Summary
Background. For the burn surgeon, the treatment of necrotizing soft tissue infections is one of the most demanding surgical emergencies, requiring "radical excisions" of the infected tissue and reconstruction. During the infection period, the excised sites are treated with application of gauzes soaked in saline solution. When the septic period is over, the excision sites are usually covered by sterile paraffin gauze dressing. Our aim was to evaluate a new calcium polyuronate dressing enriched with zinc and manganese ions (test group) versus the reference therapeutic combination (control group) from the septic period to the grafting of skin. Materials and methods. A multicentre, prospective, controlled, randomized clinical trial was conducted from November 2003 to July 2005. The primary endpoint was the waiting period for carrying out the skin graft and the percentage of grafted patients at 28 days after the last excision. The secondary endpoints were blood loss, exudates amounts, and pain during dressing changes. Results. Twenty-five patients were included, 14 with the new dressing and 11 with the reference therapeutic combination. The average waiting period for skin graft was 18 days in the test group versus 27.1 days in the control group (p = 0.128). All the patients in the test group received their grafts within 28 days after the last excision, compared with 60% (p = 0.043) in the control group. Bleeding during dressing change was statistically lower in the test group: 45.5% of the patients did not bleed compared with 0% in the control group (p = 0.045). Treatments were well tolerated. Conclusion. The properties of this new calcium polyuronate enriched with zinc and manganese ions seem to accelerate granulation tissue development, allowing skin grafting earlier in favourable conditions with less bleeding and less pain during dressing renewal.
Keywords: necrotizing dermohypodermitis, calcium polyuronate, dressing, zinc, manganese
Abstract
Données de base. Le traitement des dermohypodermites nécrosantes est une urgence chirurgicale nécessitant l'exérèse des tissus nécrosés. Pendant la période septique des compresses imbibées de sérum physiologique sont appliquées sur les lésions. Une fois le sepsis résolu, le traitement se poursuit par des pansements gras jusqu'à obtention d'un tissu de granulation permettant la greffe cutanée. Le but de ce travail était de comparer le traitement habituel (sérum physiologique puis pansement gras) à l'utilisation d'un nouveau pansement, polyuronate de calcium enrichi en ions zinc et manganèse, de la période septique à la réalisation de la greffe. Matériels et méthodes. Étude prospective multicentrique et randomisée avec période d'inclusion de novembre 2003 à juillet 2005. Le critère d'évaluation principal était le délai pour réaliser la greffe de peau. Le pourcentage de patients greffés dans les 28 jours suivant la dernière excision a été calculé. Les critères secondaires étaient les caractères hémorragique et exsudatif des lésions ainsi que le niveau de douleur des patients au renouvellement des pansements. Résultats. Vingt-cinq patients ont été inclus, dont 14 ont reçu le nouveau pansement et 11 le traitement comparateur (sérum physiologique puis pansement gras). L'analyse des critères d'efficacité a porté sur 20 patients en raison de déviations majeures au protocole. La durée moyenne avant la greffe de peau était de 18 jours avec le nouveau pansement contre 27,1 jours avec le pansement de référence (p = 0,128). Tous les patients traités avec le nouveau pansement ont reçu leur greffe de peau dans les 28 jours versus 60% des patients dans le groupe comparateur (p = 0,043). Les saignements lors du renouvellement des pansements étaient significativement réduits avec le nouveau pansement: 45,5% des patients n'ont pas saigné versus aucun patient sans saignement dans le groupe comparateur (p = 0,045). Aucune intolérance aux traitements n'a été rapportée. Conclusion. Les propriétés spécifiques du polyuronate de calcium enrichi en ions de zinc et manganèse semblent avoir un effet bénéfique sur le délai de greffe ainsi que sur le saignement et la douleur lors du renouvellement des pansements.
Introduction
Local management of wounds should provide optimal healing conditions and reduce the risk of infections. Unambiguous clinical evidence is lacking regarding the use of topical agents for local wound care and their effect on wound healing. The authors of a recent systematic review concluded that the clinical trials performed to support the use of these topical agents in general were very small and of very poor quality. 1, 2
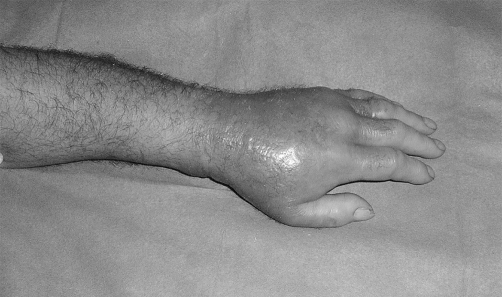
Bacterial necrotizing dermohypodermitis (BNDH) is a bacterial infection of the hypodermis causing necrosis without affecting the fascia (thus differentiating it from necrotizing fasciitis) 3, 4constitutes "gangrene of cellular tissues with thrombosis of local subcutaneous vessels". In such cases, there is no abscess formation or frank pus present. 5, 6In western countries, the condition affects 1 person in 100,000, with a mortality rate of 30% ( Fig. 1 ).
Fig. 1 A. Pre-operative view of upper limb. Bacterial necrotizing dermohypodermitis.
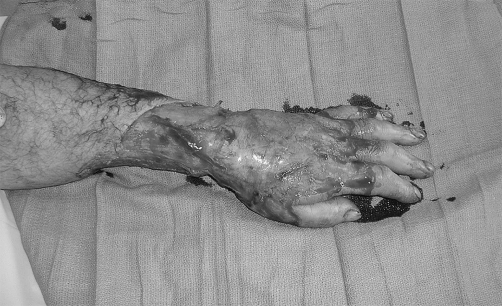
Fig. 1 B. First debridement.
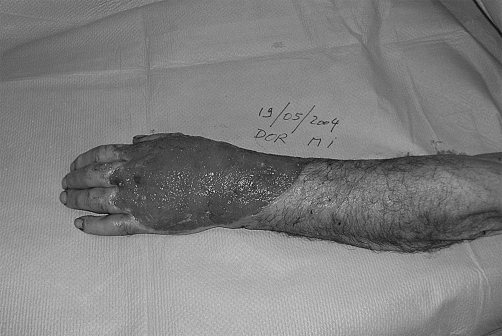
Fig. 1 C. New calcium polyuronate compound microdosed with zinc and manganese ions dressings to obtain granulation.
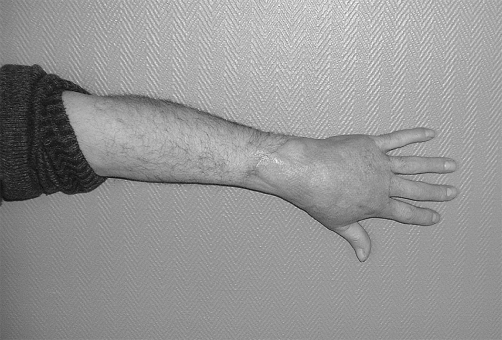
Fig. 1 D. View after skin graft.
This pathology creates a health administration problem owing to the lengthy hospitalization in a regional burn centre and to the dressing changes, which are difficult to perform properly in an out-patient setting.
During the infectious stage, also referred to as the septic period, repeated surgical debridement is often necessary to re-establish a healthy tissue bed free of necrotic, infected material. The open wound resulting from these excisions was suitable for testing our new infected wound healing modulators.
Calcium polyuronate has haemostatic properties and appears to produce favourable scarring (phenomena scientifically objectified during the 1990s. 7, 8, 9Its highly hydrophilic nature allows it to maximize wound drainage while retaining exudate and fibrinous debris. The zinc and manganese ions act on haemostasis by potentiating platelet aggregation and stabilizing factor XII. 1, 10, 11, 12These ions have known and proven effects during the essential stages of tissue repair, 13, 14, 15acting as enzymatic inductors and co-factors which stabilize certain key molecules.
Our aim is to shorten the time to obtain proper granulation tissue and to improve the trophic qualities of the tissues in order to decrease hospitalization times before definitive skin grafting.
Our objective is also to decrease bleeding and pain during dressing changes, thus helping to avoid the use of general anaesthesia.
A prospective multi centre randomized study was developed to compare the usual treatment of these types of wounds with a novel dressing composed of a new calcium polyuronate compound micro-dosed in zinc and manganese ions. This new type of dressing could be used not only during the septic phase but also during the tissue repair stage.
Materials and method
Ethics
The study was conducted in four French plastic and burns surgery centres, following French ethical regulations for clinical research, with both insurance backing and the approval of the ethical committee located in the coordinating site of the study. In compliance with the French Huriet-Sérusclat law, 16the patient's written consent was obtained one or two days following the first surgical excision as long as it was possible to communicate information about the study.
The sample size was calculated with a hypothesis of a difference of 8 days (standard deviation of 11 days) between the two groups before skin grafting, with α = 0.05 and β = 0.20. Forty patients (20 in each group) were necessary according to this hypothesis with a maximum follow up of 70 days for each patient included.
Inclusion
We proposed this study to all adult patients who presented with BNDH in an extremity, without evidence of fascial infection, and who had benefited from surgical excision with subsequent open wound dressing and eventual skin grafting. Patients presenting pancytopenia or hypersensibility to any of the treatments, as well as patients participating in any past (last 30 days) or present biomedical studies, were excluded.
Design
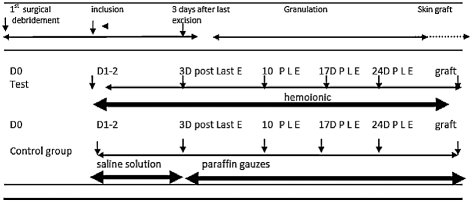
The study comprised six visits ( Table I ):
Table I. Study design.
- D1-2 (inclusion visit the next day or, at the latest, two days after first surgical excision)
- DDE+3 (three days after final excision: patient now considered to be out of the septic period when three days have passed without the need for further surgical intervention)
- DDE+10, DDE+17, DDE+24
- final visit of the study, DSTUDY END (DGRAFT or D70 in the absence of skin grafting)
During the inclusion visit, patients were randomly divided into two groups (block randomization): control group and trial group.
Control group patients. The classic treatment was used, 17beginning at D1-2 (during the septic period), with the use of NaCl 0.9% dressings on the entire wound surface followed by a secondary dry gauze dressing: this was changed once daily. Starting at DDE+3 (tissue repair period), treatment was continued through the use of sterile paraffin gauze dressing, pro-inflammatory (Vaselitulle dressing) on the entire wound surface with a secondary dry gauze dressing. These were changed either daily or every other day at the investigator's discretion. At the end of the tissue repair period, before skin grafting (approximately day 3 or 4), the investigator could use Corticotulle® Lumière (anti-inflammatory) to limit overgranulation and/or favour early skin grafting (up to a maximum of eight days).
Trial group patients. Treatment began at D1-2 and remained the same during the septic and the tissue repair periods. The new calcium polyuronate compound, micro-dosed in zinc and manganese ion (Trionic®) powder, was pulverized directly onto the entire surface of the excised tissue zone in a very thin, homogenous layer followed by the application of one or several Trionic® dressings. This initial coverage was then sealed with a secondary dressing. Daily dressing changes were performed during the septic period followed by every 2 days during the tissue repair period.
At the inclusion stage of the study, demographic data, history of the current illness, biological data, bacteriological information, and the surface area of tissue loss were documented.
During subsequent follow-up visits, data were gathered on the evolution of tissue loss up to final skin grafting, amount of blood loss and exudates, and pain during dressing changes. Standardized photographs were taken at each follow-up visit. Analgesia use and recourse to general anaesthesia were also documented.
The principal criterion for evaluating efficiency in the study was the delay between day 3 following the final excision (DDE+3) and the day of skin grafting. The effective day of skin grafting was indicated by the investigator as well as the day when tissue granulation (continuous, rosy-red, and homogenous) was ready to receive the skin graft. The delay before 'graftability' and the percentage of patients grafted 28 days after DDE+3 were compared in both groups.
The following were used as secondary evaluation criteria:
- number of times general anaesthesia was used for dressing changes
- blood loss and amount of exudate during dressing changes
- pain during dressing changes
The results were analysed by a university mathematics laboratory independent of the promoters of this study.
Results
Twenty-five patients were included in our study from December 2003 to July 2005, 14 in the trial group (calcium polyuronate enriched with zinc and manganese) and 11 in the control group (NaCl - Vaselitulle).
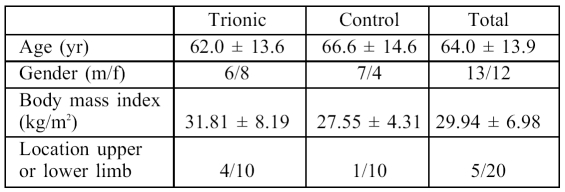
The demographic characteristics of this series and the localization of lesions are described in Table II . The most common portal of entry for these infections was a superficial cutaneous trauma. It is important to note that clinical signs became evident on average later in the trial group, i.e. after 6.6 ± 4 days versus 5.1 ± 3 days in the control group.
Table II. The patients.
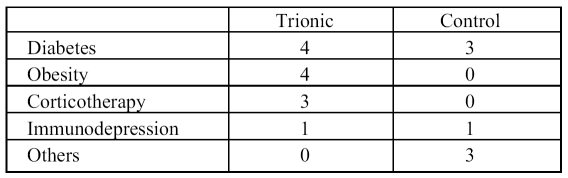
The most important risk factors identified were diabetes, obesity, and corticosteroid therapy ( Table III ). Obese and corticodependent patients were found only in the trial group. Table III summarizes the initial biological data.
Table III. Risk factors.
The results from bacteriological cultures in the two groups were comparable: Gram-positive bacilli in 44% of cases, Gram-negative bacilli in 36% of cases, and absence of bacteria in 12% of cases.
During the septic period, the number of surgical excisions per patient was one except in two patients who required two excisions (one in each group). The 'beta-lactams' (80% of the series) were the most prescribed antibiotics during the post-operative period.
Primary efficiency evaluation criteria
An analysis was made of the results in 20 patients. Five patients were excluded owing to major deviations from the primary criteria of the study: in three patients, this consisted of a longer delay than three days following final tissue excision, before initiation of the new treatment, while in two patients there was a prolonged delay (over 15 days) between the date of graftability and the effective skin graft date because of temporary contraindications to general anaesthesia (poorly controlled diabetes in one patient, myocardial infarction in the other). In these 20 patients, the average wound surface area was 361 ± 280 cm 2in the trial group versus 300 ± 224 cm 2. The average delay before skin grafting was 18 days in the trial group versus 27.1 days in the control group. However, the difference was not statistically significant.
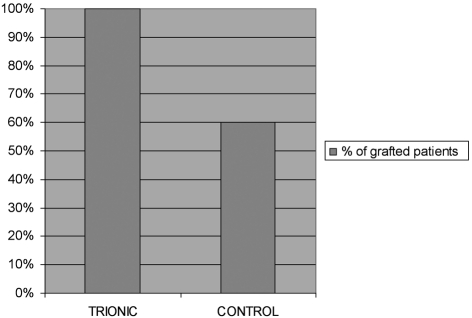
A comparison of variance between the two groups showed a higher homogeneity of delay before grafting in the trial group than in the control group: standard deviation for the trial group was 6.76 vs 22.15 in the control group (p = 0.003). The percentage of patients grafted 28 days after DDE+3 was 100% in the trial group versus 60% in the control group (p = 0.043 -2test) ( Table IV ).The average delay before 'graftability' (time until granulation tissue allowing skin grafting) was 16.7 ± 6.3 days in the trial group and 25.0 ± 20.8 days in the control group (p = 0.885, Wilcoxon test).
Table IV. Proportion of skin graft on day 28 after JDE+3.
Secondary evaluation criteria
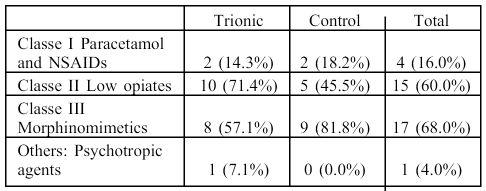
Between DDE+3 (3 days after the final surgical excision) and DDE+17 in the trial group, the most used analgesias were class I (71.4% of the patients) while in the control group, they were primarily class II (81.8% of patients) ( Table V ). Only one patient in the control group required general anaesthesia for a dressing change, between DDE+3 and DDE+10. Pain measured on the EVA scale (0 to 10) diminished progressively with time in both groups, although at each time point it was greater in the control group during visits DDE+10 (3.14 ± 0.97 in the trial group versus 3.61 ± 2.27) and DDE+17 (2.26 ± 0.68 in the trial group versus 2.35 ± 1.99) but these differences were not statistically significant.
Table V. Analgesic.
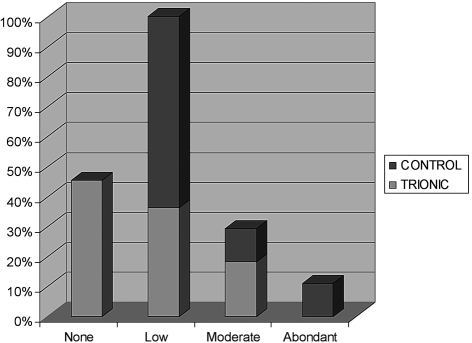
At DDE+3, blood loss was graded on four levels (absent, mild, moderate, abundant) and was found to be significantly less pronounced during dressing changes in the trial group: bleeding was noted in all patients in the control group whereas approximately half the patients in the trial group showed no bleeding whatsoever (p = 0.045, Fisher test) ( Table VI ). The amounts of exudates were also graded on four levels (absent, mild, moderate, abundant) and diminished with time, while remaining comparable between the two groups. However, the number of dressing changes was on average less numerous in the trial group 8 14than in the control group (22,2) (p = 0.205, Wilcoxon test).
Table VI. Bleeding.
Among the 25 patients included in the study, a total number of four undesirable events were reported: a death due to lymphoma, a myocardial infarction with full remission, an allergic reaction to an antibiotic, and a case of malignant hyperthermia four days after the first surgical excision. In this last patient, the calcium polyuronate treatment was interrupted for two days and then restarted, with a favourable evolution and a skin graft on day 24. None of these events was directly related to the products used in this study.
Discussion
Bacterial necrotizing dermohypodermititis is an extremely serious but rare pathology affecting less than 1 in 100,000 people in western countries. This series is one of the biggest trials to this effect in the current literature. 18The type of patients in our series was consistent with those described in the literature. The cause of cutaneous disruption was not detailed in approximately 20% of cases, but the most frequently affected region was the lower extremity. Factors which had a potential negative influence on healing were diabetes, corticotherapy, and immunosuppression. In our study, patients who were being treated by corticotherapy were all in the trial group, which may have had a considerable impact on the delay to wound healing.
For these types of infections, the current consensus agrees on the importance of urgent wide debridement of all involved tissue in order to save the patient's life.
The goal of our study was to compare a novel therapy using a calcium polyuronate enriched with zinc and manganese ions during both the septic and the tissue repair periods of wound healing compared to traditional wound dressings.
The duration of the septic period and the number of excisions were comparable in the two groups and there was good tolerance of the trial product.
During the tissue repair phase, we were unable to show any significant difference between the two groups concerning delay to skin grafting, probably as a consequence of the relatively small sample size.
Nonetheless, the proportion of patients skin grafted 28 days after DDE+3 was significantly greater in the trial group (100% versus 60%, p = 0.043) even though the average surface area of the lesions was considerably larger.
We believe that the patients in the trial group benefited from the favourable healing properties of calcium polyuronate. These are well established and have been proven over a long time period. The healing action of calcium polyuronate begins during the ionic exchange between the calcium in its fibres and the sodium found in blood and exudates in the lesion. This ionic exchange induces platelet activity and accelerates fibrin formation and cellular activation of key molecular processes (macrophages, fibroblasts, endothelial cells, and epithelial cells). Also, zinc and manganese ions added to the calcium polyuronate formula have been known for the past few decades to increase resistance to infection. In 1973 Rabinovitch and Destefano 14showed that manganese ions induced macrophage activity. Other researchers such as Agren at the Swedish University of Linkoping 10, 13discovered that zinc increased resistance of cultured fibroblasts to oxidative stress, further activating secretion of glycosaminoglycans which intervened in the cicatricial process. Zinc appears to be an important factor in the structure and function of chromatin, thus making it an important inductor of epithelial cell proliferation.
Results from our study also showed that bleeding was less important during dressing changes in the trial group, in which 45.5% of patients in the trial group had no blood loss versus 0% in the control group (p = 0.045), even though the same patients in the trial group had an initial platelet level inferior to that of the control group. This was certainly due to the haemostatic activity of calcium polyuronate, a well-known property of the compound for many years which has been demonstrated in skin graft donor sites. 19Zinc seems to potentiate platelet aggregation by stabilizing its receptors to fibrinogen. It also decreases coagulation time (fibrin formation), while amplifying the effect of calcium. 11, 12
Pain (EVA) during dressing removal was slightly less present with the trial dressing and the analgesia used was from a lower class (class I versus class II) in the trial group. We believe this to be related to the gelification properties of the calcium polyuronate fibres which initiate as soon as the dressing comes into contact with blood or exudates from the wound. 1The average number of dressing changes was lower in the trial group but the difference was not statistically significant. This could be explained by the highly hydrophilic properties of the calcium polyuronate fibres.
Conclusion
The idea of undertaking a scientific study to test an external treatment on such an aggressive pathology remains a highly controversial topic of discussion, yet no similar study has been found in the literature. Developing such a study, it was necessary to treat dressings as veritable medication.When considering necrotizing bacterial dermohypodermititis, the use of calcium polyuronate enriched with zinc and manganese was found to significantly reduce blood loss in the tissue repair phase following radical surgical debridement of the wound.
Owing to the limited number of patients recruited to our study we were unable to draw any definitive conclusion as to the main question of the delay in skin grafting. Yet one important element brought to light was the increased proportion of patient's skin grafted at 28 days after the final surgical excision in the trial group: 100% versus 60% in the control group. This conclusion is all the more interesting in light of the initial demographic conditions, which were unfavourable, and of the average wound surface area, which was more substantial in the trial group.
References
- 1.Agren M, et al. A randomized, double-blind, placebo-controlled multicenter trial evaluating topical zinc oxide for acute open wounds following pilonidal disease excision. Wound Repair and Regeneration. 2006;14:526–35. doi: 10.1111/j.1743-6109.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- 2.Vermeulen H, et al. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg. 2005;92:665–72. doi: 10.1002/bjs.5055. [DOI] [PubMed] [Google Scholar]
- 3.Chosidow O. Subacute forms of necrotizing fasciitis and necrotizing cellulitis: Diagnosis criteria and surgical decision-making. Ann Dermatologie et Vénéréologie. 2001;128:390–3. [PubMed] [Google Scholar]
- 4.Lortat-Jacob A. Subcutaneous infection and necrotizing fasciitis of the limbs in adults. Surgical treatment. Ann Dermatologie Vénéréologie. 2001;128:404–10. [PubMed] [Google Scholar]
- 5.Bosshardt TL, Henderson VJ, Organ CH. Necrotizing soft-tissue infections. Arch Surg. 1996;131:846–52. doi: 10.1001/archsurg.1996.01430200056011. [DOI] [PubMed] [Google Scholar]
- 6.Fontes RA, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthopaed Surg. 2000;8:151–8. doi: 10.5435/00124635-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Li J, et al. Addition of an alginate to a modified zeolite improves hemostatic performance in a swine model of lethal groin injury. J Trauma. 2009;66:612–20. doi: 10.1097/TA.0b013e318160ff4d. [DOI] [PubMed] [Google Scholar]
- 8.Brehant O, et al. Healing of stoma orifices: Multicenter, prospective, randomized study comparing calcium alginate mesh and polyvidone iodine mesh. World J Surg. 2009;33:1795–801. doi: 10.1007/s00268-009-0106-3. [DOI] [PubMed] [Google Scholar]
- 9.Murakami K, et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials. 2009;31:83–90. doi: 10.1016/j.biomaterials.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Agren MS. Studies on zinc in wound healing. Acta Dermatovenereologica (supplement) 1990;154:1–36. [PubMed] [Google Scholar]
- 11.Schousboe I. Factor XIIa activation of plasminogen is enhanced by contact activating surfaces and Zn2+. Blood Coagulation & Fibrinolysis. 1997;8:97–104. doi: 10.1097/00001721-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Head DM, Matthews IT, Tones MA. Effect of divalent metal ions on the binding of tissue factor and activated factor VII. Thrombosis Research. 1997;85:327–39. doi: 10.1016/s0049-3848(97)00018-2. [DOI] [PubMed] [Google Scholar]
- 13.Agren MS, Mirastschijski U. The release of zinc ions from and cytocompatibility of two zinc oxide dressings. J Wound Care. 2004;13:367–9. doi: 10.12968/jowc.2004.13.9.26705. [DOI] [PubMed] [Google Scholar]
- 14.Rabinovitch M, Destefano MJ. Macrophage spreading in vitro. II. Manganese and other metals as inducers or as co-factors for induced spreading. Experimental Cell Research. 1973;79:423–30. doi: 10.1016/0014-4827(73)90462-x. [DOI] [PubMed] [Google Scholar]
- 15.Richard M, et al. Effect of zinc supplementation on resistance of cultured human skin fibroblasts toward oxidant stress. Biological Trace Element Research. 1993;37:187–99. doi: 10.1007/BF02783794. [DOI] [PubMed] [Google Scholar]
- 16.Revision of Huriet-Sérusclat law:Position of medical and patient associations. Ann Chirurgie. 2001;126:568–71. [PubMed] [Google Scholar]
- 17.Bonnetblanc J-M, Bdane C. Erysipelas: Recognition and management. Amer J Clin Dermatol. 2003;4:157–63. doi: 10.2165/00128071-200304030-00002. [DOI] [PubMed] [Google Scholar]
- 18.Huang WS, Hsieh SC, Hsieh CS, Schoung JY, Huang T. Use of vacuum-assisted wound closure to manage limb wounds in patients suffering from acute necrotizing fasciitis. Asian J Surg. 2006;29:135–9. doi: 10.1016/S1015-9584(09)60072-5. [DOI] [PubMed] [Google Scholar]
- 19.Davey RB, Sparnon AL, Byard RW. Unusual donor site reactions to calcium alginate dressings. Burns. 2000;26:393–8. doi: 10.1016/s0305-4179(99)00140-0. [DOI] [PubMed] [Google Scholar]