Abstract
Objectives
The aim of this study is to investigate the effects of ovariectomy on bone mineral density (BMD) and oxidative state in rats, and the alterations in these effects that vitamin C supplementation may produce.
Materials and methods
Twenty female Wistar albino rats were randomly divided into three groups: control (C, n=6); ovariectomy (O, n=7); and ovariectomy+vitamin C supplement (OV, n=7). Oxidative stress (OS) was assessed 100 days postovariectomy by measuring the activity of several enzymes, including catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase, as well as the concentrations of malondialdehyde (MDA), nitric oxide (NO), and total sulfhydryl groups in plasma and bone homogenates.
Results
A significant decrease in BMD was observed in O group compared with C group (p=0.015), and a significant increase was observed in OV compared with O group (p=0.003). When groups were compared with respect to parameters of OS, MDA and NO levels in bone tissue were significantly higher in O than in C (p=0.032, p=0.022) and were significantly lower in OV than in O (p=0.025, p=0.018). SOD activity was significantly higher in O than in C (p=0.032). In plasma, MDA activity was significantly higher in O than in C (p=0.022) and NO level was significantly higher in O than in C and OV (p=0.017, p=0.018).
Conclusions
Our results suggest that ovariectomy may produce osteoporosis and OS in females, and vitamin C supplementation may provide alterations regarding improvement in OS and BMD values. We assume that studies including more subjects are needed to make a decisive conclusion about OS–BMD relation.
Keywords: rat, ovariectomy, osteoporosis, oxidative stress, vitamin C, ascorbic
Osteoporosis (OP) is a disease characterized by low bone mass without alteration in bone composition, which leads to increased risk of fracture. Being most common in women after menopause, the ovariectomized rats are used specifically as the animal model to observe the effects of OP since the biological mechanisms related with bone resorption in ovariectomized rats highly resemble to the postmenopausal bone loss in women (1, 2). A close relation was observed between increased bone turnover and OP formation at early and late periods after ovariectomy in rats (3).
Oxidative stress (OS) is a state of imbalance in favor of pro-oxidants versus antioxidants, resulting in cellular damage that is often irreversible (4, 5) and can be observed in diseases such as OP, respiratory distress syndrome, atherosclerosis, chronic renal failure, rheumatoid arthritis, diabetes, sepsis, and Alzheimer's disease. OS can be evaluated by determining the levels of antioxidants, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione-S-transferase, catalase (CAT), and glutathione reductase, that inhibit the detrimental effects of reactive oxygen species (ROS) and free radicals by determining the thiobarbituric acid reactive substance and nitric oxide synthetase (NOS) levels in aerobic organisms (4–6).
Although estradiol is known to function as an antioxidant and as a free radical scavenger, the underlying mechanism remains obscure. It is reported that OS is increased and some antioxidants are decreased (6, 7) or increased (8, 9) in relation to menopause in which estrogen diminishes. Some studies have found relationship between OS and the decrease in bone mineral density (BMD) (10). These findings suggest that OS may play a role in the pathology of postmenopausal OP and that supplementation with antioxidants may be beneficial in the treatment of this condition.
Vitamin C, a potent antioxidant, was shown to decrease bone resorption in laboratory studies. The mechanism by which vitamin C (ascorbic acid) exerts effect on bone density is unclear, but it may be related to its effects on collagen formation and osteoblast development or to the increase in calcium absorption (11, 12).
Interesting results have been reported in studies evaluating the role of oxidants and antioxidants in OP (6–9). There are only a few studies available that reported the effect of vitamin C treatment on BMD (13). Therefore, in the present study, we evaluated the relation between OS and OP in ovariectomized rats. We measured plasma and femur bone tissue levels of nitric oxide (NO), total sulfhydryl (t-SH), malondialdehyde (MDA), SOD, CAT, and GPx. We also evaluated the effects of vitamin C supplementation on the parameters of OS and bone density.
Materials and methods
Twenty old female Wistar albino rats raised in the same environment and weighing 175–215 g were used in this study (14). They were maintained in a well-ventilated controlled room at 20–22°C on a 12-h light/12-h dark schedule. The study protocol was approved by the Institutional Ethical Committee. The rats were randomly divided into three groups: control group (C, n=6), ovariectomy group (O, n=7), and ovariectomy+vitamin C group (OV, n=7). The rats had access to food and water until 2 h prior to the anesthesia.
After anesthesia (intraperitoneal ketamine, 50 mg/kg), bilateral ovariectomy was performed with a ventral approach. Surgical procedure was not performed to the control group. After surgical procedure, vitamin C (L-ascorbic acid) was added to the drinking water of the OV group at a concentration of 1 g/500 ml water. A period of 100 days was allowed for the development of OP (3, 4).
BMD measurements
Bone mineral densities of all rats were measured 100 days after ovariectomy prior to euthanasia, using dual-energy X-ray absorptiometry (Norland XR-36; Norland Inc., Fort Atkinson, WI) with small subjects’ program (1×1 mm resolution, 60 mm/s sweep speed). BMD was determined as the amount of mineral per cubic centimeters of bone (g/cm3).
Biochemical measurements
Anesthesia was performed with intraperitoneal injection of ketamine (50 mg/kg), and 5 ml blood sample was collected from intra-abdominal region prior to euthanasia.
Blood samples with ethylenediaminetetraacetic acid were centrifuged and the plasma was stored at –80°C together with bone samples until analyzed. Bone samples were homogenized in the 0.1 M phosphate buffer. The homogenates were centrifuged and the supernatant was used for analysis. To assess OS, the levels of CAT, SOD, GPx, MDA, NO, and t-SH were measured both in plasma and bone homogenates.
Cayman (Ann Arbor, MI) kits were used in CAT analysis and Randox (Crumlin, UK) kits in SOD and GPx analysis. All samples were analyzed in the laboratories of the Biochemistry Department.
Statistical analysis
Statistical Package for the Social Sciences (Chicago, IL) v12.0 program was used for statistical analysis. Descriptive statistics were conducted on all variables. Variations in bone and plasma oxidative state parameters between study groups were assessed by using Kruskal–Wallis test. Bonferroni-adjusted Mann–Whitney U test was used after significant Kruskal–Wallis to determine which group differs from the other. Spearman's rank correlation test was used to determine the relation between BMD and oxidative state parameters. Results were expressed as mean±standard deviation (Mean±SD) and minimum–maximum (min–max). Statistical significance was set at p<0.05 for all analysis and p<0.033 (0.1/3) for Bonferroni-adjusted Mann–Whitney U test.
Results
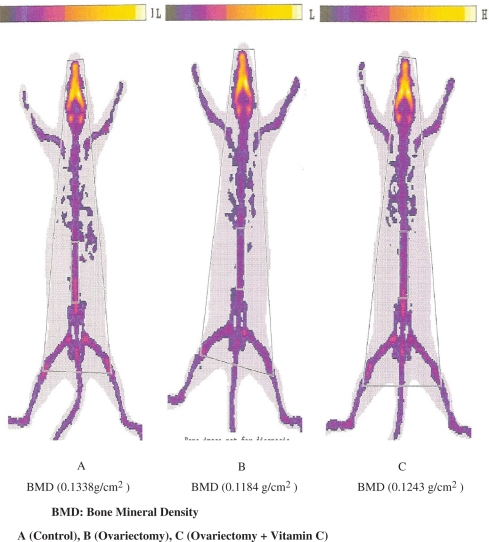
There was no statistically significant difference observed in body weights of rats at onset and at conclusion of the study within and between groups (Table 1). A significant decrease in BMD was observed in O group compared with C group (p=0.015) and a significant increase was observed in OV compared with O group (p=0.003). BMD values are given in Table 2 and Fig. 1.
Table 1.
Comparison of body weights at the beginning and end of treatment period
| Weight (g) | C (n=6); Mean±SD (min–max) | O (n=7); Mean±SD (min–max) | OV (n=7); Mean±SD (min–max) | p* | C-O (p**) | C-OV (p**) | O-OV (p**) |
|---|---|---|---|---|---|---|---|
| Beginning | 182.5±6.7 (177–195) | 183.7±10.5 (175–203) | 182.5±7.3 (175–194) | 0.960 | 0.829 | 0.829 | 0.846 |
| End | 206.7±5.1 (200–210) | 214.3±12.7 (200–230) | 199.3±11.7 (175–210) | 0.092 | 0.285 | 0.153 | 0.051 |
C: Control, O: Ovariectomy, OV: Ovariectomy +vitamin C
Mean±SD: Mean± standard deviation, min–max: minimum–maximum
Statistical significance was set at p< 0.05 with Kruskal–Wallis test
Statistical significance was set at p< 0.033 with Bonferroni-adjusted Mann–Whitney U test
Table 2.
Comparison of bone mineral densities
| C (n=6) Mean±SD (min–max) | O (n=7) Mean±SD (min–max) | OV (n=7) Mean±SD (min–max) | p* | C-O (p**) | C-OV (p**) | O-OV (p**) | |
|---|---|---|---|---|---|---|---|
| Vertebrae (g/cm2) | 0.120±0.007 (0.116–0.133) | 0.113±0.003 (0.109–0.118) | 0.121±0.004 (0.114–0.125) | 0.004 | 0.015 | 0.253 | 0.003 |
C: Control, O: Ovariectomy, OV: Ovariectomy +vitamin C
Mean±SD: Mean± standard deviation, min–max: minimum–maximum
Statistical significance was set at p< 0.05 with Kruskal–Wallis test
Statistical significance was set at p< 0.033 with Bonferroni-adjusted Mann–Whitney U test
Fig. 1.
Bone mineral density of lumbar vertebrae.
When groups were compared regarding oxidant and antioxidant parameters, MDA levels in bone tissue were significantly higher in group O than in C and OV (p=0.032 and p=0.025, respectively). There was no difference between groups C and OV. NO levels were significantly higher in group O than in either group C or group OV (p=0.022, p=0.018, respectively). There was no difference between groups C and OV. SOD activity was significantly higher in group O than in group C (p=0.032). There were no statistically significant differences in bone t-SH, CAT, and GPx levels (Table 3)
Table 3.
Oxidative stress parameters in femur bone tissue
| C (n=6) Mean±SD (min–max) | O (n=7) Mean±SD (min–max) | OV (n=7) Mean±SD (min–max) | p* | C-O (p**) | C-OV (p**) | O-OV (p**) | |
|---|---|---|---|---|---|---|---|
| MDA (nM/mg prt) | 12.85±6.01 (3.58–20.76) | 24.21±10.21 (5.35–35.27) | 12.63±3.56 (8.55–18.58) | 0.036 | 0.032 | 0.775 | 0.025 |
| t-SH (?M/g prt) | 113.33±57.14 (40–212) | 76.0±42.06 (24–140) | 96.0±42.45 (58–187) | 0.336 | 0.199 | 0.250 | 0.562 |
| NO (?M/g prt) | 8.32±1.75 (5.19–9.9) | 16.03±6.73 (7.04–26.36) | 8.78±1.69 (6.24–11.24) | 0.023 | 0.022 | 0.668 | 0.018 |
| SOD (U/mg prt) | 2.28±0.57 (1.5–2.78) | 3.85±1.25 (2.44–5.88) | 2.50±0.44 (1.55–2.92) | 0.049 | 0.032 | 0.668 | 0.048 |
| CAT (nM/min/mg prt) | 40.83±5.10 (35.9–49.3) | 38.34±14.78 (21.4–65.3) | 36.26±9.79 (19.7–52.4) | 0.448 | 0.390 | 0.253 | 0.749 |
| GPx (U/g prt) | 16.25±4.35 (12.15–24.02) | 17.85±6.01 (12.84–29.86) | 14.80±2.82 (11.87–20.31) | 0.490 | 0.668 | 0.475 | 0.225 |
C: Control, O: Ovariectomy, OV: Ovariectomy +vitamin C
Mean±SD: Mean± standard deviation, min–max: minimum–maximum
Statistical significance was set at p<0.05 with Kruskal–Wallis test
Statistical significance was set at p<0.033 with Bonferroni-adjusted Mann–Whitney U test
The plasma MDA activity was significantly higher in O than in C (p=0.022); NO level was significantly higher in O than in C or OV (p=0.017, p=0.018, respectively). There was no statistically significant difference in plasma t-SH, SOD, CAT, and GPx levels (Table 4). No correlation was observed between BMD and the measured parameters of OS.
Table 4.
Plasma oxidant stress parameters
| C (n=6); Mean±SD (min–max) | O (n=7);p Mean±SD (min–max) | OV (n=7); Mean±SD (min–max) | p* | C-O (p**) | C-OV (p**) | O-OV (p**) | |
|---|---|---|---|---|---|---|---|
| MDA (nM/ml) | 9.28±3.36 (6.61–15.9) | 14.37±3.81 (10.83–20.96) | 11.88±3.36 (7.24–16.53) | 0.044 | 0.022 | 0.086 | 0.277 |
| t-SH (nM/ml) | 0.42±0.09 (0.28–0.56) | 0.44±0.13 (0.21–0.63) | 0.38±0.12 (0.23–0.58) | 0.608 | 0.668 | 0.568 | 0.338 |
| NO (nM/ml) | 46.75±6.28 (41.9–56.5) | 82.90±30.62 (41.9–123.5) | 47.01±6.33 (37.7–55.8) | 0.020 | 0.017 | 0.774 | 0.018 |
| SOD (U/ml) | 0.53±0.08 (0.44–0.63) | 0.70±0.18 (0.56–1.08) | 0.59±0.14 (0.45–0.84) | 0.105 | 0.032 | 0.390 | 0.250 |
| CAT (nM/min/ml) | 57.5±10.3 (39.2–70.1) | 63.8±12.2 (42.8–76.1) | 61.13±8.23 (53.2–76.1) | 0.511 | 0.199 | 0.886 | 0.522 |
| GPx (U/L) | 2.61±0.70 (1.46–3.28) | 3.45±0.73 (2.71–4.93) | 2.57±1.29 (0.57–4.02) | 0.217 | 0.073 | 0.830 | 0.249 |
C: Control, O: Ovariectomy, OV: Ovariectomy +vitamin C
Mean±SD: Mean± standard deviation, min–max: minimum–maximum
Statistical significance was set at p<0.05 with Kruskal–Wallis test
Statistical significance was set at p<0.033 with Bonferroni-adjusted Mann–Whitney U test
Discussion
This study revealed that BMD was significantly reduced in ovariectomized rats and that vitamin C supplement restored BMD to control levels. OS was induced in bone and plasma with ovariectomy and levels of antioxidants raised in response to decrease in OS. Vitamin C supplement was also shown to reduce OS. No correlation was found between BMD and pro-oxidant and antioxidant parameters.
OP is generally accepted as the pathological condition due to aging. Also, OS is intimately connected to the process of aging. The relation between OP and OS has been examined in many studies. It is suggested that by reducing or eliminating OS, the loss of BMD and OP could be prevented (10).
There is an increase in superoxide formation by the osteoclasts that accompanies active bone resorption and loss of bone density (14).
It is considered that oxidative state is important in OP and coherent with the relation between diseases like OP and arteriosclerosis in which OS plays a major role. In addition, it is stated that OS and BMD are correlated in women (urine 8-iso-P6F2 levels indicates OS) (7, 15).
Recently, in vitro or animal studies showed that OS has an important role in osteoclast differentiation and functions. On the other hand, it was indicated that ROS plays an important role in osteoblastic functions (16).
In vivo and in vitro studies proved that NO (NO is a free radical that causes OS) plays a role in osteoblastic and osteoclastic activities (8, 15).
Animal and human studies showed that NO is an important regulator in bone metabolism (17, 18). But the outcomes of these experimental results are debated and the roles of the mechanisms are unclear. It is known that bone cell produces NO as a response of estrogens, proinflammatory cytokines, and mechanic stress, during this process different NOS isoforms take part (17, 19). Van't Hof et al. (19) noticed that neuronal NOS (nNOS) in knockout rats plays a role on increased bone mass regulation and bone circle. Caballero et al. (18) emphasized a similar result in their study. Contrast to this result, Baecker et al. (20) reported that, in early postmenopause, L-arginine treatment (natural NO percussor) is not effective in bone resorption or in bone formation.
The relationship between NO and BMD is controversial. Aguirre (21) advocated that resection of NO gene in guinea pigs decreased bone volume, bone formation rate, and mineral density, whereas Wimalawansa (22) found that NO donors are protected from postmenopause bone loss.
Ozgocmen et al. (8) found a significant relationship between erythrocyte NO and proximal femur BMD rats, but not with lumbar BMD. In addition, compared with non-porotic controls, females with postmenopausal OP have an increased NO level, but they found no difference in plasma NO levels. Same investigators in another study found increased NO levels in women with OP compared with non-porotic controls. On comparison between calcitonin, risedronat, and raloksifen treatment in osteoporotic patients, NO level is decreased only in the risedronat group (23).
Yalin et al. (24) investigated the effect of antioxidants and free radicals on postmenopausal OP and found that NO levels in OP patients were higher. In our study, we found that in the ovariectomized group NO level in femur bone tissue and plasma was higher than the ovariectomized+vitamin C group. Although it has been reported that there was a negative correlation between NO levels and BMD, we could not find a correlation. We believe that a correlation could be found if we increase the number of subjects.
Studies showed that the effect of NO on bone formation is related to other stimuli, which regulates the bone formation cycle. In situations with high stress, like increased osteoblastic activity, NO levels increase in order to eliminate O2. –. ONOO– is a very potent oxidant, which can affect deoxyribonucleic acid (DNA) and fatty acids and initiate lipid peroxidation. This mechanism can explain the increase in NO levels in osteoporotic females. We believe that our findings support these results.
Many ROS, which are made in cells and tissues, occur in very low levels. ROS is a very reactive molecule when it is very high and it affects the defensive systems, causes tissue damage, and starts lipid peroxidation (25). Lipid peroxidation is a well-known tissue damage mechanism in humans and is used in tissue and cells as an OS indicator. Lipid peroxides are made of polyunsaturated fatty acids; they are unstable and they decompose to make complex compounds. MDA is the biggest group of this compound and for this reason measuring the level of MDA is the criteria to understand the lipid peroxidation (26).
Recently it is shown that oxide lipids affect osteoclastic and osteoblastic bone cells (27).
Levels of the femur hydrogen peroxide (H2O2) and lipid peroxidation in rats with overectomy indicate the deficiency of estrogen levels and activate ROS, which leads to bone loss. H2O2 increases osteoblastic differentiation and function and is necessary for osteoblastic differentiation as well. It is well known that osteoclasts express NADPH and produce high levels of ROS during bone resorption (28).
Ozgocmen et al. (8) showed that increase in MDA levels (in plasma and erythrocyte) was a result of lipid peroxidation in osteoporotic females compared with the non-porotic control group. In another study, Ozgocmen et al. (23) notified an increase in MDA levels in osteoporotic females and a decrease in MDA levels in the group which was treated with calcitonin, risedronat, and raloksifen. Similarly, Chavan et al. (29) marked the increase in MDA levels in osteoporotic females, but there was no change in alkaline phosphatase level.
Although there are studies that investigated the role of free radicals on bone resorption, Muthusami et al. (6) noted that the data of the bone loss in ovariectomized rats are limited and they also found that the levels of MDA increase, which is induced by lipid per oxidation. Increased production of H2O2 in femur may cause an increase in the lipid per oxidation. It was thought that increased H2O2 induced peroxidation of the polysaturated lipid acids. MDA, which has high reactivation against the amino groups, inhibits the protein synthesis and deactivates the enzymes. For this reason, it is reported that decreased antioxidant enzymes of the femur corresponded to increased lipid peroxidation.
Basu et al. (10) investigated the lipid peroxidation levels by assessing the urine levels of the 8-iso-PGF(2α) in osteoporotic patients; they indicated that there is a correlation between lipid peroxidation and BMD.
Experimental and clinical studies about OP point different results in other findings, except Maggio et al. (7), besides increased lipid peroxidation and as a result of this increased MDA activity is indicated (14, 30). In our study, we found increased MDA activity in femur compared with other groups and increased MDA activity in plasma group compared with the control group.
The results of the earlier studies on animals showed that osteoclastic functions and differentiations are stimulated by the ROS, especially H2O2 and O2 – (6, 15, 16, 28, 31). Oxygen radicals, which appear on cell levels, are detoxified by the SOD. SOD is inhibited by H2O2, which is a product of the reaction. Formation of SOD increases the speed of dismutation by 104 times. Consequently, reaction of the radicals with the potential substrates and formation of hydroxyl, which is a kind of toxic product, inhibits SOD (32).
Osteoclasts produce high levels of O2 – and calciotropic hormones which stimulate or inhibit the osteoclasts have also stimulating or inhibiting effect on cell's production of anion. H2O2 functions both as intra- and inter-cellular pulses and has the most appropriate characteristic besides the other reactive oxygen types. From this point of view, ROS, which is responsible for bone loss due to estrogen deficiency, is asserted as H2O2. Increased OS is related with the risk factors of OP, like smoking, hypertension (HT), and diabetes mellitus (DM). Estrogen deficiency decreases the defense of the thiol antioxidants and induces TNF-α expressions, which result in bone loss (6).
Muthusami et al. (6) indicated increased lipid peroxidation and H2O2 levels and decreased enzymatic antioxidants like SOD, GPx, and glutathione-S-transferase in preovariectomized rats compared with the control group. They claimed that H2O2 is necessary for osteoclast differentiation. In another study, the major intracellular enzyme for H2O2 GPx and its expression comparing macrophages is amplified and the levels of enzymes increased by 17-β-eustradiol are shown (31).
Sontakke and Tare (14) in a group of PMO, renal osteo dystrophy, and patients with fractured bone have found decreased GPx and SOD activity compared with the control group, and this indicates the potential role of ROS in bone metabolism.
Bednarek-Tupikowska et al. (30) found similar erythrocyte SOD results in women with both premenopausal and postmenopausal OP.
Ozgocmen et al. (8) found that in patients with OS, SOD enzyme activity was almost same compared with the control group. But on the other hand, osteoporotic women have high SOD enzyme activity in plasma and that there was a correlation between erythrocyte SOD enzyme activity and femur BMD.
Yalin et al. (24), in their study about the role of antioxidants and free radicals in postmenopausal OP, reported that SOD activity in patients with OP was similar compared with the control group. On the other hand, Chavan et al. (29) showed that serum SOD activity is notably higher than the control group.
SOD is accepted as the characteristic of antioxidant defense mechanism. Maggio (7) and Sontakke (14)and Tare found SOD and GPx levels to be low in the osteoporotic patients and they believed that as a result of this the antioxidant defense mechanisms were reduced in OP.
In our study, like Ozgocmen et al. (8) and Chavan et al. (29), we found increased SOD activity in the femur bone tissue in ovariectomized group compared with the control group. Plasma SOD activities increased in the ovariectomized group, but it was not statistically significant.
Among various studies about the evaluation of GPx, which is one of the enzymatic antioxidant enzymes, Maggio et al. (7) reported that plasma GPx activity is significantly low in osteoporotic patients.
Muthusami et al. (6) showed that levels of enzymatic antioxidants decreased like GPx and glutathione-S-transferase in ovariectomized rats compared with the control group and pronounced that H2O2 is necessary for osteoclast differentiation.
Bednarek-Tupikowska et al. (30) compared premenopausal and postmenopausal women and showed a significant decrease in erythrocyte GPx activity in postmenopausal women. Similarly, Sontakke and Tare (14), in a group of PMO, found decreased GPx activity in patients with renal dystrophy and bone fracture compared with the control group.
Ozgocmen et al. (8) found similar results in plasma GPx enzyme activities in OP patients and the control group. Yalin et al. (24) found reduced GPx activity in PMO group. The same investigators (9) showed that male OP patients have significantly low GPx activities, but the difference is not statistically significant. But in osteoporotic patients, compared with the control group, SOD/GPx was higher. Differences in SOD/GPx ratio are very important in cellular resistance over cell death and damage caused by oxidants and also cause increased ROS formation.
In our study, like Ozgocmen et al. (8) and Yalin et al. (9), we found that, in femur bone tissue and in plasma, GPx activity was similar in all the three groups. Causes of increase in SOD activity and unchanged GPx activity in ovariectomized group had higher SOD/GPx ratio. We think that it explains the increase in ROS formation.
Compared with other studies which evaluated another antioxidant named CAT, Ozgocmen et al. (8) reported that erythrocyte CAT activity is meaningly low in patients with postmenopausal OP and also reported that they found a correlation between erythrocyte CAT enzyme activity and proximal femur BMD.
In our study, we found that SOD activity was increased and on the contrary CAT activity was the same in femur antioxidant defenses. We thought that OS level was not very high. Catalase, which speeds up the H2O2 formation with a catalytic reaction transforms H2O2 to two molecules of water and in this way removes it from the environment. In our study, the formation speed of H2O2 might not be so high and for that reason we might have found CAT activities alike.
Plasma thiols are cleaners of the free radicals and in some instances they can be antioxidant. In pathological and physiological situations, to evaluate the overproduction of free radicals, t-SH is measured (33). Protein thiols in some mechanisms can protect their antioxidant function; in the beginning of peroxidation they can remove the antioxidants in a preemptive way (33). We could not find any study in the literature that evaluated thiol in plasma, but there are some studies in which the t-SH is measured (especially in illnesses which are related with OS) (33, 34).
In our study, we found t-SH levels were almost same in each group. Although femur bone tissue t-SH level in ovariectomized group decreased from 113.33+57.14 (mm/g prt) to 76.0+42.16 (mm/g prt), this is probably due to increased standard deviation and low number of subjects in the study.
A study has shown that vitamin C therapy increases the t-SH levels (34). In our study, vitamin C supplementation has increased the t-SH levels in femur bone tissue and in plasma, but this increase is not statistically significant and further studies will make us understand the role of t-SH in OP.
Vitamin C is an influential antioxidant that decreases the effects of free radicals. It limits antioxidants’ bone resorption. Other mechanisms in which vitamin C assists BMD are still not clear, but it is known that it takes place in collagen formation growth of osteoblasts or increase in Ca absorption (35).
In various studies, it was shown that, in postmenopausal females there is a relationship between dietary vitamin C intake and BMD (36). Hall and Greendale (36) showed a consistent positive association of vitamin C with BMD in postmenopausal women with dietary calcium intake of at least 500 mg. Ilich et al. (37) in a cross-sectional study showed significant relationship between BMD and several critical nutrients such as: energy, protein, calcium, magnesium, zinc, and vitamin C. It was shown that people who smoke and take low doses of vitamin C and E in their diets have a higher incidence of hip fracture. It was shown that vitamin C and niacin intake in postmenopausal females and iron and zinc intake in premenopausal females are related closely with the forearm bone mineral content. On the other hand, Wolf et al. (12) did not find any correlation between vitamin C and BMD, but with hormone therapy they found a relation. They also found that in females with high total vitamin C concentration it plays a good role.
Vitamin C deficiency is known in rats and pigs (38), and when they are feeded with low levels of ascorbate many deformities have been pointed out:shortness in the legs, multiple fractures, OP, growth retardation, and tendencies to haemoragies.
Melhus et al. (39) investigated the potential role of antioxidating vitamin on the hip fractures in people who smoke. They reported that if vitamin C and E are taken in low quantities, besides other factors such as age, weight, and other OP risk factors, they cause as much as five times more hip fractures. They concluded that sufficient vitamins intake could protect people from the side effects of smoking.
Morton et al. (13) studied 994 postmenopausal women, in which 277 of them were regularly taking vitamin C support. Daily intake of vitamin C was 100–5,000 (average 745 mg) and the average of the year of intake was 12.4; 85% of them reported that they were taking vitamin C support for more than 3 years. As a result we can say that vitamin C intake has beneficial effects in BMD, especially if they are combined with hormone therapy. In this study, optimal doses were not determined. But best results (high BMD level) were by obtained/ taking 1,000 mg/day or higher.
Simon and Hudes (40) showed a linear relationship between ascorbic acid intake with diet and BMD in premenopausal women. A relationship was found between decreased spontaneous fractures and ascorbic acid levels in females who took estrogen and smoke, but surprisingly they reported a correlation in females who do not use estrogen or smoke.
Maggio et al. (7) pronounced that in elderly osteoporotic patients, there is a significant decrease in vitamin levels, SOD, and GPx activity. They also reported that taking vitamin C and E with diet is protective against hip fractures. Wolf et al. (12) in a cohort study indicated a correlation between dietary antioxidant intake, to total intake serum concentrations and BMD.
With all these results, decreasing on bone resorption and on BMD, vitamin C plays an important role. Further studies need to be done on this subject.
In our study, we added 1 g vitamin C (l-ascorbic acid) to 500 ml of water. We changed the water every day and vitamin C was administered (as explained earlier) as rats consumed 10–12 ml (ml/100 g/day) water every day (41). In this case, our rats took 50–75 g of vitamin C every day.
In our study, BMD was totally restored and OS was prevented in female rats.
In our study we did not measure the vitamin C level. On the other hand, Li and Schellhorn (42) indicated that understanding the effect of vitamin C on various illnesses, serum vitamin C level along with serum glucose level must be measured. It is pointed that hormones and glucose levels affect the process of measuring serum vitamin C level.
For the aforementioned reasons, vitamin C level must specially be measured in studies on humans. In diabetes, blood sugar must be controlled in hyperglycemic states and vitamin C must be increased.
Wronski et al. (3) characterized the bone deficiencies in rats with postmenopausal or ovarian deficiency in 12 control and 12 ovariectomized rats (90 days old) in a 18-month period. They observed that in 6-month-old rats cancellous bone volume were almost stable and reduced later. In contrast to this, in ovariectomized rats the proximal tibia bone volume was dramatically lost in 2 weeks and in 100 days callose bone volume was 5–7% and on day 270 bone volume was 1–2%. In ovariectomized rats, osteoblast level, osteoclast level, and bone formation were higher compared with control group (especially in 100 days in ovariectomized rats) As a result, they said ovariectomy in mature rats causes a rapid bone loss in conjunction with maximum increase in bone circle.
For that reason we used mature rats (100 days old) in our study. After this period we also found that there was a statically significant reduction in BMD.
Our study has some limitations. First, the number of rats used is not sufficient. Because of this, a relationship between BMD and oxidant parameters could not be found. If the study was done with large number of animals, we could have been more successful. On the other hand, vitamin C level intake and output liquid were not measured in our study, and these were our limitations.
To conclude, our results suggest that ovariectomy may produce OP and OS in females, and vitamin C supplementation may provide alterations regarding improvement in OS and BMD values. We assume that studies including more subjects are needed to make a decisive conclusion about OS–BMD relation.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Frost HM, Jee WS. On the rat model of human osteopenias and osteoporoses. Bone Miner. 1992;18:227–36. doi: 10.1016/0169-6009(92)90809-r. [DOI] [PubMed] [Google Scholar]
- 2.Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:175–91. doi: 10.1016/0169-6009(91)90124-i. [DOI] [PubMed] [Google Scholar]
- 3.Wronski TJ, Dann LM, Scott KS, Cintron M. Long-term effects of ovariectomy and aging on the rat skeleton. Calcif Tissue Int. 1989;45:360–6. doi: 10.1007/BF02556007. [DOI] [PubMed] [Google Scholar]
- 4.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 5.Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38:995–1014. doi: 10.1590/s0100-879x2005000700003. [DOI] [PubMed] [Google Scholar]
- 6.Muthusami S, Ramachandran I, Muthusamy B, Vasudevan G, Prabhu V, Subramaniam V, et al. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta. 2005;360:81–6. doi: 10.1016/j.cccn.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88:1523–7. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- 8.Ozgocmen S, Kaya H, Fadillioglu E, Aydogan R, Yilmaz. Z. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol Cell Biochem. 2007;295:45–52. doi: 10.1007/s11010-006-9270-z. [DOI] [PubMed] [Google Scholar]
- 9.Yalin S, Bagis S, Polat G, Dogruer N, Cenk Aksit S, Hatungil R, et al. Is there a role of free oxygen radicals in primary male osteoporosis? Clin Exp Rheumatol. 2005;23:689–92. [PubMed] [Google Scholar]
- 10.Basu S, Michaelsson K, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. 2001;288:275–9. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- 11.Nieves JW. Osteoporosis: the role of micronutrients. Am J Clin Nutr. 2005;81:1232S–1239S. doi: 10.1093/ajcn/81.5.1232. [DOI] [PubMed] [Google Scholar]
- 12.Wolf RL, Cauley JA, Pettinger M, Jackson R, Lacroix A, Leboff MS, et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women's Health Initiative. Am J Clin Nutr. 2005;82:581–8. doi: 10.1093/ajcn.82.3.581. [DOI] [PubMed] [Google Scholar]
- 13.Morton DJ, Barret-Connor EL, Schneider DL. Vitamin C supplement use and bone mineral density in postmenopausal women. J Bone Miner Res. 2001;16:135–40. doi: 10.1359/jbmr.2001.16.1.135. [DOI] [PubMed] [Google Scholar]
- 14.Sontakke AN, Tare RS. A duality in the roles of reactive species with respect to bone metabolism. Clin Chim Acta. 2002;318:145–8. doi: 10.1016/s0009-8981(01)00766-5. [DOI] [PubMed] [Google Scholar]
- 15.Lean MJ, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, et al. A crucial role for thiol antioxidants estrogen-deficiency bone loss. J Clin Invest. 2003;112:915–23. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 17.Van't Hof RJ, Ralston SH. Nitric oxide and bone. Immunology. 2001;103:255–61. doi: 10.1046/j.1365-2567.2001.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caballero-Alias AM, Loveridge N, Lyon A, Das-Gupta V, Pitsillides A, Reeve J. NOS isoforms in adult human osteocytes: multiple pathways of NO regulation? Calcif Tissue Int. 2004;75:78–84. doi: 10.1007/s00223-003-0161-y. [DOI] [PubMed] [Google Scholar]
- 19.Van't Hof RJ, Macphee J, Libouban H, Helfrich MH, Ralston SH. Regulation of bone mass and bone turnover by neuronal nitric oxide synthase. Endocrinology. 2004;145:5068–74. doi: 10.1210/en.2004-0205. [DOI] [PubMed] [Google Scholar]
- 20.Baecker N, Boese A, Schoenau E, Gerzer R, Heer M. L-arginine, the natural precursor of NO, is not effective for preventing bone loss in postmenopausal women. J Bone Miner Res. 2005;20:471–9. doi: 10.1359/JBMR.041121. [DOI] [PubMed] [Google Scholar]
- 21.Aguirre J, Buttery L, O'Shaughnessy M, Afzal F, Fernandez de Marticorena I, Hukanen M, et al. Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am J Pathol. 2001;158:247–57. doi: 10.1016/S0002-9440(10)63963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wimalawansa SJ. Restoration of ovariectomy-induced osteopenia by nitroglycerin. Calcif Tissue Int. 2000;66:56–60. doi: 10.1007/s002230050011. [DOI] [PubMed] [Google Scholar]
- 23.Ozgocmen S, Kaya H, Fadillioglu E, Yilmaz Z. Effects of calcitonin, risedronate, and raloxifene on erythrocyte antioxidant enzyme activity, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Arch Med Res. 2007;38:196–205. doi: 10.1016/j.arcmed.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Yalin S, Bagis S, Aksit SC, Arslan H, Erdogan C. Effect of free radicals and antioxidants on postmenopausal osteoporosis. A J of Chem. 2006;18:1091–6. [Google Scholar]
- 25.Halliwell B. Free radicals, antioxidants and human disease: curiosity, cause or consequence? Lancet. 1994;344:721–4. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 26.Gambhir JK, Lali P, Jain AK. Correlation between blood antioxidant levels and lipid peroxidation in rheumatoid arthritis. Clin Biochem. 1997;30:351–5. doi: 10.1016/s0009-9120(96)00007-0. [DOI] [PubMed] [Google Scholar]
- 27.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–37. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 28.Steinbeck MJ, Appel WH, Jr, Verhoeven AJ, Karnovsky MJ. NADPH-oxidase expression and in situ production of superoxide by osteoclasts actively resorbing bone. J Cell Biol. 1994;126:765–72. doi: 10.1083/jcb.126.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chavan SN, More U, Mulgund S, Saxena V, Sontakke AN. Effect of supplementation of vitamin C and E on oxidative stress in osteoporosis. Ind J of Clin Biochemi. 2007;22:101–5. doi: 10.1007/BF02913324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bednarek-Tupikowska G, Bohdanowicz-Pawlak A, Bidzinska B, Milewicz A, Antonowicz-Juchniewicz J, Andrzejak R. Serum lipid peroxide levels and erythrocyte glutathione peroxidase and superoxide dismutase activity in premenopausal and postmenopausal women. Gynecol Endocrinol. 2001;15:298–303. [PubMed] [Google Scholar]
- 31.Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclasts formation. Endocrinology. 2005;146:728–35. doi: 10.1210/en.2004-1021. [DOI] [PubMed] [Google Scholar]
- 32.Bolann BJ, Ulvik RJ. Improvement of a direct spectrophotometric assay for routine determination of superoxide dismutase activity. Clin Chem. 1991;37:1993–9. [PubMed] [Google Scholar]
- 33.Pasaoglu H, Sancak B, Bukan N. Lipid peroxidation and resistance to oxidation in patients with type 2 diabetes mellitus. Tohoku J Exp Med. 2004;203:211–18. doi: 10.1620/tjem.203.211. [DOI] [PubMed] [Google Scholar]
- 34.Yousef MI, Awad TI, Elhag FA, Khaled FA. Study of the protective effect of ascorbic acid against the toxicity of stannous chloride on oxidative damage, antioxidant enzymes and biochemical parameters in rabbits. Toxicology. 2007;235:194–202. doi: 10.1016/j.tox.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Macdonald HM, New SA, Golden MH, Campbell MK, Reid DM. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr. 2004;79:155–65. doi: 10.1093/ajcn/79.1.155. [DOI] [PubMed] [Google Scholar]
- 36.Hall SL, Greendale GA. The relation of dietary Vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998;63:183–9. doi: 10.1007/s002239900512. [DOI] [PubMed] [Google Scholar]
- 37.Ilich JZ, Brownbill RA, Tamborini L. Bone and nutrition in elderly women: protein, energy, and calcium as main determinants of bone mineral density. Eur J Clin Nutr. 2003;57:554–65. doi: 10.1038/sj.ejcn.1601577. [DOI] [PubMed] [Google Scholar]
- 38.Kawai T, Nishikimi M, Ozawa T, Yagi K. A missense mutation of l-gulono-gamma-lactone oxidase causes the inability of scurvy-prone osteogenic disorder rats to synthesize l-ascorbic acid. J Biol Chem. 1992;267:21973–6. [PubMed] [Google Scholar]
- 39.Melhus H, Michaelsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins and risk of hip fracture. J Bone Miner Res. 1999;14:129–35. doi: 10.1359/jbmr.1999.14.1.129. [DOI] [PubMed] [Google Scholar]
- 40.Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am J Epidemiol. 2001;154:427–33. doi: 10.1093/aje/154.5.427. [DOI] [PubMed] [Google Scholar]
- 41.Van Zutphen LFM, Baumans V, Beynen AC. Principles of Laboratory Animal Science. 2nd ed. Elsevier Science Publishers; 2001. pp. 19–30. [Google Scholar]
- 42.Li Y, Schellhorn E. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137:2171–84. doi: 10.1093/jn/137.10.2171. [DOI] [PubMed] [Google Scholar]