Abstract
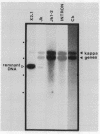
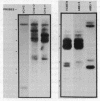
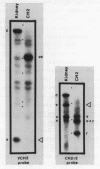
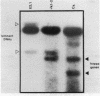
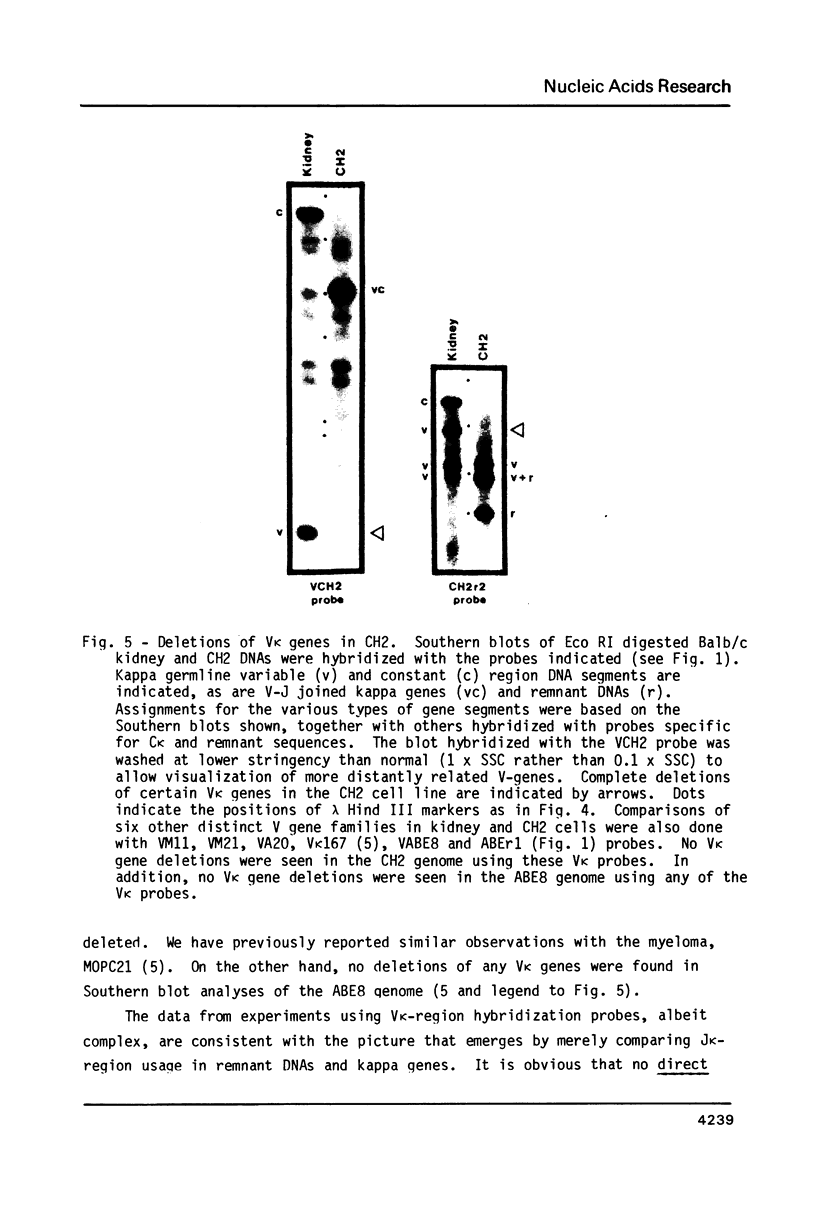
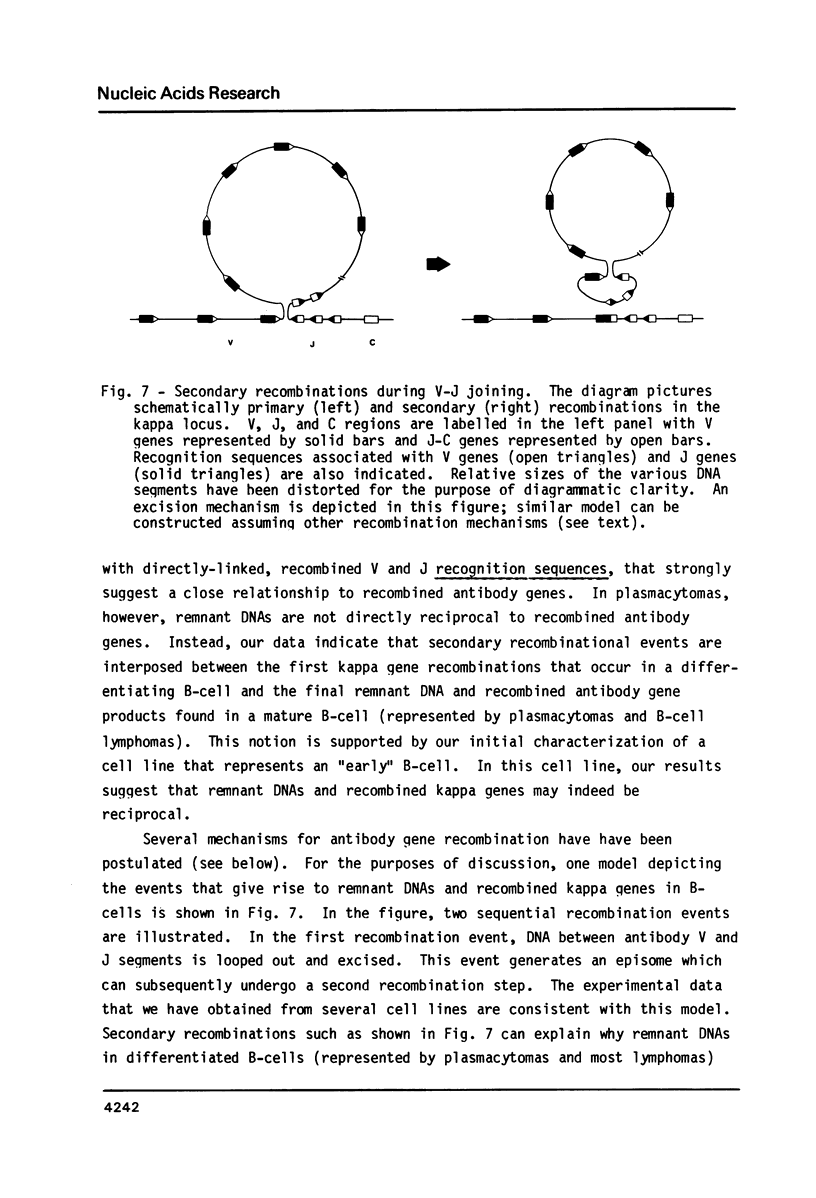
Many immunoglobulin (Ig)-producing cells retain the DNA that separates Ig variable (V) and constant (C) region genes in the germline. This "remnant" DNA must be moved during the recombination process that joins V and C genes via a joining (J) segment. We have analyzed remnant DNAs in several Ig-producing cell lines. The nucleotide sequences of kappa (kappa) light chain remnant DNAs indicate close relationships to V-J joining. We find fused V kappa and J kappa recognition sequences in five remnant DNAs, suggesting reciprocal relationships to the fused V kappa and J kappa segments produced by V-J joining. However, of sixteen plasmacytoma remnant DNAs analyzed, all involve only recombination with J kappa l. Thus, in most cell lines, remnant DNAs are not directly reciprocal to recombined kappa-genes. On the other hand, our analyses of some myelomas do indicate indirect relationships between remnant DNAs and kappa-genes. Our results suggest that multiple steps of DNA recombination occur during Ig-gene rearrangement. Because remnant DNA joining sites do not exhibit the flexibility that has been observed in Ig-gene V-J joining, our findings also suggest that the joining mechanism may involve endonuclease, exonuclease and ligase activities.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Enea V., Bothwell A. L., Baltimore D. Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell. 1980 Aug;21(1):1–12. doi: 10.1016/0092-8674(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Altenburger W., Steinmetz M., Zachau H. G. Functional and non-functional joining in immunoglobulin light chain genes of a mouse myeloma. Nature. 1980 Oct 16;287(5783):603–607. doi: 10.1038/287603a0. [DOI] [PubMed] [Google Scholar]
- Andres C. M., Maddalena A., Hudak S., Young N. M., Claflin J. L. Anti-phosphocholine hybridoma antibodies. II. Functional analysis of binding sites within three antibody families. J Exp Med. 1981 Nov 1;154(5):1584–1598. doi: 10.1084/jem.154.5.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Rosenberg N., Witte O. N. Transformation of immature lymphoid cells by Abelson murine leukemia virus. Immunol Rev. 1979;48:3–22. doi: 10.1111/j.1600-065x.1979.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Durdik J., Moore M. W., Selsing E. Novel kappa light-chain gene rearrangements in mouse lambda light chain-producing B lymphocytes. Nature. 1984 Feb 23;307(5953):749–752. doi: 10.1038/307749a0. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Korsmeyer S. J., Waldmann T. A., Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981 Apr 2;290(5805):368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Hozumi N., Wu G. E., Murialdo H., Roberts L., Vetter D., Fife W. L., Whiteley M., Sadowski P. RNA splicing mutation in an aberrantly rearranged immunoglobulin lambda I gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7019–7023. doi: 10.1073/pnas.78.11.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höchtl J., Müller C. R., Zachau H. G. Recombined flanks of the variable and joining segments of immunoglobulin genes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1383–1387. doi: 10.1073/pnas.79.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S., Rosenberg N., Alt F., Baltimore D. Continuing kappa-gene rearrangement in a cell line transformed by Abelson murine leukemia virus. Cell. 1982 Oct;30(3):807–816. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- Lynes M. A., Lanier L. L., Babcock G. F., Wettstein P. J., Haughton G. Antigen-induced murine B cell lymphomas. I. Induction and characterization of CH1 and CH2. J Immunol. 1978 Dec;121(6):2352–2357. [PubMed] [Google Scholar]
- Max E. E., Maizel J. V., Jr, Leder P. The nucleotide sequence of a 5.5-kilobase DNA segment containing the mouse kappa immunoglobulin J and C region genes. J Biol Chem. 1981 May 25;256(10):5116–5120. [PubMed] [Google Scholar]
- Pech M., Höchtl J., Schnell H., Zachau H. G. Differences between germ-line and rearranged immunoglobulin V kappa coding sequences suggest a localized mutation mechanism. Nature. 1981 Jun 25;291(5817):668–670. doi: 10.1038/291668a0. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. A mutant immunoglobulin light chain is formed by aberrant DNA- and RNA-splicing events. Nature. 1980 Aug 21;286(5775):779–783. doi: 10.1038/286779a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Max E. E., Leder P. A kappa-immunoglobulin gene is formed by site-specific recombination without further somatic mutation. Nature. 1979 Aug 2;280(5721):370–375. doi: 10.1038/280370a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Nau M. M., Norman B., Kwan S. P., Scharff M., Leder P. Immunoglobulin V/J recombination is accompanied by deletion of joining site and variable region segments. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6022–6026. doi: 10.1073/pnas.77.10.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsing E., Miller J., Wilson R., Storb U. Evolution of mouse immunoglobulin lambda genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4681–4685. doi: 10.1073/pnas.79.15.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsing E., Storb U. Mapping of immunoglobulin variable region genes: relationship to the 'deletion' model of immunoglobulin gene rearrangement. Nucleic Acids Res. 1981 Nov 11;9(21):5725–5735. doi: 10.1093/nar/9.21.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsing E., Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981 Jul;25(1):47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Altenburger W., Zachau H. G. A rearranged DNA sequence possibly related to the translocation of immunoglobulin gene segments. Nucleic Acids Res. 1980 Apr 25;8(8):1709–1720. doi: 10.1093/nar/8.8.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ness B. G., Coleclough C., Perry R. P., Weigert M. DNA between variable and joining gene segments of immunoglobulin kappa light chain is frequently retained in cells that rearrange the kappa locus. Proc Natl Acad Sci U S A. 1982 Jan;79(2):262–266. doi: 10.1073/pnas.79.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfield A., Selsing E., Arp B., Storb U. Misalignment of V and J gene segments resulting in a nonfunctional immunoglobulin gene. Nucleic Acids Res. 1981 Mar 11;9(5):1101–1109. doi: 10.1093/nar/9.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Ziegler S. F., Treiman L. J., Stafford J. I., Witte O. N. Differentiation of cloned populations of immature B cells after transformation with Abelson murine leukemia virus. Cell. 1983 Mar;32(3):903–911. doi: 10.1016/0092-8674(83)90075-2. [DOI] [PubMed] [Google Scholar]