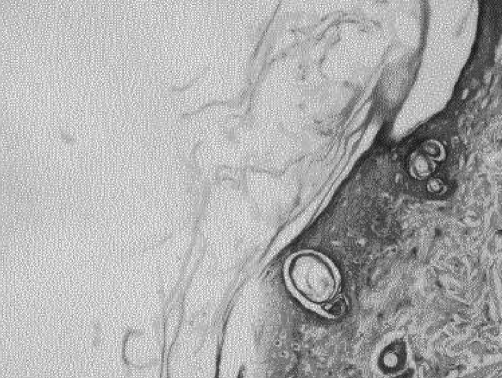
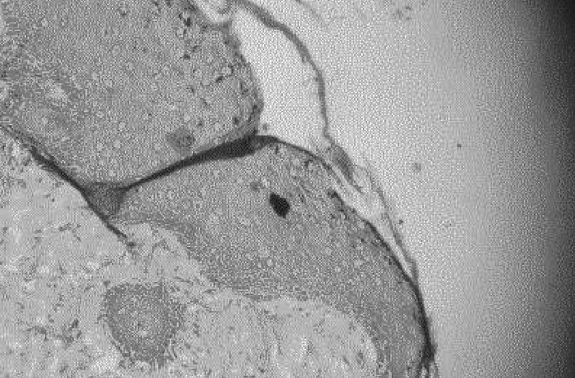
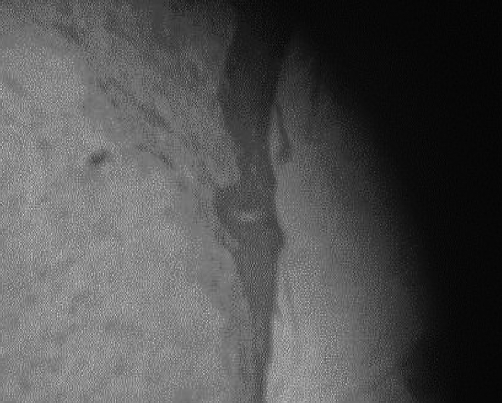
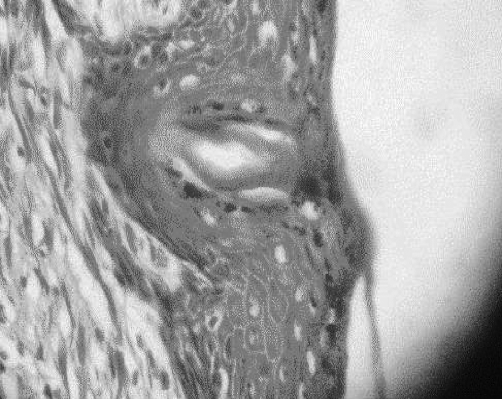
The characteristic histological finding was denudation of the epidermis with the dermis generally intact. Reepithelialization proceeded by proliferation in deep layers (Fig. 1). Beneath a uniform covering of deep staining keratinocytes, two cell layers were recognizable. Stratum basale cells were transformed into layers of mitotically active polyhedral cells. In all specimens, epidermal repair was at an advanced stage by the termination of the experiment. Mean epidermal depth measured from the centre of the wound at 150 total magnification was 12 mm in the MEBO group and 10 mm in the SSD group, compared to 25 mm in healthy skin. Inflammatory cells were rarely seen, but were more frequent in the MEBO group (Figs. 2 - 4).
Fig. 1. Healing area in MEBO group. Note epidermal denudation inferior to cut hair follicle, peeling scab, and intact dermis. Mag. X 600.
Fig. 2. Healing area in MEBO group. Note transition between healthy and burned skin in lower part of slide. Note also mitotic activity in basal area of epidermis in burned area. Mag. X 150.
Fig. 4. Healing area in SSD group. Note extensive epidermal denudation. Mag. X 150.
Fig. 3. Healing area in SSD group. Note migrating keracinocytes on the surface and intense mitotic activity in lower strata. Clear cells are melanocytes. Mag. X 600.
Discussion and conclusion
Burn wounds, even of partial depth, can present challenging clinical problems if the surface involved is sufficiently extensive. Excision and early wound closure are the recommended treatment at the present time. However, topical treatment is required in most burn patients with superficial burns. It is also needed in deep burns pending more definitive closure modalities because it plays a vital role in preventing microbial invasion.9 Silver compounds have been the mainstay of topical burn care since the early 1960s.10 Silver ion, which is released from SSD, kills virtually all known microbes, including yeasts. It kills on contact by blocking their cellular respiratory pathways.11 However, silver nitrate causes electrolyte imbalance. It is absorbed into the bloodstream, where it alters acid-base levels. Mafenide acetate causes pain and may increase opiate requirements, while SSD causes neutropenia.12 MEBO on the other hand soothes wounds by the action of a moisture-retaining oil, berberine, one of its active agents. It also suppresses microbial proliferation and promotes rapid re-epithelialization.7 In this study, rapid re-epithelialization occurred in MEBO-treated animals, which is consistent with previous findings.7 This is believed to be aided by B-sitosterol, a member of a family of plant steroids found in several plants, especially soya. MEBO reduces exposure of the burn surface, which - as shown by Smahel et al. - limits tissue damage and leads to better healing outcomes, even if healing times may be altered as a consequence.8 Although in vitro antimicrobial activity has not been demonstrated with MEBO, it has been shown that in experimental and clinical use it prevents wound infection just as much as SSD, possibly by blocking penetration of the eschar by virulent microbes.13 Retention of biological fluids on the wound surface is believed to prevent desiccation of tissue and promote the migration of keratincoytes across the wound. It also pro motes the function of natural cytokines and growth factors in the healing process by mechanisms that are yet to be explained.14,15 Worries that moist environments will increase tissue maceration and infection have not been supported by experimental or clinical evidence. Conventional mois ture-retaining devises such as Sofra-tulle and Tegaderm are time-consuming and labour-intensive to apply.
Although mean healing times were virtually identical in this study, a number of other studies show that SSD may delay healing, especially in deeper burns. In one such study, healing time was normal when SSD was used in split-thickness donor sites but significantly slower in deep er wounds.16,17 The mechanism by which this unique effect of delayed healing occurs is not yet clear. Invasive wound infection continues to be a route for much of the mortal ity from burns, especially in children.18 The search for suit able topical burn therapy will continue to focus on prepa rations that can kill microbes and promote tissue regener ation while limiting tissue damage. Concern about the tox icity of purified chemicals will continue to make people turn to complementary and alternative medicines (CAM) such as MEBO. Low toxicity, cheaper costs, easier handling, and availability are all factors that may promote the use of CAM such as MEBO.19 Our study shows that MEBO will be found to be an effective burn treatment, at least in partial-thickness cases and may be a useful alternative to conventional therapies such as SSD, especially in cases where infection is not a major consideration.
References
- 1.Pruitt B.A., Goodwin C.W., Pruitt S.K. In: Textbook of Surgery, The Biologic Basis of Modern Surgical Practice (15th ed.) Sabiston D., editor. Sabiston D. (ed.), W.B. Saunders; Philadelphia: 1995. pp. 221–263. [Google Scholar]
- 2.Badoe E.A., Achampong E.Q. In: Principles and Practice of Surgery (3rd ed.) Rocha, editor. University of Ghana Medical School; Accra: 1994. [Google Scholar]
- 3.Demling R.H., Wong S.O., Jin L.J. Early lung dysfunction after major burns. Role of edema and vasoactive mediators. J. Trauma. 1985;25:959–961. [PubMed] [Google Scholar]
- 4.Demling R.H., Pomposelli J.J. In: Surgical Infections. Meakins J.L., editor. New York: Scientific American Medicine; 1994. pp. 369–373. [Google Scholar]
- 5.Pruitt B.A.. Burn wound. In: Cameron J.L., editor. Current Surgical Therapy (5th ed.) St Louis: Mosby Year Book; 1995. pp. 872–872. [Google Scholar]
- 6.Subrahmanyam M.A. Prospective randomized clinical and histological study of superficial burn wound healing with honey and silver sulphadiazine. Burns. 1998;24:157–161. doi: 10.1016/s0305-4179(97)00113-7. [DOI] [PubMed] [Google Scholar]
- 7.Ang E.S., Lee S.T., Gan C.S., See P.I., Chan Y.H. The role of alternative therapy in the management of partial-thickness burns of the face; experience with use of moist exposed burn ointment (MEBO) compared with silver sulphadiazine. Ann. Acad. Med. Singapore. 2000;29:7–10. [PubMed] [Google Scholar]
- 8.Smahel J. The problem of dehydration and healing of burn wounds. Burns. 1993;19:511–512. doi: 10.1016/0305-4179(93)90009-w. [DOI] [PubMed] [Google Scholar]
- 9.Yin H., Langford R., Burrel R. Comparative evaluation of the antimicrobial activity of Acticoat, an antimicrobial barrier dressing. J. Burn Care Rehabil. 1999;20:193–200. doi: 10.1097/00004630-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Fox C. Silver sulphadiazine, a new topical therapy for Pseudomonas in burns. Arch. Surg. 1968;19:184–184. doi: 10.1001/archsurg.1968.01330200022004. [DOI] [PubMed] [Google Scholar]
- 11.Gilman & Goodman (eds), editor. Pharmacologic Basis of Therapeutics (5th ed.) New York: McMullin; 1975. pp. 930–930. [Google Scholar]
- 12.Jarnet F., Demling E. Acute leucopenia during topical burn therapy with silver sulphadiazine. Am. J. Surg. 1978;35:818–818. doi: 10.1016/0002-9610(78)90173-3. [DOI] [PubMed] [Google Scholar]
- 13.Mieduin R. Inhibition of wound healing by antiseptics. Br. J. Derm. 1986;15:41–41. doi: 10.1111/j.1365-2133.1986.tb02106.x. [DOI] [PubMed] [Google Scholar]
- 14.Vogt P., Andrea C., Breuing K., Liu P. Dry, moist and wet skin wound repair. Ann. Plast. Surg. 1995;1:493–499. doi: 10.1097/00000637-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Grinnel R. Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 1994;124:40–44. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reutering C.O., Agren M.S., Sodenberg T.A., Tengap I. The effects of occlusive dressings on inflammation and granulation tissue formation in excised wounds in rats. Scand. J. Plast. Reconstr. Surg. 1989;23:89–96. doi: 10.3109/02844318909004499. [DOI] [PubMed] [Google Scholar]
- 17.Atiyeh B.S., Al-Am C.A., El-Musa K.A., Dham R. The effect of moist exposed dressing: Healing, barrier function and restoration of partial-thickness burn wounds. Eur. J. Plast. Surg. 2003;26:5–11. [Google Scholar]
- 18.Ferrara J.J., Dyess D.L., Leuterman A.A. The suppressive effects of sub-eschar fluid on in vitro cell-mediated immunologic function. J. Burn Care Rehabil. 1998;9:584–588. doi: 10.1097/00004630-198811000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Yeh G.Y., Eisenberg D.M., Kaptchuk T.J. Systematic review of herbs and dietary supplements used in glycemic control in diabetes. Diabetes Care. 2003;26:1277–1294. doi: 10.2337/diacare.26.4.1277. [DOI] [PubMed] [Google Scholar]