Summary
Background. Thermal injury causes the destruction of the physical skin barrier that normally protects the body from invasion by micro-organisms and induces an immunocompromised state that predisposes burn patients to infection, sepsis, and multiple organ failure. Reactive oxygen species contribute to burn-mediated immune suppression, and as the use of antioxidants has a positive effect on immune function, this may reduce the incidence of wound infection and related complications in burn patients. Patients and methods. One hundred and eighty burn patients of either sex and different ages, suffering from burns of varying percentage, were involved in the study. They were allocated to six groups: A, B, C, D, E, and F, each of 30 patients. Groups B, C, D, E, and F were treated with antioxidants (vitamins E and C, zinc sulphate, allopurinol, melatonin, and N-acetylcysteine, respectively) while group A was treated without antioxidants, according to our hospital policy. Thirty healthy subjects (group G) were also involved in the study as a control group for comparison. In each group, serum malondialdehyde and serum glutathione levels, microbiological values, healing time, and the mortality rate were measured using standard methods. Results. Administering antioxidants to burn patients produced significant improvements in the parameters studied compared with burn patients not given antioxidants. Conclusion. The study clearly showed the beneficial effect of antioxidants in the treatment of burn patients, as evidenced by the reduced incidence of wound infection and the shortening of healing time, in addition to the lower mortality rate. It is therefore recommended to add antioxidants to the treatment list of burn patients.
Keywords: BURN, ANTIOXIDANTS, OXIDATIVE STRESS, WOUND INFECTION, WOUND HEALING, MORTALITY,
Abstract
Données générales. Les lésions thermiques détruisent la barrière physique cutanée qui normalement fait obstacle à l’invasion de micro-organismes et induisent un état d’immunodépression qui prédispose les patients brûlés à l’infection, à la septicémie et à une défaillance multiorganique. Les espèces réactives de l’oxygène contribuent à la suppression immunitaire causée par les brûlures, et puisque l’utilisation des antioxydants exerce un effet positif sur la fonction immunitaire, il est possible en cette manière de réduire l’incidence de l’infection des lésions et des relatives complications chez les patients brûlés. Patients et méthodes. Les 180 patients brûlés des deux sexes et d’âge variable, atteints de brûlures de pourcentage variable, qui ont participé à l’étude ont été divisés en six groupes: A, B, C, D, E et F, chacun composé de 30 patients. Les patients inclus dans les groupes B, C, D, E et F ont été traités avec des antioxydants (respectivement avec les vitamines E et C, le sulfate de zinc, l’allopurinol, la mélatonine et la N-acétylcystéine). Les patients inclus dans le groupe A ont été traités sans utiliser les antioxydants, selon la politique de notre hôpital. Trente sujets sains (groupe G) ont également participé à l’étude comme groupe témoin de comparaison. Dans chaque groupe, les niveaux de malondialdéhyde sérique et de glutathion sérique, les valeurs microbiologiques, les temps de guérison et le taux de mortalité ont été mesurés moyennant les méthodes standard. Résultats. L’administration d’antioxydants aux patients brûlés a produit des améliorations significatives dans les paramètres étudiés par rapport aux patients qui n’ont pas reçu les antioxydants). Conclusion. Les Auteurs ont démontré en manière bien claire l’effet bénéfique des antioxydants dans le traitement des patients brûlés. Ce résultat est mis en évidence par l’incidence diminuée des infections des lésions et la réduction des temps de guérison, comme aussi par la réduction du taux de mortalité. Conséquemment, ils recommandent d’ajouter les antioxydants à la liste des traitements qu’il faut suivre dans le traitement des patients brûlés.
Introduction
Infection in burn patients is a leading cause of morbidity and mortality and remains one of the most challenging concerns for the burn team. 1The development of infection depends on the presence of three conditions: a source of organisms; a mode of transmission; and patient susceptibility. 2A source of organisms is found in the patient’s normal endogenous flora, in exogenous sources in the environment, and in health care personnel. Exogenous organisms from the hospital environment are generally more resistant to antimicrobial agents than endogenous organisms. 3The modes of transmission include contact, droplets, and airborne spread. As for patient susceptibility, a patient has three principal defences against infection: physical defences, nonspecific immune responses, and specific immune responses. Changes in these defences determine patients’ susceptibility to infection. 4
In normal body conditions there exists a balance between free radicals and the natural scavengers of the body, but during a traumatic state the balance is lost and reactive oxygen species (ROS) are greater in number. While a major source of ROS in burn trauma is exogenous, adherent and activated neutrophils produce a burst of free radicals. 6Also, while burn trauma upregulates free radical production, this type of traumatic injury also impairs antioxidant defence mechanisms, rendering burn patients more susceptible to ROS-mediated injury. Furthermore, it has been reported that ROS contribute to burn-mediated immune suppression and that the use of antioxidants has a positive effect on immune function, which may reduce the incidence of wound infection and related complications. 7
Patients and methods
This study was carried out in 180 burn patients of either sex, age range 20-45 yr, and varying burn percentage (15-40% total body surface area estimated according to the rule of nine). Consent was obtained from all patients when admitted to the burn unit in our department of surgery; the study was approved by the Iraqi Ministry of Health Research Board and followed the instructions of the Diyala Health Directorate ethical committee of Baquba Teaching Hospital.
The patients were allocated to six groups and treated as follows:
Group A (30 patients): this group was already present in the burn unit and was treated according to our standard hospital policy (antioxidants not given);
Group B (30 patients): treated with vitamin E 400 mg capsule and vitamin C 500 mg tablet daily;
Group C (30 patients): treated with 75 mg/day zinc sulphate capsule;
Group D (30 patients): treated with 100 mg/day allopurinol tablet;
Group E (30 patients): treated with 3 mg/day melatonin capsule, taken at night;
Group F (30 patients): treated with 500 mg/day N-acetylcysteine (NAC) capsule;
Group G (30 healthy subjects): of same age range as the patients and selected to serve as control for purposes of comparison.
All the treated groups took their antioxidants in addition to other drugs prescribed according to hospital drug policy, including a topical antimicrobial agent (silver sulphadiazine 1% cream was used in this study).
Blood samples were collected from all subjects by venipuncture. Ten millilitres were taken on admission to the burn unit within the first 24 h post-burn; before starting drug treatment, which was considered zero time; and at days 3, 7, 14, and discharge day, to check changes in the parameters studied; in addition, wound swabs were taken at the same time intervals as the blood samples, and microbiological analyses were performed.
Serum malondialdehyde (MDA) levels were measured according to the standard method of Stocks and Dormandy, 8as modified by Gilbert et al. 9Serum glutathione levels were measured according to the method of Godin et al. 10Wound swabs for microbiological examination and the characterization of the micro-organisms present were taken according to the method of Revathi et al. 11
The statistical analyses used were Student’s t-test and the ANOVA test in order to examine the degree of significance. A p value less than 0.05 was considered significant, and the results were expressed as mean ± standard deviation.
Results
The results presented in Table I show that serum MDA levels were significantly increased (p > 0.05) at zero time in burn patients compared with control: in group A (no antioxidant used), serum MDA levels were non-significantly decreased at days 3, 7, and 14 compared with zero time, but at discharge day they were significantly decreased by 61.37% compared with zero time, although this result was still significantly higher than control (group G). Results in groups B, C, and D showed a significant reduction in serum MDA levels at day 7; in contrast, groups E and F showed a significant reduction in serum MDA level at day 3 compared with zero time; also, in all groups treated with antioxidant, serum MDA levels were significantly reduced at discharge day compared with zero time and group A (no antioxidant).
Table I. Effects of drug treatment on serum malondialdehyde (MDA) and serum glutathione (GSH) in the patients.
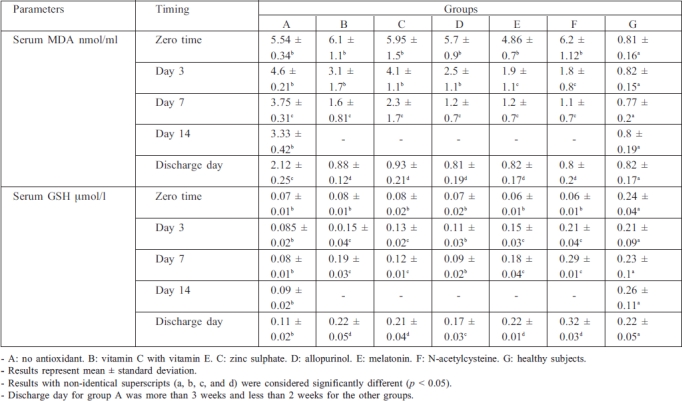
In the case of serum glutathione (GSH), Table I shows that serum GSH levels were significantly reduced in burn patients at zero time compared with healthy subjects: in group A, serum GSH levels remained significantly lower than control until discharge day, while in the antioxidant-treated groups serum GSH levels were significantly increased at discharge day compared with zero time.
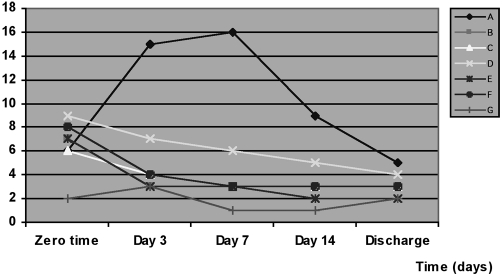
Table II and Fig. 1 show the incidence and distribution of the invading micro-organisms isolated from the burn patients. The percentage of positive swabs at zero time in all the groups studied was significantly higher (p < 0.05) than in control. The results in group A (no antioxidant) showed a significant increase in the number of positive swabs at day 3, 7, and 14 but a significant reduction at discharge day compared with zero time, while results in groups B, E, and F showed a significant decrease in the number of positive swabs at days 3 and 7 and at discharge time compared with zero time. In addition, group C showed a significant reduction at day 7 and at discharge day compared with zero time, while in group D the number of positive swabs was significant only at discharge day compared with zero time.
Table II. Effects of drug treatment on the incidence and distribution of invading micro-organisms isolated from the patients.
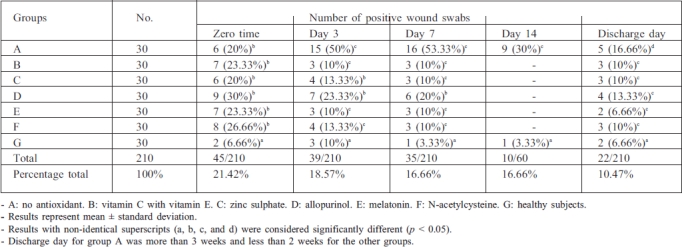
Fig. 1. Effects of drug treatment on the incidence of invading micro-organisms isolated fromt he patients.
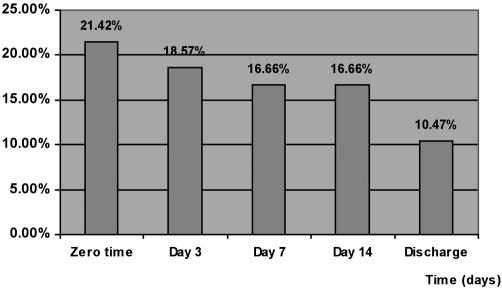
The total percentage of the incidence of positive swabs in all groups is shown in Table II and Fig. 2 . The percentage incidence decreased from 21.42% at zero time to 18.57% at day 3, decreasing to 16.66% at days 7 and 14 and becoming 10.47% at discharge day.
Fig. 2. Incidence of invading micro-organisms isolated from the patients.
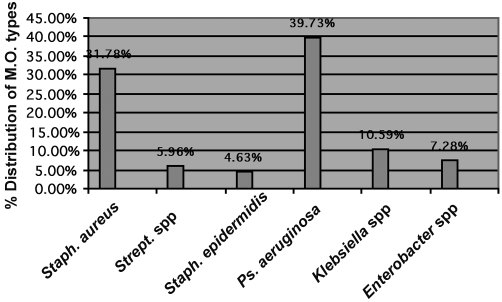
Table III and Fig. 3 demonstrate the distribution of micro-organism types isolated from the patients - the incidence of Staphylococcus aureus at zero time was the highest percentage (57.77%) of all types, while Pseudomonas aeruginosa presented the highest percentages at day 3 (43.58%) and day 7 (54.28%) compared with other types. The total percentages indicate that P. aeruginosa presented the highest percentage (39.73%) compared with other types, followed by S. aureus (31.78%). Other types found were Streptococcus spp., S. epidermides, Klebsiella spp., and Enterobacter spp.
Table III. Distribution of micro-organism types in positive burn wound swabs isolated from the patients.
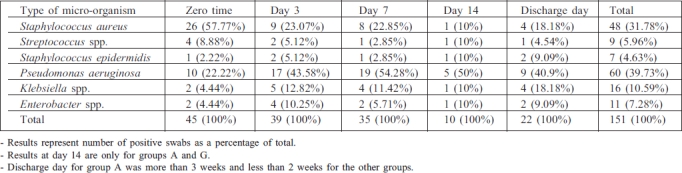
Fig. 3. Distribution of micro-organism types isolated from the patients.
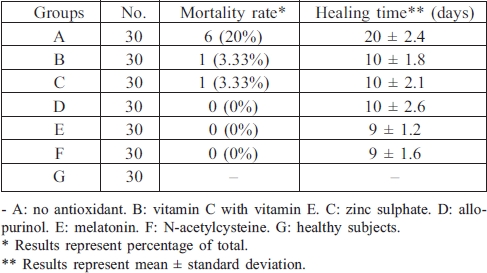
Table IV shows that the mortality rate fell from 20.0% in group A to 3.33% in groups B and C, while in groups D, E, and F the mortality rate was 0%. Table IV also shows that in group A the mean healing time was 20.0 ± 2.4 days ± SD, which was reduced by 50% in groups B, C, and D and in groups E and F was reduced to 9 ± 1.2 and 9 ± 1.6 days respectively.
Table IV. Effects of drug treatment on patient mortality rate and healing time.
Discussion
Thermal injury of the skin is an oxidation process associated with biological and metabolic alterations. It generates free radicals from various cellular populations through numerous pathways, and the modulation of generated free radical activity with antioxidants seems to play an important part in the pharmacological treatment of burns. 12In burn patients alterations in antioxidant micronutrient status and endogenous antioxidant defences against the deleterious effects of free radicals appear to be crucial, as indicated in recent studies. 13Data reported by Cetinkale et al. 14demonstrated that in addition to loss of plasma antioxidants such as vitamin E and sulphydryl groups in burn patients, vitamin C was diminished early post-burn. Recently, Traber et al. 15suggested that a-tocopherol should be administered to burn patients to prevent vitamin E depletion and to protect against oxidative stress from burn injury. While vitamin E effectively scavenges free radicals within the cells, vitamin C would serve to scavenge free radicals within the extracellular space. 16There is substantial experimental and clinical evidence to show an interacting dependence of vitamins E and C in antioxidant defence. 17It has been shown that trace elements play an important role in oxygen metabolism and therefore in the formation of free radicals. Zinc is regarded as an antioxidant owing to its ability to protect the cell from the effects of oxidative damage through its interaction with cellular thiols, preventing their oxidative inactivation, and by competing with the metal ions that produce ROS. 18In the case of allopurinol, it is clear that circulating exogenous activity is detected after skin lesions - this is the major source of free radicals in the serum of burn patients. 19
It has been reported that the pineal hormone melatonin may function as a powerful antioxidant, functioning as a scavenger of hydroxyl, peroxyl, and superoxide radicals. 20
Furthermore, melatonin has the ability to stimulate the activity of antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase (GSH-Px), and glutathione reductase (GSH-R). 21This explains the strong indication of melatonin as a rational antioxidant therapy in the treatment of burns - the rich free radical environment.
Regarding NAC’s antioxidant effect, it has been shown that NAC acts as a source of sulphydryl groups and facilitates GSH synthesis; it is also it is a direct scavenger for ROS. 22
It is clear that the findings obtained in this study regarding the reduction of MDA serum levels and the increase of serum GSH levels - i.e. of the natural antioxidant - in burn patients are compatible with those obtained in the other above-cited results.
Thermal injury destroys the physical skin barrier that normally prevents the invasion of micro-organisms; it also induces an immunocompromised state that predisposes burn patients to infection, sepsis, and multiple organ failure. 23Skin, as the first line of defence against invading microbes, is equipped with an array of immune mediators capable of recruiting inflammatory cells to enable neutralization and clearance of bacteria and fungi. 24Hence, burn patients with immune failure who have lost the skin barrier are vulnerable to infection and offer new sites for bacterial colonization and sepsis. The burn wound surface is generally considered to be initially free of major microbial contamination; Gram-positive bacteria in the depths of hair follicles and sweat glands may however survive the heat of the initial injury. 25These bacteria can heavily colonize the wound within the first 48 h post-burn. The micro-organisms present in the wound of hospitalized patients change with time after the injury: Gram-positive organisms (Staphylococcus aureus, Streptococcus pyogenes) are usually present during the first week post-burn 26while, as time passes, Gram-negative bacteria (Pseudomonas aeruginosa, E. coli) are present in the wound during the second week. 2These data are compatible with the findings of this study, as shown in Table III and Fig. 2 .
Burn patients can become colonized with Staphylococcus aureus by direct spread from infected healthcare workers (nasal and pharyngeal colonization) or indirectly by contact with contaminated objects. 27It has been shown that in burn wounds, molecules such as fibronectin, fibrinogen, collagen, and many others are exposed at the wound surface; many bacterial species harbour specific receptors for such molecules, and burn wound surfaces are therefore easily colonized by bacteria. In addition, Staphylococcus aureus encodes many proteins that specifically interact with human cellular matrix components. These microbial surface compounds, recognizing adhesive matrix molecules, make Staphylococcus aureus one of the most common microbes found to be colonizing burn wounds. 28The results reported in Table II and Fig. 1 show that treating burn patients with antioxidants significantly decreased the incidence of invading micro-organisms at day 3 post-burn compared with group A patients in whom antioxidants were not used; also, the percentage incidence of invading micro-organisms in burn patients, as shown in Fig. 2 , clearly showed a reduction as time progressed. This result is consistent with that obtained by Cetinkale et al., 7who demonstrated that antioxidants had a positive effect on immune function and that ROS contributed, in part, to burn-mediated immune suppression; it was also shown that treatment with allopurinol, desferrioxamine, catalase, NAC, and vitamin C significantly restored cell-mediated immunity in burn injury. Along with these findings, it was demonstrated that, in vivo, high exogenous doses of melatonin caused a general stimulation of immune system, increasing T cell activity, lymphocyte growth, and humoral responses and possibly inhibiting thymus involution with age; it was been suggested that these effects may occur via a direct action of melatonin on its receptor in various tissues of the immune system, such as the thymus, spleen, lymphocytes, and T helper cells. 29
It is also known that zinc is essential for the immune system and that zinc’s effect on the immune cells mainly depends on its concentration. All types of immune cells show decreased function after zinc depletion, and impaired immune function due to zinc deficiency has been shown to be reversed by adequate zinc supplementation, which must be adapted to the patients’ actual requirements. 30It has also been shown that early trace element supplementation appears to be beneficial after major burns, being associated with a significant decrease in the number of bronchopneumonia infections and with a shorter hospital stay. 31Berger et al. 32demonstrated that in the critical phase of illness oxidative stress was proportional to the severity of the patient’s condition and that this was particularly marked in burn patients. Patients with major burns suffered acute early trace element depletion caused by the extensive exudative trace element losses, which persisted until wound closure. Oxidative stress was worsened by these trace element deficiencies, particularly of zinc, and this was linked to impaired immune response. Berger et al. 32 also reported that trace element supplementation was associated with a significant reduction of infectious pulmonary complications, mainly related to a lower rate of nosocomial pneumonia in critically ill burn patients - the likely underlying mechanism was a reinforcement of the endogenous antioxidant defences.
It has been shown that low levels of antioxidants accompanied by raised levels of markers of free radical damage play a significant role in delaying wound healing, that ROS produced in response to cutaneous injury impede the healing process by causing damage to cellular membranes, DNA, proteins, and lipids, and that antioxidants promote wound healing. 33
It has been reported that infection is one of the most important causes of the systemic inflammatory response syndrome (SIRS) developing in burn patients; 34it has also been hypothesized that the aetiology of the multiple organ dysfunction syndrome (MODS) may be related to uncontrolled infection - control of the source of infection is thus considered the major therapeutic aim, 35since the condition may develop further into progressive organ failure and a possibly fatal outcome. 36
Another finding is that oxidative stress plays an important role both in the activation of the inflammatory response and in tissue damage, resulting in SIRS, which is the leading cause of MODS. Several antioxidant substances have been proposed as treatment for SIRS, and it has been shown that antioxidants, given post-burn, restore antioxidant defences and prevent mortality. 37The results of the present study are consistent with these findings.
Conclusion
In conclusion, the results obtained in this study showed the beneficial effect of the antioxidants used, i.e. vitamin E with vitamin C, zinc sulphate, allopurinol, melatonin, and N-acetylcysteine, in the treatment of burn patients, as shown by the reduction in the incidence of wound infection, the reduction in healing time, and the improvement in the mortality rate compared with the results in other patients treated classically according to our hospital policy, without the addition of antioxidants to the treatment list.
It is recommended to consider antioxidants as an effective addition to the arsenal of weapons available for combating morbidity and mortality in burn patients.
References
- 1.Mason AD, McManus AT, Pruitt BA. Association of burn mortality and bacteraemia: A 25-year review. Arch Surg. 1986;121:1027–31. doi: 10.1001/archsurg.1986.01400090057009. [DOI] [PubMed] [Google Scholar]
- 2.Vindenes H, Bjerkens R. Microbial colonization of large wounds. Burns. 1995;21:575–9. doi: 10.1016/0305-4179(95)00047-f. [DOI] [PubMed] [Google Scholar]
- 3.Haley RW, Culver DH, White JW. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985;121:182–205. doi: 10.1093/oxfordjournals.aje.a113990. [DOI] [PubMed] [Google Scholar]
- 4.Weber J, McManus AT. Infection control in burn patients. Burns. 2004;30:A16–A24. doi: 10.1016/j.burns.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Latah B, Babu M. The involvement of free radicals in burn injury. A review. Burns. 2001;27:309–17. doi: 10.1016/s0305-4179(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 6.Horton JW, White DJ. Role of xanthine oxidase and leukocytes in post-burn cardiac dysfunction. J Am Coll Surg. 1995;181:129–37. [PubMed] [Google Scholar]
- 7.Cetinkale O, Senel O, Bulan R. The effect of antioxidant therapy on cell-mediated immunity following burn injury in an animal model. Burns. 1999;25:113–8. doi: 10.1016/s0305-4179(98)00124-7. [DOI] [PubMed] [Google Scholar]
- 8.Stocks J, Dormandy TL. The auto-oxidation of human red cell lipids induced by hydrogen peroxide. Br J Haematol. 1971;20:95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert HS, Stemp DD, Roth EF. A method to correct errors caused by generation of interfering compounds during lipid peroxidation. Ann Biochem. 1984;173:282–6. doi: 10.1016/0003-2697(84)90086-1. [DOI] [PubMed] [Google Scholar]
- 10.Godin DV, Wahaieb SA, Garnet ME. Antioxidant enzyme alteration in experimental and clinical diabetes. Mol Cell Biochem. 1988;84:223–31. doi: 10.1007/BF00421057. [DOI] [PubMed] [Google Scholar]
- 11.Revathi G, Puri J, Jain BK. Bacteriology of burns. Burns. 1998;24:347–9. doi: 10.1016/s0305-4179(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 12.Arturson G. Pathophysiology of the burn wound and pharmacological treatment: The Rudi Hermans Lecture, 1995. Burns. 1996;22:255–74. doi: 10.1016/0305-4179(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 13.Horton JW. Free radicals and lipid peroxidation mediated injury in burn trauma: The role of antioxidant therapy. Toxicology. 2003;189:75–88. doi: 10.1016/s0300-483x(03)00154-9. [DOI] [PubMed] [Google Scholar]
- 14.Cetinkale O, Demir M, Sayman HB, et al. Effects of allopurinol, ibuprofen, and cyclosporine A on local microcirculatory disturbances due to burn injuries. Burns. 1997;23:43–9. doi: 10.1016/s0305-4179(96)00079-4. [DOI] [PubMed] [Google Scholar]
- 15.Traber MG, Shimoda K, Murakami K, et al. Burn and smoke inhalation injury in sheep depletes vitamin E: Kinetic studies using deuterated tocopherols. Free Radic Biol Med. 2007;42:1421–9. doi: 10.1016/j.freeradbiomed.2007.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinnell SR. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J Am Acad Dermatol. 2003;48:1–19. doi: 10.1067/mjd.2003.16. [DOI] [PubMed] [Google Scholar]
- 17.Huang HY, Appel LJ, Croft KD, et al. Effects of vitamin C and vitamin E on in vivo lipid peroxidation: Results of a randomized controlled trial. Am J Clin Nutr. 2002;76:549–55. doi: 10.1093/ajcn/76.3.549. [DOI] [PubMed] [Google Scholar]
- 18.Delima R, Trinder D, Olynyk JK. Potential protective effects of zinc in iron overload. Liver Inter. 2007;27:4–5. doi: 10.1111/j.1478-3231.2006.01428.x. [DOI] [PubMed] [Google Scholar]
- 19.Burton LK, Velasco SE, Patt A, et al. Xanthine oxidase contributes to lung leak in rats subjected to skin burn. Inflammation. 1995;19:31–8. doi: 10.1007/BF01534378. [DOI] [PubMed] [Google Scholar]
- 20.Zang LY, Cosma G, Gardner H, et al. Scavenging of reactive oxygen species by melatonin. Biochim Biophys Acta. 1998;1425:469–77. doi: 10.1016/s0304-4165(98)00099-3. [DOI] [PubMed] [Google Scholar]
- 21.Karbowink M, Reiter R. Antioxidant effects of melatonin in protection against cellular damage caused by ionizing radiation. PSEBM. 2000;225:9–22. doi: 10.1177/153537020022500102. [DOI] [PubMed] [Google Scholar]
- 22.Benrahmoune M, Therond P, Abedinzadeh Z. The reaction of superoxide radical with N-acetylcysteine. Free Radic Biol Med. 2000;29:775–82. doi: 10.1016/s0891-5849(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 23.Still JM, Belcher K, Law EJ. Experience with polymicrobial sepsis in a regional burn unit. Burns. 1993;19:434–6. doi: 10.1016/0305-4179(93)90069-k. [DOI] [PubMed] [Google Scholar]
- 24.Steinstraesser L, Oezdogan Y, Wang SC, et al. Host defense peptides in burns. Burns. 2004;30:619–27. doi: 10.1016/j.burns.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Luttherman A, Dacso CC, Curreri PW. Infections in burn patients. Am J Med. 1986;81:45. doi: 10.1016/0002-9343(86)90513-9. [DOI] [PubMed] [Google Scholar]
- 26.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244–69. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khojasteh VJ, Jones VE, Childs C, et al. Prevalence of toxin producing strains of Staphylococcus aureus in a pediatric burns unit. Burns. 2007;33:334–40. doi: 10.1016/j.burns.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Smid MK, Dijk S, Beerthuizen G, et al. Molecular epidemiology of Staphylococcus aureus colonization in a burn center. Burns. 2004;30:27–33. doi: 10.1016/j.burns.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Simonneaux V, Ribelayga C. Generation of the melatonin endocrine message in animals: A review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 1993;55:325–395. doi: 10.1124/pr.55.2.2. [DOI] [PubMed] [Google Scholar]
- 30.Ibs KH, Rink L. Zinc-altered immune function. J Nutr. 2003;133:1452S–56S. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- 31.Berger MM, Spertini F, Shenkin A, et al. Trace element supplementation modulates pulmonary infection rates after major burns: A double-blind, placebo-controlled trial. Am J Clin Nutr. 1998;68:365–71. doi: 10.1093/ajcn/68.2.365. [DOI] [PubMed] [Google Scholar]
- 32.Berger MM, Eggimann P, Heyl DK, et al. Reduction of nosocomial pneumonia after major burns by trace element supplementation: Aggregation of two randomized trials. Crit Care. 2006;10:R153. doi: 10.1186/cc5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta A, Singh RL, Raghubir R. Antioxidant status during cutaneous wound healing in immunocompromised rats. Mol Cell Biochem. 2002;241:1–7. doi: 10.1023/a:1020804916733. [DOI] [PubMed] [Google Scholar]
- 34.Miller PR, Munn DD, Wayne MJ. Systemic inflammatory response syndrome in the trauma intensive care unit: Who is infected? J Trauma Injury Infection Crit Care. 1999;47:1004–8. doi: 10.1097/00005373-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Fry DE, Pearlstein L, Fulton RL, et al. Multiple system organ failure. The role of uncontrolled infection. Arch Surg. 1980;115:136–40. doi: 10.1001/archsurg.1980.01380020006003. [DOI] [PubMed] [Google Scholar]
- 36.Tran DD, Cuesta MA, Leeuwen PA, et al. Risk factors for multiple organ system failure and death in critically injured patients. Surgery. 1993;114:21–30. [PubMed] [Google Scholar]
- 37.LaLonde C, Nayak U, Hennigan J, et al. Excessive liver oxidant stress causes mortality in response to burn injury combined with endotoxin and is prevented with antioxidants. J Burn Care Rehabil. 1997;18:187–92. doi: 10.1097/00004630-199705000-00002. [DOI] [PubMed] [Google Scholar]