Summary
Wound infections are common problems in burn units and mostly originate from nosocomial contamination. The development of infections in burn cases is serious because of their effects on the course of the disease and patient outcomes. Many burned patients die as a result of infection during their hospital courses. The rate of infection in burn cases is extremely high in developing countries. For these reasons, we carried out a study of the bacteriological profiles and a comparison of antimicrobial resistance patterns of predominant bacteria, over a period of one year. The study was conducted during a period of 12 months (from May 2008 to April 2009) at the Sulaimani Burn, Reconstructive and Plastic Surgery Hospital, Sulaimani, Iraq. In the present study, a total of 1126 samples of swabs and blood were processed from 760 admitted patients (505 female and 255 male). Bacteria isolates were found in 935 samples (83%) and only 180 wound swabs were sterile (16%); the others became contaminated in the lab and no test was performed (1%). Total bacteria isolated: 1402 organisms (average number of organisms per sample, 1.5). Staphylococcus aureuswas the commonest cause of bacterial burn wound invasion, accounting for 34% of cases, while Pseudomonas aeruginosacaused 18%. The total percentage of multidrug-resistant organisms among all organisms was 51% (715/1402); a percentage of 87.1% was recorded with reference to methicillin-resistant Staphylococcus aureusamong all cases of Staphylococcus aureus(176/202); non-fermenting bacteria among all non-fermenting bacteria were 28.8%.
Keywords: INFECTION, BURN PATIENTS, SULAIMANI
Abstract
Les infections qui se produisent dans les lésions des patients brulés constituent un problème très commun dans les unités de brûlures et dans la plupart des cas elles sont provoquées par des contaminations d’origine nosocomiale. Le développement des infections chez les patients brûlés est grave en raison des effets sur l’évolution de la maladie et la condition finale des patients. Beaucoup de patients brûlés meurent à la suite d’une infection au cours de leur hospitalisation. Le taux d’infection parmi les patients brûlés est extrêmement élevé dans les pays en voie de développement. Pour ces raisons, nous avons effectué une étude des profils bactériologiques et une comparaison de la résistance aux agents antimicrobiens des bactéries prédominantes, sur une période d’un an. L’étude a été effectuée pendant une période de 12 mois (mai 2008 à avril 2009) à l’Hôpital pour la Reconstruction et la Chirurgie Plastique de l’Hôpital Sulaimani, Irak. Dans la présente étude, un nombre total de 1126 échantillons de sang et des prélèvements ont été traitées à partir de 760 patients admis (505 femmes et 255 hommes). Des isolats bactériens ont été trouvés dans 935 échantillons (83%) et seulement 180 tampons ont été trouvés stériles (16%); les autres ont été contaminés en laboratoire et aucun test n’a été effectué (1%). Nombre total des bactéries isolées: 1402 organismes (nombre moyen d’organismes par échantillon, 1,5). Le Staphylococcus aureusa été la cause la plus fréquente de l’invasion bactérienne de la lésion, ce qui représente 34% des cas, tandis que la Pseudomonas aeruginosaa causé 18% des cas. Le pourcentage total des organismes multirésistants, parmi tous les organismes, était 51% (715/1402); un pourcentage de 87,1% a été observé parmi les cas de Staphylococcus aureusrésistant à la méthicilline parmi tous les cas de Staphylococcus aureus(176/202); les Enterobacteriaceae- bêta-lactamases à spectre étendu (BLSE) - entre toutes les entérobactéries avaient un taux de 80,4% (111/138) et les bactéries non fermentantes BLSE démontraient un taux de 28,8% (62/215) entre toutes les bactéries non fermentantes.
Introduction
Burn injury is a major public health problem in many countries of the world. The burn wound represents a susceptible site for opportunistic colonization by organisms of endogenous and exogenous origin. Patient factors such as age, extent of injury, and depth of burn in combination with microbial factors determine the likelihood of invasive burn wound infection.1,2 Septicaemia is a serious, rapidly progressing, life-threatening infection that can arise from infections throughout the body, including the lungs, abdomen, and the urinary tract.3
Initially, the burned area is considered free of major microbial contamination. However, Gram-positive bacteria in the depths of sweat glands and hair follicles may survive the heat of the initial injury, and unless topical antimicrobial agents are used, these bacteria heavily colonize the wounds within the first 48 h after injury.4,5The topical agents reduce the microbial overgrowth, but they seldom prevent further colonization with other potentially invasive bacteria and fungi. These are mainly derived from the patient’s gastrointestinal and upper respiratory tracts as well as from the hospital environment.6Following colonization, the organisms on the surface start to penetrate the burn eschar to a variable extent, depending on their invasive capacity, local wound factors, and the degree of the patient’s immunosuppression. If viable sub-eschar tissue becomes invaded, disseminated infection is likely to occur.7It is common knowledge that the spectrum of infective agents varies from time to time and from place to place. It is therefore desirable to carry out periodic reviews of the bacterial flora of burn wounds in order to modify preventive strategies as necessary. For these reasons we carried out a study of bacteriological profiles and compared the antimicrobial resistance patterns of predominant bacteria over a period of one year.
Materials and method
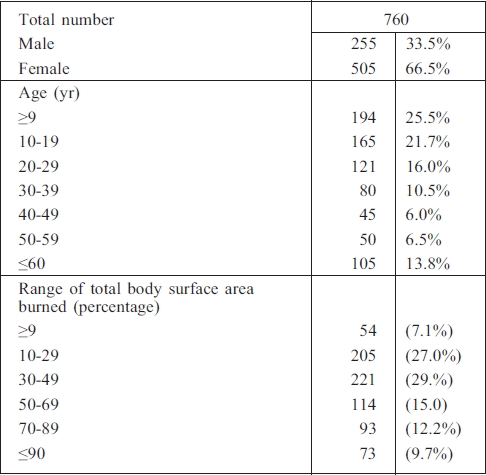
The study was conducted over a period of 12 months (from May 2008 to April 2009) at the Sulaimani Burn, Reconstructive and Plastic Surgery Hospital, Sulaimani, Iraq. A total of 1126 samples of swabs and blood were processed from 760 admitted patients (505 female and 255 male) ( Table I).
Table I. Patients.
All the patients received immediate care and resuscitation therapy. Silver sulphadiazine was used topically and the dressings were changed daily. Clindamycin and amikacin were administered (single dose) as prophylactic antibiotics during debridement or early excision of the burn eschar and grafting. The wound was inspected daily during the dressing changes.
Culture swabs from the burn wounds were taken at the time of admission for all patients and were swabbed on the third and seventh day on the basis of clinical judgment of infection.
Infection and septicaemia were monitored according to the guidelines advocated by the National Nosocomial Infections Surveillance system8when a patient showed signs of disorientation, hyperpyrexia or hypothermia, circulatory embarrassment, petechial haemorrhages, black and dark discoloration in a previously clean-looking burn wound, early and rapid eschar separation, bleeding into the subcutaneous tissues, increasing oedema in surrounding areas, or leucocytosis in white blood cell counts.
Identification of isolates was performed according to conventional bacteriological methods or using a commercial ATB identification kit (ATB System, BioMerieux, France). The antimicrobial disk susceptibility test was performed according to the National Committee for Clinical Laboratory Standards guidelines.9
Results
Bacteria isolates were found in 935 samples (83%) and only 180 wound swabs (16%) were sterile; others were contaminated within the lab and tests were not performed (1%). Total bacteria isolated: 1402 organisms (average number organisms per sample, 1.5).
Pattern of burn wound microbial colonization
The various types of bacteria isolated from burn wound culture and biopsies of total invasive burn wound infection are shown in Table II. The table clearly indicates that Staphylococcus aureus was the commonest bacterial cause of invasive burn wound invasion, accounting for 34% of cases, with Pseudomonas aeruginosa causing 18%.
Table II. Pattern of burn wound microbial colonization.
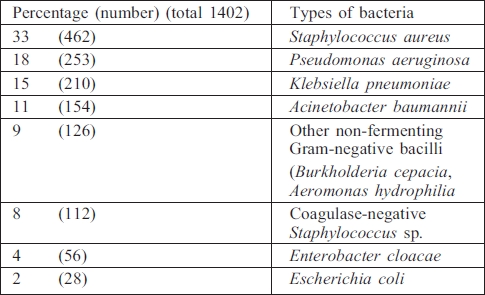
Gram-negative bacteria
Pseudomonas aeruginosa was the commonest pathogen isolated (30.5%), followed by Klebsiella pneumoniae (25.5%) (Table III).
Table III. Types of Gram-negative bacteria.
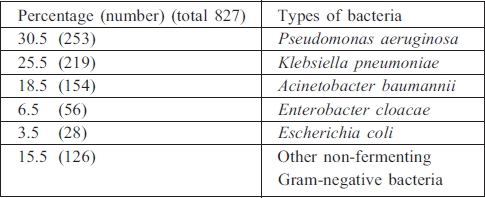
Gram-positive bacteria
Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus (MRSA), was the commonest pathogen isolated in Gram-positive bacteria (80.5%) (Table IV).
Table IV. Types of Gram-positive bacteria.
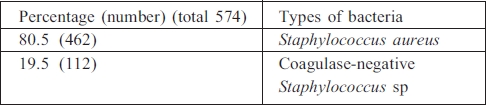
Multidrug-resistant organisms
Total multidrug-resistant organisms (MDROs) among the organisms were 50.4% (715/1402); MRSA among all Staphylococcus aureus, 87.1% (176/202); extended-spectrum beta-lactamase (ESBL) Enterobacteriaceae among all Enterobacteria, 80.4% (111/138); and ESBL non-fermenting bacteria among all non-fermenting bacteria, 28.8% (62/215) (Table V).
Table V. Types of multidrug-resistant organism bacteria.
Staphylococcus aureus resistance
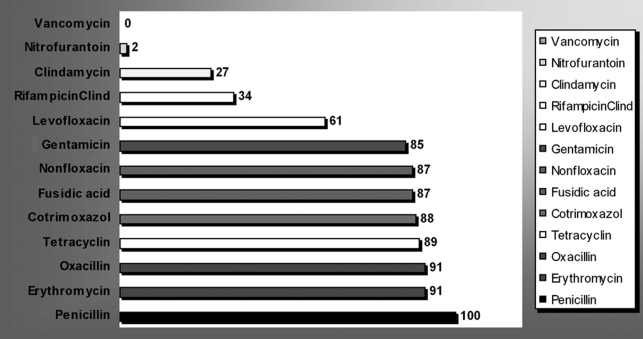
Staphylococcus aureus was completely penicillin resistant (100%) and resistance was marked with other antimicrobials: erythromycin, 91%); oxacillin, 91%; gentamicin, 85% (Fig 1). On the other hand, Staphylococcus aureus sp were found to be more sensitive to antimicrobials such as minocyclin (4%) and nitrofurantoin (2%). However, no single strain of S. aureus was found to be resistant to vancomycin.
Fig 1. Antibiotic resistance of major micro-organisms from nosocomial infections in burns unit.
Non-fermenting Gram-negative bacilli
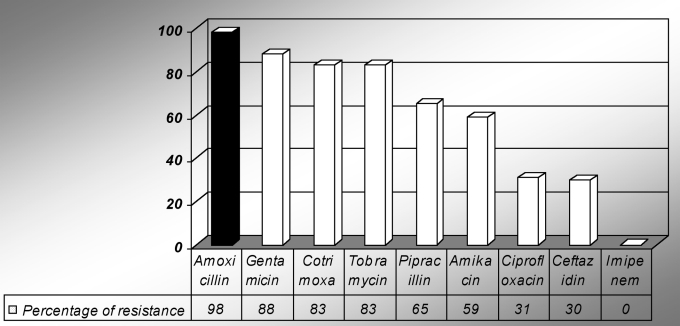
Among Gram-negative bacilli, the resistance rate varied from 98% (amoxicillin) to 83% (tobramycin). However, no strain belonging to non-fermenting Gram-negative bacilli sp was found to be resistant to imipenem (Fig 2).
Fig 2. Antibiotic resistance among Gram-negative bacilli.
Acinetobacter baumanii resistance
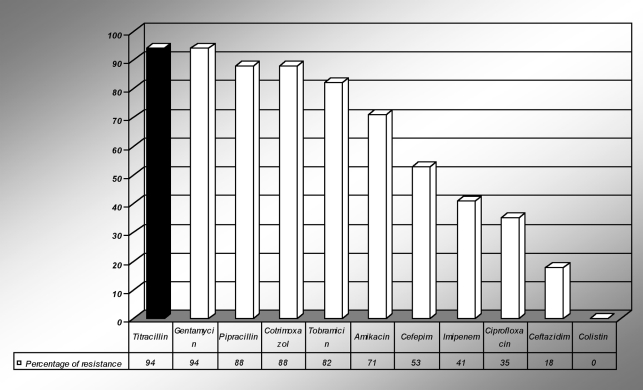
Acinetobacter sp were found to be resistant to imipenem (41%). The resistance to pipracillin and gentamicin of Acinetobacter was 94%. Ciprofloxacin resistance was seen in only 35% of Acinetobacter. However, no single strain belonging to Acinetobacter baumanii sp was found to be resistant to colistin (Fig 3).
Fig 3. Antibiotic resistance among Acinetobacter sp.
Discussion and conclusion
Wound infections are common problems in burn units, mostly originating from nosocomial contamination. The development of infections in burn cases is serious because of their effects on the course of the disease and patient outcomes. Many burn patients die as a result of infection during their hospital courses. The rate of infection in burn cases is extremely high in developing countries.10,11 This may be due to the prevalence of low-level socioeconomic groups of patients in whom poor hygienic conditions prevail; malnutrition may also play a role in the earlier establishment of the infection.12 Families and hospital personnel frequently touch hospitalized patients. Infection is easily spread by means of this close contact. Inadequate measures to prevent cross-infection by burn unit workers and visitors may also be implicated. Poor compliance with hygiene rules and inadequate disinfection or sterilization of mattresses, bed sheets, dressing materials, and other equipment used for patient care, prolonged catheterization, central or peripheral lines, inefficient isolation of infected patients, overcrowding, decreased host resistance, and inappropriate antibiotic use are the most important causes for nosocomial infections in burn units.13
In our study, the most frequent micro-organisms from burn wound infections were found to be Staphylococci (34%), Pseudomonas spp (18%), E. coli (18%), Klebsiella pneumoniae (15%), and Acinetobacter baumannii (11%).
Our finding that Staphylococcus aureus was the most common isolate coincides with many previous reports14,15 but contrasts with some other studies which report Pseudomonas aeruginosa as the predominant organism.16-18
Staphylococcus aureus is carried in the nose, and occasionally in other sites, by up to 30% of the general population, and over 50% of hospital workers.19 The prevalence of MRSA within the general population is less well documented. An open wound is an easy target for bacterial colonization either from the patients themselves or from their environment. Reports suggest that Staphylococcus aureus is currently responsible for the majority of septic complications in burn patients and that frequently the strain causing infection is that cultured on swabs at the time of admission.20
Pseudomonas aeruginosa, which is the most important and dangerous bacterium in burn sepsis and septicaemia, shows variable incidences. Pseudomonas aeruginosa has emerged as an important pathogen during the past two decades. It causes between 10 and 20% of infections in most hospitals. Pseudomonas infection is especially prevalent among patients with burn wounds.21
A. baumannii are Gram-negative, non-fermentative bacteria commonly present in the soil and water as free-living saprophytes. Although Acinetobacter spp are frequently isolated as commensals from human skin, throat, and various secretions in healthy people, they are increasingly being held responsible for hospital infections, predominantly in intensive care units. Multidrug-resistant A. baumannii have emerged as an important pathogens in burn units in several hospitals around the globe.22-24
Successful infection control measures depend on a burn unit being built with exacting specifications, well-trained burn unit workers, surveillance of the bacteria prevalent in the burn unit and their antimicrobial resistance patterns, and an efficient hospital infection control programme.25 To control infection in the burn unit, overcrowding must be avoided, and strict hand washing both before and after handling patients must be implemented, as well as restriction of movement within the burn unit.
The pattern of bacterial resistance is important for epidemiologic surveillance and treatment options in patients with burns. The antimicrobial treatment closely depends on the bacterial resistance pattern. The antimicrobial resistance patterns seen in this study give serious cause for concern because the predominant bacterial isolates were highly resistant to the commonly available antimicrobial agents in many burn units. Antimicrobial prophylaxis was not given on the basis of rational indications in the burn unit. An effective infection control programme should be established in all burn units. The prevention of nosocomial burn wound infections can be minimized with fastidious wound care. It is important that the burn unit staff and infection control units work together to control infections. Team effort can help avoid infections and solve problems. The training of the burn unit staff and the establishment of a follow-up programme are important for the prevention of nosocomial infections.
References
- 1.Manson WL, Pernot PC, Fidler V, et al. Colonization of burns and the duration of hospital stay of severly burned patients. J Hosp Infect. 1992;22:55–63. doi: 10.1016/0195-6701(92)90130-e. [DOI] [PubMed] [Google Scholar]
- 2.Pruitt BA, McManus AT, Kim SH, et al. Burn wound infections. Current status. World J Surg. 1998;22:135–45. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- 3.Linares HA. The Burn Problem: A Pathologist’s Perspective. In: Herndon DN, editor. “Total Burn Care”. Vol. 371 WB Saunders Company; London: 1996. [Google Scholar]
- 4.Luterman A, Dacso CC, Curreri PW. Infections in burn patients. Am J Med. 1986;81 (Suppl. 1A):45–52. doi: 10.1016/0002-9343(86)90513-9. [DOI] [PubMed] [Google Scholar]
- 5.Mooney DP, Gamelli RL. Sepsis following thermal injury. Comp Ther. 1989;15:22–29. [PubMed] [Google Scholar]
- 6.Monafo WW, Freedman B. Topical therapy for burns. Surg Clin North Am. 1987;67:133–45. doi: 10.1016/s0039-6109(16)44137-x. [DOI] [PubMed] [Google Scholar]
- 7.Hansbrough JF. Burn wound sepsis. J Intens Care Med. 1987;2:313–27. [Google Scholar]
- 8.National Nosocomial Infections Surveillance (NNIS) System Report. Data summary from January 1992-June 2002, issued August 2002. Am J Infect Control. 2002;30:458–75. doi: 10.1067/mic.2002.130032. [DOI] [PubMed] [Google Scholar]
- 9.Performance standards for antimicrobial disk susceptibility tests. Approved Standards M2-A6. NCCLS, Villanova, PA. National Committee for Clinical Laboratory Standards (NCCLS). 1999 [Google Scholar]
- 10.Lari AR, Alaghehbandan R, Nikui R. Epidemiological study of 3341 burns patients during three years in Tehran, Iran. Burns. 2000;26:49–53. doi: 10.1016/s0305-4179(99)00102-3. [DOI] [PubMed] [Google Scholar]
- 11.Mabogunje OA, Khwaja MS, Lawrie JH. Childhood burns in Zaria, Nigeria. Burns. 1987;13:298–304. doi: 10.1016/0305-4179(87)90050-7. [DOI] [PubMed] [Google Scholar]
- 12.Esposito S. Immune system and surgical site infection. J Chemother. 2001;3:12–16. doi: 10.1179/joc.2001.13.Supplement-2.12. [DOI] [PubMed] [Google Scholar]
- 13.Pandit DV, Gore MA, Saileshwar N, et al. Laboratory data from the surveillance of a burns ward for the detection of hospital infection. Burns. 1993;19:52–8. doi: 10.1016/0305-4179(93)90101-d. [DOI] [PubMed] [Google Scholar]
- 14.Taylor GD, Kibsey P, Kirkland T, et al. Predominance of Staphylococcus organisms in infections occurring in a burns intensive care unit. Burns. 1992;18:332–5. doi: 10.1016/0305-4179(92)90158-q. [DOI] [PubMed] [Google Scholar]
- 15.Lesseva MI, Hadjiiski OG. Staphylococcal infections in the Sofia Burn Center, Bulgaria. Burns. 1996;22:279–86. doi: 10.1016/0305-4179(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 16.Revathi G, Puri J, Jam BK. Bacteriology of burns. Burns. 1998;24:347–9. doi: 10.1016/s0305-4179(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 17.Panit DV, Gore MA, Saileshwar N, et al. Laboratory data from the surveillance of a burns ward for the detection of hospital infection. Burns. 1993;19:52–5. doi: 10.1016/0305-4179(93)90101-d. [DOI] [PubMed] [Google Scholar]
- 18.Lari AR, Alaghehbandan R. Nosocomial infections in an Iranian burn care center. Burns. 2000;26:737–40. doi: 10.1016/s0305-4179(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 19.Fekey FR. The epidemiology and prevention of staphylococcal infection. Medicine. 1964;43:593–613. [PubMed] [Google Scholar]
- 20.Taylor GD, Kibsey P, Kirkland T, et al. Predominance of staphylococcal organisms in infections occurring in a burns intensive care unit. Burns. 1992;18:332–5. doi: 10.1016/0305-4179(92)90158-q. [DOI] [PubMed] [Google Scholar]
- 21.Tredget EE, et al. Pseudomonas infection in the thermal injured patient. Burns. 2004;30:3–26. doi: 10.1016/j.burns.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Ang SW, Lee ST . Emergence of a multiply-resistant strain of Acinetobacter in a burns unit. Ann Acad Med Singapore. 1992;21:660–3. [PubMed] [Google Scholar]
- 23.Frame D, Kangesu L, Malik WM. Changing flora in burn and trauma units: Experience in United Kingdom. J Burn Care Rehabil. 1992;2:281–6. doi: 10.1097/00004630-199203000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Sheretz RJ, Sullivan ML. An outbreak of infections with Acinetobacter calcoaceticus in burn patient: Contamination of patient’s mattress. J Infect Dis. 1985;2:252–8. doi: 10.1093/infdis/151.2.252. [DOI] [PubMed] [Google Scholar]
- 25.Ozumba UC, Jiburum BC. Bacteriology of burn wounds in Enugu, Nigeria. Burns. 2000;26:178–80. doi: 10.1016/s0305-4179(99)00075-3. [DOI] [PubMed] [Google Scholar]