Abstract
The secondary structures of poly(dG-dC).poly(dG-dC) in the presence of alcaline , alcaline earth and first row transition metal ions (Na+, Mg2+, Co2+, Ni2+) are investigated by infrared spectroscopy. The conformational transitions are studied as a function of the hydration of the polynucleotide and counter-ion nature and content. The use of selectively deuterated poly(dG-dC).poly(dG-dC) on the 8-carbon of guanines allows to show that a direct interaction occurs between the N7 site of guanines and the transition metal ions Co2+ and Ni2+. In the case of Mg2+, for high ion/nucleotide ratios, the interaction occurs essentially at the level of the phosphate groups. This interaction leads to a modification of the left-handed conformation. Based on the IR spectroscopy results, an explanation is proposed for the different efficiencies of these various ions to induce the B----Z transition.
Full text
PDF

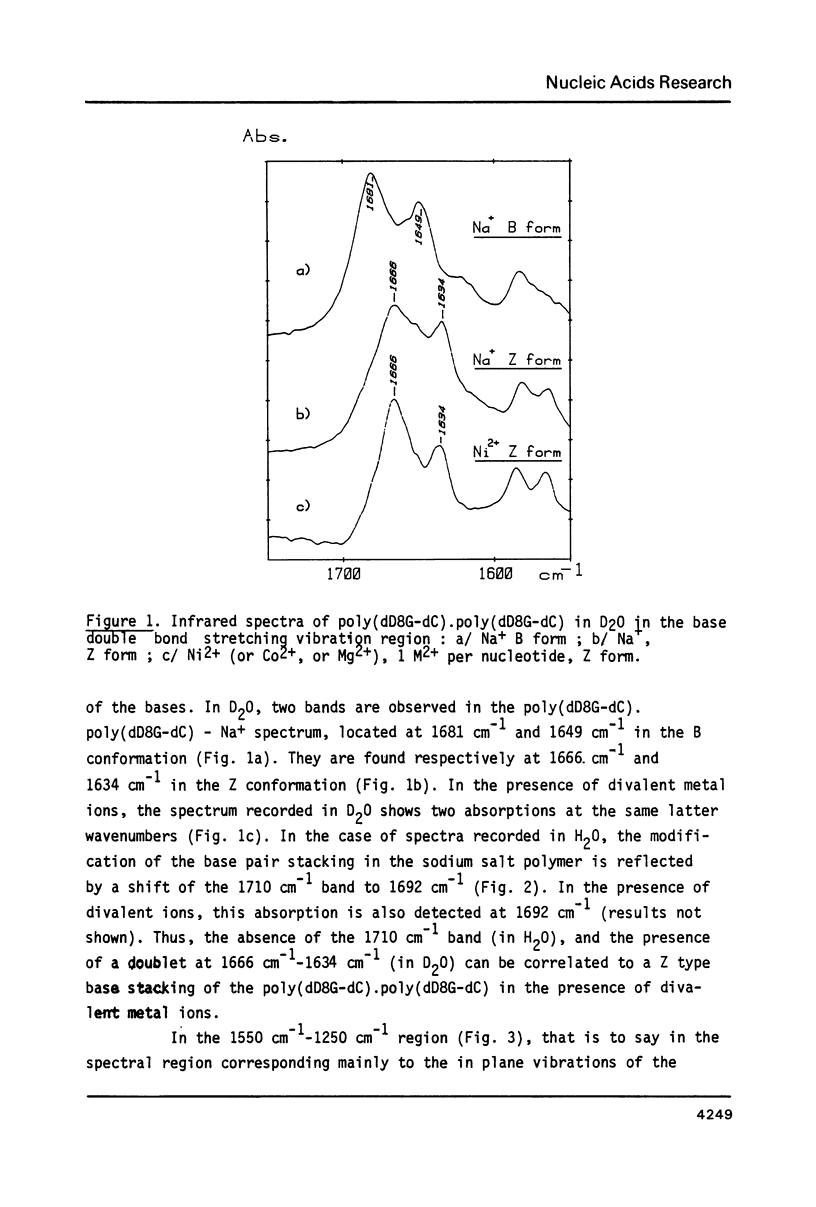
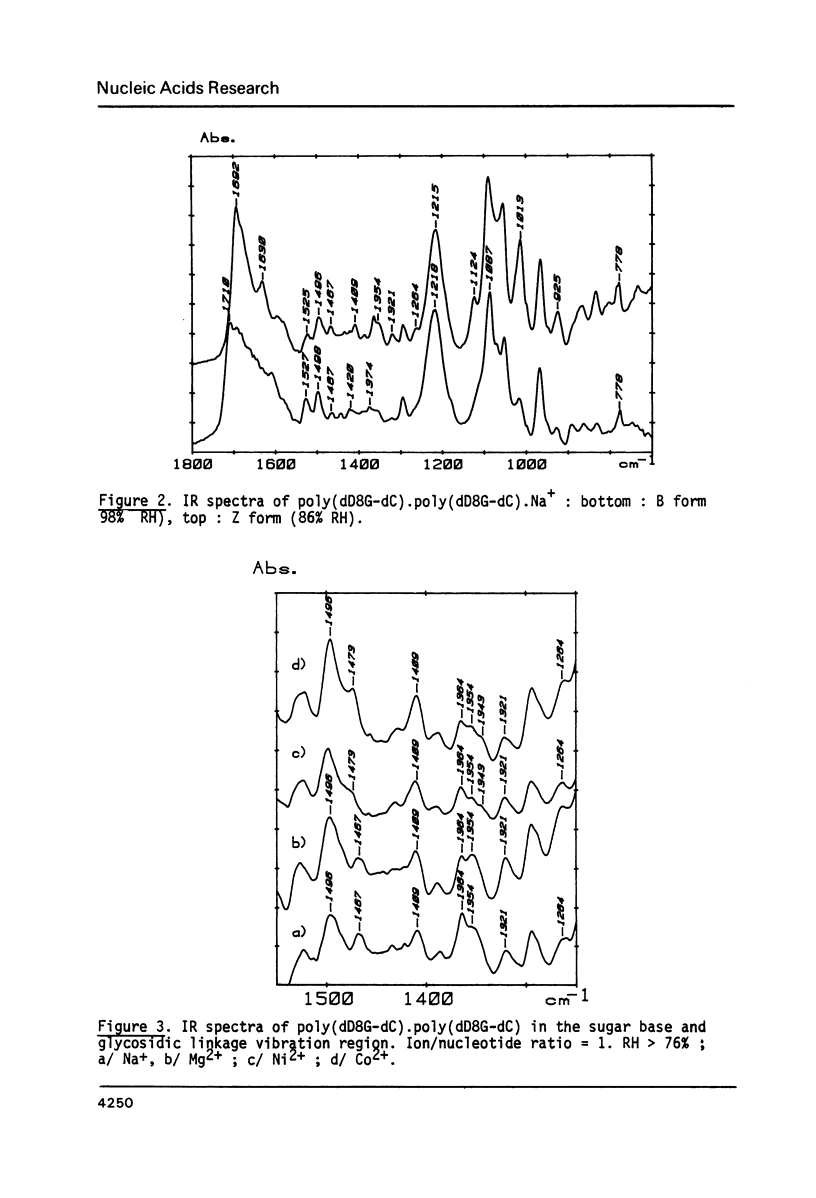
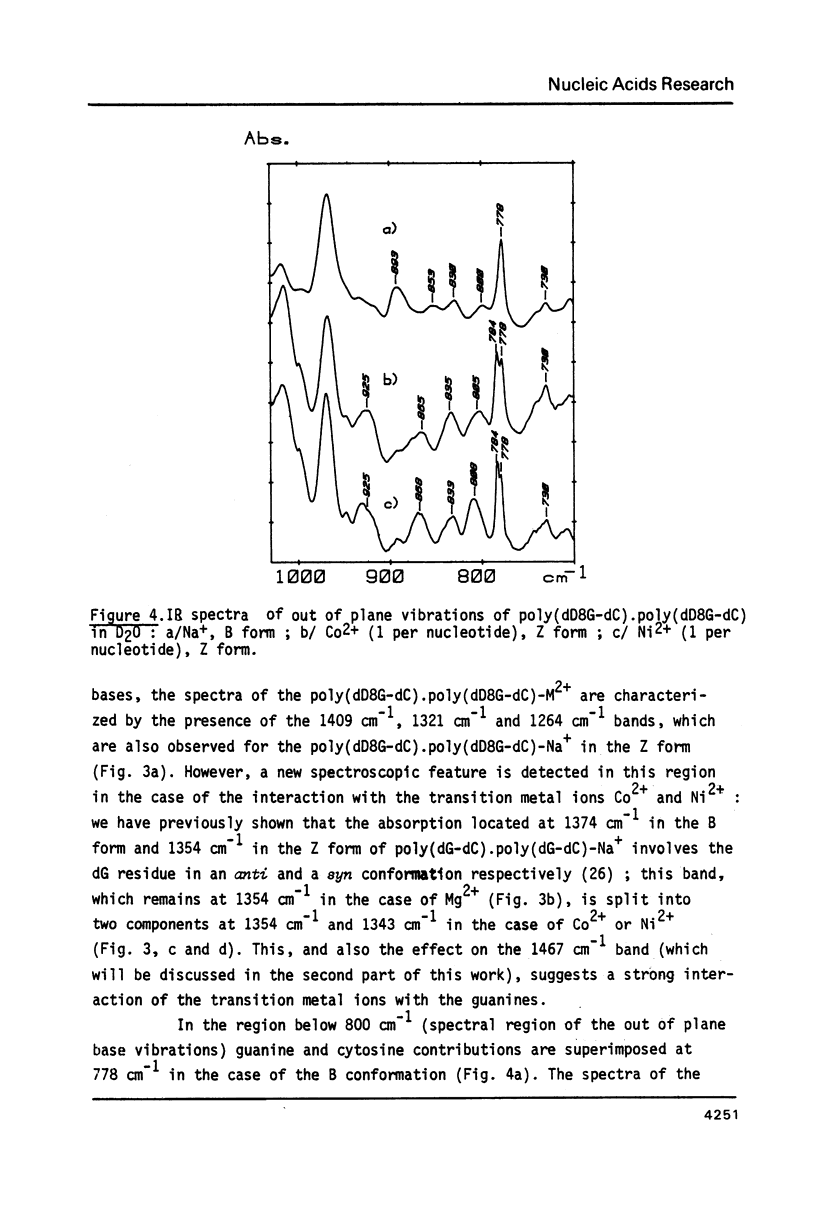
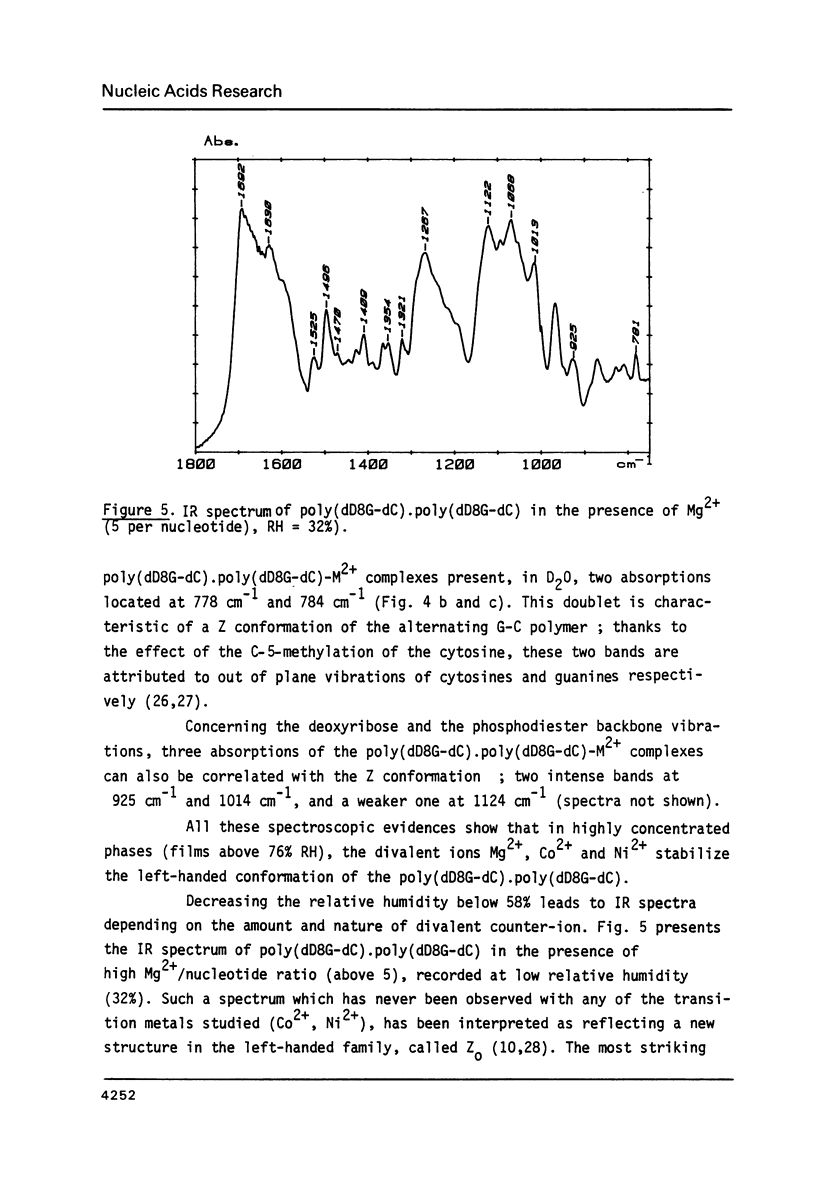





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. S., Wooten J. B., Chatterjee C. L. Characterization of alternating deoxyribonucleic acid conformations in solution by phosphorus-31 nuclear magnetic resonance spectroscopy. Biochemistry. 1981 May 26;20(11):3049–3055. doi: 10.1021/bi00514a010. [DOI] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Feigon J., Wang A. H., van der Marel G. A., Van Boom J. H., Rich A. A one- and two-dimensional NMR study of the B to Z transition of (m5dC-dG)3 in methanolic solution. Nucleic Acids Res. 1984 Jan 25;12(2):1243–1263. doi: 10.1093/nar/12.2.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot J., Feigon J., Kearns D. R. Interactions of DNA with divalent metal ions. I. 31P-NMR studies. Biopolymers. 1982 Jan;21(1):181–201. doi: 10.1002/bip.360210115. [DOI] [PubMed] [Google Scholar]
- Harvey S. C. DNA structural dynamics: longitudinal breathing as a possible mechanism for the B in equilibrium Z transition. Nucleic Acids Res. 1983 Jul 25;11(14):4867–4878. doi: 10.1093/nar/11.14.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane M. J., Thomas G. J., Jr Kinetics of hydrogen-deuterium exchange in guanosine 5'-monophosphate and guanosine 3':5'-monophosphate determined by laser-Raman spectroscopy. Biochemistry. 1979 Sep 4;18(18):3839–3846. doi: 10.1021/bi00585a002. [DOI] [PubMed] [Google Scholar]
- Lemeunier F., Derbin C., Malfoy B., Leng M., Taillandier E. Identification of left-handed Z-DNA by indirect immunofluorescence in polytene chromosomes of Chironomus thummi thummi. Exp Cell Res. 1982 Oct;141(2):508–513. doi: 10.1016/0014-4827(82)90245-2. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Lafer E. M., Möller A., Stollar B. D., Rich A. Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature. 1981 Dec 3;294(5840):417–422. doi: 10.1038/294417a0. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilet J., Leng M. Comparison of poly(dG-dC).poly(dG-dC) conformations in oriented films and in solution. Proc Natl Acad Sci U S A. 1982 Jan;79(1):26–30. doi: 10.1073/pnas.79.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Rose D. M., Bleam M. L., Record M. T., Bryant R. G. Mg NMR in DNA solutions: Dominance of site binding effects. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6289–6292. doi: 10.1073/pnas.77.11.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillandier E., Taboury J., Liquier J., Sautière P., Couppez M. Structural transitions in DNAs and nucleohistones studied by I.R. spectroscopy. Biochimie. 1981 Nov-Dec;63(11-12):895–898. doi: 10.1016/s0300-9084(82)80282-4. [DOI] [PubMed] [Google Scholar]
- Thamann T. J., Lord R. C., Wang A. H., Rich A. The high salt form of poly(dG-dC).poly(dG-dC) is left-handed Z-DNA: Raman spectra of crystals and solutions. Nucleic Acids Res. 1981 Oct 24;9(20):5443–5457. doi: 10.1093/nar/9.20.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Livramento J. Kinetics of hydrogen-deuterium exchange in adenosine 5'-monophosphate, adenosine 3':5'-monophosphate, and poly(riboadenylic acid) determined by laser-Raman spectroscopy. Biochemistry. 1975 Nov 18;14(23):5210–5217. doi: 10.1021/bi00694a030. [DOI] [PubMed] [Google Scholar]
- Viegas-Péquignot E., Derbin C., Lemeunier F., Taillandier E. Identification of left-handed Z-DNA by indirect immunomethods in metaphasic chromosomes of a mammal, Gerbillus nigeriae (Gerbillidae, Rodentia). Ann Genet. 1982;25(4):218–222. [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]
- Zacharias W., Larson J. E., Klysik J., Stirdivant S. M., Wells R. D. Conditions which cause the right-handed to left-handed DNA conformational transitions. Evidence for several types of left-handed DNA structures in solution. J Biol Chem. 1982 Mar 25;257(6):2775–2782. [PubMed] [Google Scholar]
- Zimmer C., Luck G., Triebel H. Conformation and reactivity of DNA. IV. Base binding ability of transition metal ions to native DNA and effect on helix conformation with special reference to DNA-Zn(II) complex. Biopolymers. 1974;13(3):425–453. doi: 10.1002/bip.1974.360130302. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Jovin T. M. Z* DNA, the left-handed helical form of poly[d(G-C)] in MgCl2-ethanol, is biologically active. EMBO J. 1982;1(1):115–120. doi: 10.1002/j.1460-2075.1982.tb01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sande J. H., McIntosh L. P., Jovin T. M. Mn2+ and other transition metals at low concentration induce the right-to-left helical transformation of poly[d(G-C)]. EMBO J. 1982;1(7):777–782. doi: 10.1002/j.1460-2075.1982.tb01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


