Summary
This study was designed to evaluate the frequency and profile of bloodstream infection (BSI) in a burn intensive care unit (BICU) in Tripoli, Libya, from 1st January 2000 to 31st December 2007 and to determine the prevalence of different bacteria involved in such infections and their antimicrobial susceptibilities. During the eight-year study period, 995 patients were admitted to the BICU. Blood cultures were collected from each septicaemic case and reviewed for age, sex, total body surface area burned, isolated micro-organisms, and antibiotic sensitivity. There were 430 episodes of BSI among 830 cases; the annual true positive rate varied between 40.0 and 59.4%, the majority (87.9%) being caused by one species only. However, 22% had two or more episodes with different pathogens during hospitalization. The leading isolate was Staphylococcus aureus (40.4%) (methicillinresistant, 55.7%). Pseudomonas spp ranked second (23.9%). Klebsiella spp were third, responsible for 7.4%; the rate of extended spectrum beta lactamase among Klebsiella isolates was 47%. Candida spp were the fourth most common pathogen (6.7%), the majority (55%) being C. albicans. Staphylococci were generally resistant to trimethoprim (91%) and fusidic acid (80%). Pseudomonas spp proved moderately resistant (38-43%) to tobramicin, ciprofloxacin, amikacin, and impenem but remained relatively susceptible to cefepime (72%). Klebsiella isolates demonstrated moderate resistance (46-58%) to most agents tested, and relatively low resistance (19-27%) to meropenem, impenem, and cefepime. We suggest that extra infection control measures should be implemented and antibiotic policy and guidelines introduced to reduce the high resistance rate among isolates such as Pseudomonas, Acinetobacter, and MRSA.
Keywords: Bloodstream infection, burn intensive care unit, MRSA, Pseudomonas
Abstract
Les auteurs de cette étude se sont proposés d'évaluer la fréquence et le profil des infections septicémiques dans une unité de soins intensifs pour les patients brûlés (USIB) à Tripoli, Libye, du premier janvier 2000 au 31 décembre 2007 et de déterminer la prévalence des différentes bactéries impliquées dans ces infections et leur sensibilité aux antimicrobiens. Au cours de la période d'étude de huit ans, 995 patients ont été admis à l'USIB. Des cultures sanguines ont été prélevées chez tous les patients atteints de septicémie et analysées du point de vue de l'âge, du sexe, de la surface totale du corps brûlée, des micro-organismes isolés et de la sensibilité aux antibiotiques. Dans 430 épisodes d'infection septicémique sur 830 cas le taux annuel de vrais positifs variait entre 40,0 et 59,4%, la majorité (87,9%) causée par une seule espèce. Toutefois, 22% des patients ont subi deux ou plusieurs épisodes, avec divers agents pathogènes, pendant l'hospitalisation. L'isolat le plus commun était Staphylococcus aureus (40,4%) (résistant à la méticilline, 55,7%), avec Pseudomonas spp au deuxième rang (23,9%), suivi par Klebsiella spp (7,4%); le taux de béta-lactamase à spectre étendu parmi les isolats de Klebsiella était de 47%. Le quatrième agent pathogène était Candida spp (6,7%), la majorité (55%) C. albicans. Les Staphylococci étaient généralement résistants au triméthoprime (91%) et à l'acide fusidique (80%). Les Pseudomonas spp ont démontré une résistance modérée (38-43%) à la tobramicine, à la ciprofloxacine, à l'amikacine, et à l'impénème mais sont restés relativement sensibles à la céfépime (72%). Les isolats de Klebsiella présentaient une résistance modérée (46-58%) à la plupart des agents testés, et une résistance relativement faible (19-27%) au méropénem, à l'impénem, et à la céfépime. Selon les auteurs, on devrait appliquer des mesures supplémentaires de contrôle des infections, introduire des politiques antibiotiques et suivre les lignes directrices mises en place pour réduire le taux de résistance élevé registré registré parmi les isolats tels que Pseudomonas, Acinetobacter, et Staphylococcus aureusrésistant à la méticilline (40,4%).
Introduction
Hospital-acquired bloodstream infection (BSI) is a serious health care problem worldwide associated with significant morbidity and mortality. 1, 2It accounts for 10-15% of hospital-acquired infections and increases costs of hospitalization. 3, 4In the United States, 10-20% of nosocomial infections are estimated to involve the bloodstream. 5Appropriate antimicrobial treatment of BSI is critical in decreasing morbidity and mortality due to BSI; 6many surveillance studies indicate a trend of increasing antimicrobial resistance among common pathogens such as staphylococcal species, the most common bacteria reported for BSI among patients in intensive care units. 7Some previous reports of patients with methicillin-resistant Staphylococcus aureus (MRSA) found higher mortality rates, increased morbidity, and longer length of hospital stay. 8, 9Risk factors for MRSA blood infections have been extensively described but vary among institutions and patient populations. 10, 11 Candida albicans was reported to be associated with the highest mortality rate, ranging from 40-70%, 12, 13and BSI with Gram-negative bacteria (17%). 14The frequency of Gram-negative sepsis has diminished over the last 20 years; however, P. aeruginosa is considered an important nosocomial BSI pathogen with a high associated mortality. 15
Identification of BSI bacterial pathogens combined with determination of antimicrobial susceptibility of the bacteria can help clinicians to select appropriate agents for rigorous empirical treatment of BSI. 16Although eradication of infection in burn patients is impossible, a well-conducted effective surveillance, infection control and a prevention measures programme can help to reduce the incidence. In recent years, studies of epidemiology, microbiological aetiology, and prognosis have been performed worldwide. 17, 18, 19, 20, 21
The aim of the current study is to evaluate the frequency and profile of bacteraemia and antimicrobial susceptibilities of BSI among patients at the Burn Plastic Surgery Centre, Tripoli, Libya.
Patients and methods
Between January 2000 and December 2007, information regarding all patients with microbiological evidence of septicaemia admitted to the burn intensive care unit (BICU) at the Burn Plastic Surgery Centre in Tripoli, Libya, was collected and reviewed for age, sex, and TBSA, isolation of micro-organisms and antibiotic sensitivity. Blood was obtained under aseptic conditions. At least two sets of blood cultures were collected for each case. Ten ml of blood were aseptically collected from adults and 2-5 ml from paediatric cases. The samples were added to bottles of blood culture medium (OXOID, England), incubated at 37 °C, and monitored for 7 days. All bottles designated positive were examined by Gram stain and subcultured on blood agar and MacConkey and Sabouraud agar (OXOID, England), and also screened on selective and differential chromogenic medium, MRSA ID medium (bioMérieux, France).Green colonies on MRSA ID plates at 24 and 48 h were identified as MRSA; all isolates were identified using standard diagnostic microbiological methods of micro-organism isolation. 22Specimens that grew more than one organism were also subcultured, the organisms being separated and identified by standard diagnostic methods. Candidaspecies that were isolated from blood cultures were identified using API-20 C AUX system (bioMérieux, Lyons, France). A single positive blood culture was necessary for a diagnosis of BSI. The detection of the same organism in one or more blood cultures from the same patient within one week was defined as a single episode. 23The antibiotic susceptibility of each isolate was tested manually according to the Clinical Laboratory Standards Institute (CLSI) guidelines, 24and all isolates were inoculated onto Mueller Hinton medium (OXOID, England). Detection of methicillin resistance was carried out according to CLSI guidelines for detection of MRSA by using disk diffusion technique (cefoxitin 30 µg and oxacillin 1 µg). All S. aureus isolates were further evaluated and confirmed by detection of PBP2a latex agglutination (bioMérieux, France); S. aureus ATCC 33591 was included as a reference strain for quality control. All Klebsiella isolates were subjected to extended spectrum beta lactamas (ESBL) detection by using chromogenic medium, ESBL ID medium (bioMérieux, France).
Results
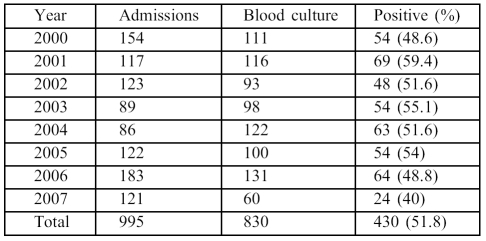
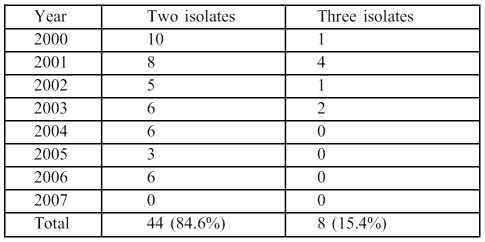
During the study period, 995 patients were admitted to BICU for periods ranging from 14-45 days. The majority had indwelling vascular catheters and respiratory intubation and were on broad-spectrum antimicrobial therapy including gentamicin, amikacin, ciprofloxacin, ceftriaxone, impenem, meropenem, or cefepime. In addition, vancomycin was used to treat all MRSA cases. There were 585 males (58.5%) and 410 females, with a male:female ratio of 1.4:1. The median age was 35 (range 2 months 85 years). The mean TBSA was 55% (range 10-90%). There were 430 (51.8%) episodes of BSI identified among 830 patients; the annual proportion of BSI varied between 40-59.4% ( Table I ). However, 22% of these cases had two or more episodes of BSI detected at different intervals and caused by different pathogens during hospitalization at BICU. Among the 430 episodes of BSI, the majority (87.9%) were caused by a single species while only 52/430 (12.1%) were caused by a combination of more than one species of micro-organism. Overall, 44/52 cases (84.6%) had simultaneous infection caused by two isolates, while a combination of three different micro-organisms was observed in 8/52 (15.4%). No infections due to two or more species were observed during the last year of the study ( Table II ).
Table I. Episodes of bloodstream infection in the burns intensive care unit (2000-07).
Table II. Polymicrobial episodes (52 episodes) of bloodstream infection (2000-07).
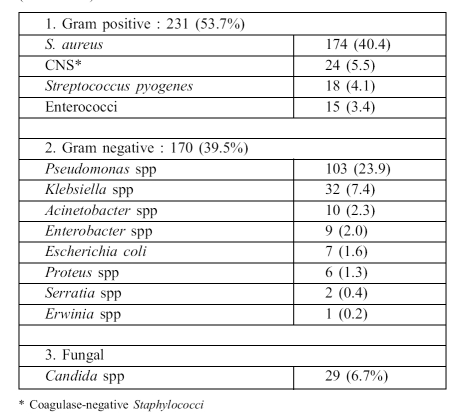
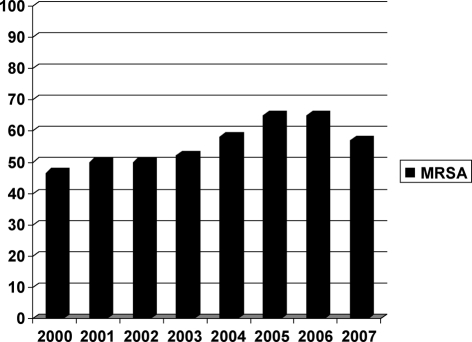
Table III summarizes the distribution of micro-organisms isolated over the eight years. Gram-positive species represented 231 of the 430 cases (53.7%), Gram-negative accounted for 170/430 (39.5%), and 29/430 (6.7%) were caused by Candida species. Among Gram-positive organisms, the leading isolate was S. aureus (174/430, 40.4%) and 97 (55.7%) were MRSA. The percentage of MRSA varied during the successive eight years of the study, as follows: 46.6%, 50%, 50%, 52%, 58%, 65%, 65%, 57% respectively; the average was 55.5% ( Fig. 1 ). Methicillin resistance was detected among coagulase-negative staphylococcal isolates (7/24, 29.1%).
Table III. Isolates from 430 episodes of bloodstream infection (2000-2007).
Fig. 1. Distribution of MRSA between 2000-2008.
Pseudomonas spp (103/430, 23.9%) were the most common Gram-negative isolate, the majority (85/103, 82.5%) being P. aeruginosa. Klebsiella spp were the second most common (32/430, 7.4%). The proportion of ESBL among Klebsiella isolates was 47%. Candida species were the fourth most common isolate (29/430, 6.7%) ( Table III ). Candida albicans was the most common (55%). Many of the candidaemia episodes (21/52, 40.3%) were associated with other isolates such as Staphylococci, Pseudomonas, and Klebsiella. Candidaemia was not detected during the last two years of the study.
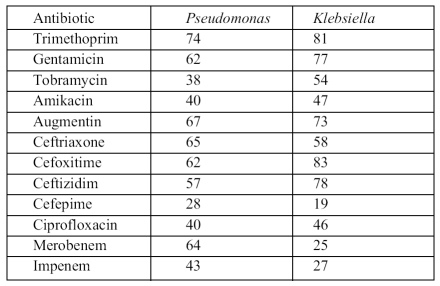
Antimicrobial sensitivity data for the most frequently isolated organisms are shown in Table IV . The results showed variability in susceptibility among pathogens and among antimicrobial agents.
Table IV. Resistance of Pseudomonas and Klebsiella isolates to antimicrobial agents .
Staphylococci were generally resistant to trimethoprim (91%) and fusidic acid (80%) but relatively less resistant to ciprofloxacin (52%). Pseudomonas spp proved moderately resistant (38-43%) to tobramicin, ciprofloxacin, amikacin and impinem but remained relatively susceptible to cefepime (72%). Klebsiella isolates demonstrated moderate resistance (46-58%) to most agents tested such as tobramicin, amikacin, cefoxatime, ceftriaxone, and ciprofloxacin, but there was relatively little resistance (19 27%) to meropenem, impenem, and cefepime.
Generally, most pathogens studied were resistant to trimethoprim (74-91%). The activity of cephalosporins against Gram-negative organisms was mixed - cefepime was often more active than the other cephalosporins. Among the aminoglycosides, amikacin was relatively active against the most commonly isolated organisms compared with gentamicin, while ciprofloxacin proved to be a moderate active agent against these organisms. No antifungal susceptibility was carried out in the study. However, all patients received fluconazole as treatment for candidaemia.
Discussion
During the eight-year study period, out of 830 blood cultures from suspected cases with clinical evidence of septicaemia referred to our laboratory, 51.8% were found to be blood-culture positive by the conventional diagnostic techniques used in the study. Data from the National Nosocomial Infections Surveillance System (NNIS) from 1995 to 2000, involving nearly 800 intensive care units (ICUs) demonstrated that BSI rates were higher in burns ICUs compared with other types of ICU. 25Similarly to our results, specialized Brazilian BICUs showed that primary BSI was the most common infection (49%). 26However, two separate studies conducted in burns care units in Iran and Turkey recently demonstrated that the BSI rate (18.6% and 19.9% respectively) was lower than in our study. 27, 28In a different setting, it was demonstrated that among haemodialysis patients the incidence of BSI among the patients who used a double-lumen central venous catheter was very high (61%), and it was concluded that the risk factors for developing BSI were the use of a catheter in the internal jugular vein, the duration of catheter use, and length of hospitalization. 29In another study, up to 49% of cancer patients admitted to an ICU developed nosocomial BSI. 30
The causative organisms of BSI have changed over time. In the 1960s and 1970s, Gram-negative bacteria were more predominant causative agents but over the last few decades there has been a shift toward predominance by Gram-positive bacteria. 31There have been reports suggesting 70-81% of the bacteria isolated from BSI are Grampositive. 32, 33Similar trends were also observed in BICUs. 26, 27, 28The distribution of micro-organisms isolated over the eight years was as follows: Gram-positive species (53.7%) figured as the leading cause of BSI, followed by Gram-negatives (39.5%), and Candida spp (6.7%). Santucci and colleagues found that the pattern of the main micro-organisms associated with BSI in BICUs was S. aureus (24%), P. aeruginosa (18%), and Acinetobacter (14%), followed by Candida (8%). 26
In this study, the majority (87.9%) of BSI episodes were caused by a single species and only 12.1% of the cases were polymicrobial. In addition, among the population studied, 22% had two or more episodes of BSI detected at different intervals caused by different pathogens during hospitalization in the BICU. No polymicrobial episodes were detected during the last year of the study. A previous study 34showed that patients with polymicrobial infection involving P. aeruginosa had worse clinical courses and developed shock more frequently. A major difficulty is the choice of an appropriate antimicrobial treatment for polymicrobial infection involving P. aeruginosa. 35This problem is even greater if Candida is the co-pathogen 36Similarly, in this study the majority of polymicrobial BSI episodes involved Candida spp as a co-pathogen associated with Staphylococci, Pseudomonas, and Klebsiella spp. Many studies have associated polymicrobial infection with higher mortality. 37, 38During this study period, candidaemia was the fourth most frequent isolate, exceeded only by Staphylococci, Pseudomonas, and Klebsiella. No candidaemia was detected during the last two years of the study. C. albicans was the most common pathogen (55%). It has been shown that it continues to account for approximately half of all episodes of candidaemia reported worldwide; nevertheless, frequencies vary widely from institution to another. 39, 40, 41, 42However, our findings were similar to those in the United States of America (52-54%), reported from surveillance studies, and indicated that many candidaemia episodes (40.3%) were associated with other isolates such as Staphylococci, Pseudomonas, and Klebsiella. 43, 44, 45
Antibiotic resistance was common, including MRSA, ESBL-producing isolates, and multiple drug-resistant (MDR) organisms, such as Acinetobacter. The three most common isolates were evaluated for susceptibility to antimicrobial agents. The results showed great variability in susceptibility among pathogens and among antimicrobial agents. With regard to pathogens, the leading isolate associated with BSI was S. aureus (40.4%); the incidence rate of S. aureus has increased over the years. 6MRSA was responsible for 55.7% of the cases. The percentage of MRSA rate increased steadily during the first seven year of the study (46.6-65%) but declined to 57% during the last year of the study. Another study in Turkey demonstrated that MRSA among BICU patients was high (40%),28 while BSI evaluated among Brazilian patients on haemodialysis identified the most frequently isolated organism as Gram-positive (49%), of which S. aureus was the most prevalent, while the MRSA rate was 43.4%. 25Our results are comparable to many studies, as multiresistant MRSA has been reported to be relatively high in African countries including Morocco, Kenya, Nigeria, and Cameroon. 47Comparable results were also found in South Africa, 48where 84.7% of MRSA isolates were resistant to at least four classes of antibiotics. The risk factors for methicillin resistance in S. aureus have been extensively described but vary among institutions and patient populations. 49, 50Recently, linezolids have been introduced into the therapeutic arsenal for combating MRSA infection in our BICU, on the basis of a study carried out on MRSA isolates obtained from burn patients, 51but their clinical value is not yet clearly established for BSI. Most Staphylococci isolates were resistant to trimethoprim (91%) and fusidic acid (80%) but susceptible to ciprofloxacin. Pseudomonas species were resistant (38-43%) to tobramicin, ciprofloxacin, amikacin, and impenem but remained relatively susceptible to cefepime. Klebsiella demonstrated resistance (46-58%) to tobramicin, amikacin, cefoxatime, cephtriaxone, and ciprofloxacin but relatively less resistance (19-27%) to meropenem, impenem, and cefepime. The activity of cephalosporins against Gram-negative organisms was mixed - cefepime was often more active than the other cephalosporins. However, clinical experience with cefepime in infections caused by ESBL-producing bacteria is limited, and evidence thus far favours the use of carbapenems. 52Among the aminoglycosides, amikacin was relatively active against the most common isolated organisms compared with gentamicin, while ciprofloxacin proved to be a moderately active agent against these organisms. It has been demonstrated that S. aureus and Pseudomonas are the most common resistant organisms identified among burn populations. 53ESBL-producing strains have become a difficult challenge for clinicians. 52, 53, 54Overall, patients with BSI caused by ESBL-producing pathogens have a higher risk of death than those with BSI caused by non-ESBL producing pathogens. A prospective study conducted in six countries analysed K. pneumonia bacteraemia, and showed that 19% of these organisms were ESBL-positive. 55The rate of infections caused by ESBL-producing organisms was higher in patients who acquired their infection in hospital (31%), particularly ICU patients (44%). Comparable results were obtained in this study, with 47% of K. pneumonia isolates being ESBL-positive. The rate of infections caused by ESBL-producing organisms varied from 59% in Argentina to 25% in the USA and 12% in Belgium. A. baumannii is becoming an increasingly significant pathogen, causing a number of infections including BSI and commonly associated with high mortality. 56Infection caused by MDR A. baumannii led to a return of colistin to clinical practice. 57, 58Nevertheless, resistance to colistin has been reported not only for A. baumannii 59but also for K. pneumonia 60and Pseudomonas spp. 61The emergence of these broadly resistant organisms may reflect selective pressure of the frequently used cephalosporins and fluoroquinilones among the patients. 62, 63Effective surveillance, proper antibiotic policy tailored to local findings, and good adherence to infection control measures may therefore reduce infection, mortality rates, the duration of hospitalization, and associated costs.
Conclusion
In view of the high incidence of MRSA and the fact that the majority of Pseudomonas, Klebsiella, and Acinetobacter isolates are resistant to most commonly used antibiotics, a comprehensive education campaign is required, together with the institution of more efficient and effective quality control measures in hospital environments. Further study is needed to fingerprint the interrelations of all MRSA isolates.
Acknowledgments
The authors wish to thank Dr Blackwell and Dr Bouri for their technical advice and would also like to extend their sincere gratitude to the Diar Assalam Company (bioMérieux) for their financial support.
References
- 1.Garrouste-Orgeas M, Timsit JF, Taffet M, et al. Excess risk of death from intensive care unit-acquired nosocomial bloodstream infections: A reappraisal. Clin Infect Dis. 2006;42:1118–26. doi: 10.1086/500318. [DOI] [PubMed] [Google Scholar]
- 2.Laupland KB, Lee H, Gregson DB, et al. Cost of intensive care unit-acquired bloodstream infections. J Hosp Infect. 2006;63:124–32. doi: 10.1016/j.jhin.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Richard P, Edmond W, Edmond MB. The impact of hospital-acquired bloodstream infections. Emerg Infect Dis. 2001;7:174–7. doi: 10.3201/eid0702.010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hugonnet S, Sax H, Eggimann P, et al. Nosocomial bloodstream infection and clinical sepsis. Emerg Infect Dis. 2004;10:76–81. doi: 10.3201/eid1001.030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Society for Microbiology Report of the ASM Task Force on Antibiotic Resistance. Antimicrob Agents Chemother (suppl) 1995:1–23. [PubMed] [Google Scholar]
- 6.Nosocomial infection rates for interhospital comparison:Limitations and possible solutions. A report from the National Nosocomial Infections Surveillance (NNIS) System. Infect Control Hosp Epidemiol. 1991;12:609–21. [PubMed] [Google Scholar]
- 7.Weinstein MP, Towns ML, Quartey SM, et al. The clinical significance of positive blood cultures in the 1990s: A prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 8.Kopp BJ, Nix DE, Armstrong EP. Clinical and economic analysis of methicillin-susceptible and -resistant S. aureus infections. Ann Pharmacother. 2004;38:1377–82. doi: 10.1345/aph.1E028. [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove S, Sakoulas G, Perencevich E. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36:53–9. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 10.Lodise TP jr, McKinnon PS, Rybak M. Prediction model to identify patients with Staphylococcus aureus bacteremia at risk for methicillin resistance. Infect Control Hosp Epidemiol. 2003;24:655–61. doi: 10.1086/502269. [DOI] [PubMed] [Google Scholar]
- 11.McHugh CG, Riley LW. Risk factors and costs associated with methicillin-resistant Staphylococcus aureus bloodstream infections. Infect Control Hosp Epidemiol. 2004;25:425–30. doi: 10.1086/502417. [DOI] [PubMed] [Google Scholar]
- 12.Zaoutis TE, Argon J, Chu J, et al. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States. Clin Infect Dis. 2005;34:1232–9. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 13.Eggimann P, Garbo J, Pittet D. Epidemiology of Candida species infection in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3:685–702. doi: 10.1016/s1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 14.Jamal WY, El-Din K, Rotimi VO, et al. An analysis of hospitalacquired bacteremia in intensive care unit patients in a university hospital in Kuwait. J Hosp Infect. 1999;43:49–56. doi: 10.1053/jhin.1999.0608. [DOI] [PubMed] [Google Scholar]
- 15.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention CDC's campaign to prevent antimicrobial resistance in health-care settings. MMWR Morb Mortal Wkly Rep. 2002;51:343. [PubMed] [Google Scholar]
- 17.Friedman ND, Kaye KS, Stout JE, et al. Heath care-associated bloodstream infections in adults: A reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–7. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 18.Valles J, Leon C, Alveraz F. Spanish collaborative group for infections in ICUs - Nosocomial bacteraemia in critically ill patients: A multicenter study evaluating epidemiology and prognosis. Clin Infect Dis. 1997;24:387–95. doi: 10.1093/clinids/24.3.387. [DOI] [PubMed] [Google Scholar]
- 19.Valles J, Calbo E, Anoro E. Bloodstream infections in adults: Importance of healthcare-associated infections. J Infect. 2008;56:27–34. doi: 10.1016/j.jinf.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Baby HA. Bacterial isolates from fatal cases of bloodstream infections at a university hospital in Central Saudi Arabia. Saudi Med J. 2007;28:231–5. [PubMed] [Google Scholar]
- 21.Ben Jaballah N, Bouziri A, Mnif K, et al. Epidemiology of hospital acquired bloodstream infections in a Tunisian pediatric intensive care unit: A 2-year prospective study. Am J Infect Control. 2007;35:613–8. doi: 10.1016/j.ajic.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Murry PR, Baron EJ, Pfaller MA, et al. Manual for Clinical Microbiology. ASM Press; Washington: 1995. [Google Scholar]
- 23.Bouza E, Perez-Molina J, Munoz P. Reports of ESGNI-001 and ESGNI-002 studies, blood stream infections in Europe. Cl Microbial Infect (suppl. 2) 1999;5:S1–S12. [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards . NCCLS approved standard M100-S15 NCCLS (M2-A8 and M7-A6) Wayne, PA; USA: 2009. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 25.National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary January 1992-June 2002, issued August 2002. Am J Infect Control. 2002;30:458–75. doi: 10.1067/mic.2002.130032. [DOI] [PubMed] [Google Scholar]
- 26.Santucci SG, Gobara S, Santos CR, et al. Infections in a burn intensive care unit: Experience of seven years. J Hosp Infect. 2003;53:6–13. doi: 10.1053/jhin.2002.1340. [DOI] [PubMed] [Google Scholar]
- 27.Ekrami A, Kalantar E. Bacterial infections in burn patients at a burn hospital in Iran. Indian J Med Res. 2007;126:541–4. [PubMed] [Google Scholar]
- 28.Oncul O, Ulkur E, Acar A, et al. Prospective analysis of nosocomial infections in a burn care unit, Turkey. Indian. J Med Res. 2009;130:758–4. [PubMed] [Google Scholar]
- 29.Grothe C, Belasco A, Bittencourt A, et al. Incidence of bloodstream infections among patients on hemodialysis by central venous catheter. Rev Latino-Am Enfermagem. 2010;18:73–80. doi: 10.1590/s0104-11692010000100012. [DOI] [PubMed] [Google Scholar]
- 30.Prabhash K, Medhekar A, Ghadyalpatil N, et al. Blood stream infections in cancer patients: A single centre experience of isolates and sensitivity pattern. Indian J Cancer. 2010;47:184–8. doi: 10.4103/0019-509X.63019. [DOI] [PubMed] [Google Scholar]
- 31.Wisplinghoff H, Seifert H, Wenzel RP. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasmas in the United States. Clin Infect Dis. 2003;63:110–1. doi: 10.1086/374339. [DOI] [PubMed] [Google Scholar]
- 32.Rubio M, Palau L, Vivas JR, et al. Predominance of gram-positive microorganisms as a cause of septicemia in patients with hematological malignancies. Infect Control Hosp Epidemiol. 1994;15:101–4. doi: 10.1086/646869. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Barca E, Fernandez-Sevilla A, Carratala J, et al. Prospective study of 288 episodes of bacteremia in neutropenic cancer patients in a single institution. Eur J Clin Microbiol Infect Dis. 1996;15:291–6. doi: 10.1007/BF01695660. [DOI] [PubMed] [Google Scholar]
- 34.Aliaga L, Mediavilla JD, Llosá J, et al. Clinical significance of polymicrobial versus monomicrobial bacteremia involving Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2000;19:871–4. doi: 10.1007/s100960000392. [DOI] [PubMed] [Google Scholar]
- 35.Marra AR, Bearman GML, Wenzel RP, et al. Comparison of the systemic inflammatory response syndrome between monomicrobial and polymicrobial Pseudomonas aeruginosa nosocomial bloodstream infections. BMC Infectious Diseases. 2005;5:94. doi: 10.1186/1471-2334-5-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahim EH, Sherman G, Ward S, et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:9–11. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 37.Pittet D, Li N, Wenzel RP. Association of secondary and polymicrobial nosocomial bloodstream infections with higher mortality. Eur J Clin Microbiol Infect Dis. 1993;12:813–9. doi: 10.1007/BF02000400. [DOI] [PubMed] [Google Scholar]
- 38.Pittet D, Li N, Woolson RF. Microbiological factors influencing the outcome of nosocomial bloodstream infections: A 6-year validated, population-based model. Clin Infect Dis. 1997;24:1068–78. doi: 10.1086/513640. [DOI] [PubMed] [Google Scholar]
- 39.Marchetti O, Bille J, Fluckiger U. Epidemiology of candidemia in Swiss tertiary care hospitals: Secular trends, 1991-2000. Clin Infect Dis. 2004;38:311–20. doi: 10.1086/380637. [DOI] [PubMed] [Google Scholar]
- 40.Rani R, Mohaprata NP, Mehta G. Changing trends of Candida species in neonatal septicemia in a tertiary north Indian hospital. Ind J Med Microbiol. 2002;20:42–4. [PubMed] [Google Scholar]
- 41.Godoy P, Tiraboschi IN, Severo LC. Species distribution and antifungal susceptibility profile of Candida spp. bloodstream isolates from Latin America hospitals. Mam Inst Oswaldo Cruz. 2003;98:401–5. doi: 10.1590/s0074-02762003000300020. [DOI] [PubMed] [Google Scholar]
- 42.Chen S, Slavin M, Nguyen Q. Australian Candidemia Study Active surveillance for candidemia. Australia Emerg Infect Dis. 2006;12:1508–16. doi: 10.3201/eid1210.060389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diekema DJ, Messer SA, Brueggemann AB. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J Clin Microbiol. 2002;40:1298–302. doi: 10.1128/JCM.40.4.1298-1302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfaller MA, Diekema DJ, Jones RN, et al. The SENTRY Participant Group International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibility to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY Antimicrobial Surveillance Program. J Clin Microbiol. 2001;39:3254–9. doi: 10.1128/JCM.39.9.3254-3259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfaller MA, Jones RN, Doern GV, et al. The SENTRY Participant Group Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997-1998. Antimicrob Agents Chemother. 2000;44:747–1. doi: 10.1128/aac.44.3.747-751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinberg JP, Clark CC, Hackman BO. Nosocomial and community acquired Staphylococcus aureus MRSA bacteremias from 1980 to 1993: Impact of intravascular devices and methicillin resistance. Clin Infect Dis. 1996;23:255–9. doi: 10.1093/clinids/23.2.255. [DOI] [PubMed] [Google Scholar]
- 47.Kesah C, Redjeb SB, Odugbemi TO, et al. Prevalence of methicillin resistant Staphylococcus aureus in eight African hospitals and Malta. Clin Microbiol Infect. 2003;9:153–6. doi: 10.1046/j.1469-0691.2003.00531.x. [DOI] [PubMed] [Google Scholar]
- 48.Shittu O, Lin J. Antimicrobial susceptibility pattern and characterization of clinical isolates of Staphylococcus aureus in Kwa Zulu Natal province, South Africa. BMC Infectious diseases. 2006;6:125–31. doi: 10.1186/1471-2334-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lodise TP jr, McKinnon PS, Rybak M. Predication model to identify patients with Staphylococcus aureus bacteremia at risk for methicillin resistance. Infect Control Hosp Epidemiol. 2003;24:655–61. doi: 10.1086/502269. [DOI] [PubMed] [Google Scholar]
- 50.Jang TN, Kue BI, Shen SH, et al. Nosocomial gram-negative bacteremia in critically ill patients: Epidemiologic characteristics and prognostic factors in 147 episodes. J Formosan Med Assoc. 1999;98:465–73. [PubMed] [Google Scholar]
- 51.Zorgani AA, Sadaa K, Tawil KA, et al. In vitro activity of methicillin resistant staphylococci to linezolid and some other antimicrobial agents. Libyan J Infect Dis. 2008;2:51–5. [Google Scholar]
- 52.Ramphal R, Ambrose PG. Extended-spectrum beta-lactamases and clinical outcomes: Current data. Clin Infect Dis. 2006;42:S164–72. doi: 10.1086/500663. [DOI] [PubMed] [Google Scholar]
- 53.Wibbenmeyer L, Danks RM, Faucher L, et al. Prospective analysis of nosocomial infection rates, antibiotic use, and patterns of resistance in a burn population. J Burn Care Research. 2006;27:152–60. doi: 10.1097/01.BCR.0000203359.32756.F7. [DOI] [PubMed] [Google Scholar]
- 54.Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: A systematic review and metaanalysis. J Antimicrob Chemother. 2007;60:913–20. doi: 10.1093/jac/dkm318. [DOI] [PubMed] [Google Scholar]
- 55.Canton R, Novais A, Valverde A, et al. Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clin Microbiol Infect. 2008;14:144–53. doi: 10.1111/j.1469-0691.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 56.Kollef MH. Broad-spectrum antimicrobials and the treatment of serious bacterial infections: Getting it right up front. Clin Infect Dis. 2008;47:S3–13. doi: 10.1086/590061. [DOI] [PubMed] [Google Scholar]
- 57.Pastewski AA, Caruso P, Parris AR, et al. Parenteral polymyxin B use in patients with multidrug-resistant Gram-negative bacteremia and urinary tract infections: A retrospective case series. Ann Pharmacother. 2008;42:1177–87. doi: 10.1345/aph.1K346. [DOI] [PubMed] [Google Scholar]
- 58.Michalopoulos A, Falagas ME. Colistin and polymyxin B in critical care. Crit Care Clin. 2008;24:377–91. doi: 10.1016/j.ccc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Matthaiou DK, Michalopoulos A, Rafailidis PI, et al. Risk factors associated with the isolation of colistin-resistant Gram-negative bacteria: A matched case-control study. Crit Care Med. 2008;36:807–11. doi: 10.1097/CCM.0B013E3181652FAE. [DOI] [PubMed] [Google Scholar]
- 60.Antoniadou A, Kontopidou F, Poulakou G, et al. Colistin-resistant isolates of Klebsiella pneumonia emerging in intensive care unit patients: First report of a multiclonal cluster. J Antimicrob Chemother. 2007;59:786–90. doi: 10.1093/jac/dkl562. [DOI] [PubMed] [Google Scholar]
- 61.Falagas ME, Rafailidis PI, Matthaiou DK, et al. Pandrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii infections: Characteristics and outcome in a series of 28 patients. Int J Antimicrob Agents. 2008;32:450–4. doi: 10.1016/j.ijantimicag.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 62.Akcam FZ, Karaaslan D, Dogan M, et al. Microbiological surveillance in the intensive care unit: A tertiary hospital experience. Med Sci Monit. 2006;12:81–5. [PubMed] [Google Scholar]
- 63.McHugh CG, Riley TG. Risk factors and costs associated with methicillin-resistant Staphylococcus aureus bloodstream infections. Infect Cont Hosp Epidemiol. 2004;25:425–30. doi: 10.1086/502417. [DOI] [PubMed] [Google Scholar]