Abstract
The synthesis, structure, and characterization of a [Yb(Tren-Me-3,2-HOPO)(H2O)2] complex are reported. As a result of its Yb(III) emission in the Near Infra-Red (NIR) region, sensitized by the Me-3,2-HOPO chromophore, this complex can be utilized for the first time to determine the hydration state, q, via the luminescence lifetimes and hence the solution structure of these Me-3,2-HOPO type ligands which have attracted significant interest in complex with Gd(III) as possible next generation magnetic resonance imaging contrast agents (MRI-CA).
It has been more than a decade since the first reports of [Gd(Tren-Me-3,2-HOPO)(H2O)2] as a potential new class of magnetic resonance imaging contrast agent (MRI-CA).2 The defining feature of these 1-methyl-3-hydroxypyridin-2-one (Me-3,2-HOPO) based compounds has been the use of a hexadentate ligand design, and hence an increase in the number of metal bound water molecules, without sacrificing complex stability compared to the typically octadentate contrast agents used commercially. Since that time, significant advances in the properties of these chelates have been steadily reported, including improvements in relaxivity,3,4 incorporation into macromolecular architectures5–7 and, recently, the first direct verification of solution structure using the discovery of Eu(III) centered luminescence with the isomeric 1-hydroxypyridin-2-one (1,2-HOPO) chelate as a sensitizing chromophore.8,9 Nonetheless, it has remained frustrating that direct measurements of the inner sphere hydration state, q, using luminescence techniques with the parent Me-3,2-HOPO compounds have remained elusive, even when direct laser excitation of weakly absorbing f-f transitions were employed (eg. for Eu(III) complexes). This failing can likely be traced to the presence of a low lying LMCT state which efficiently quenches metal based emission. Instead, estimates of the q and hence solution structure have relied on the fitting of relaxivity data to the Solomon-Bloembergen-Morgan equations or, where sufficiently soluble in aqueous solution, studies on the temperature dependence of the paramagnetic contribution to the water 17O NMR transverse relaxation rate.10 Recently, Beeby et al11 reported on a qualitative equation to determine inner sphere hydration based on the change in lifetimes for Yb(III) in going from H2O to D2O solution, and we reasoned that the lower energy accepting state of Yb(III) may lie below the LMCT state which quenches Eu(III) emission, and hence may facilitate sensitized emission from Yb(III). This hypothesis was borne out experimentally, and herein we describe for the first time sensitized luminescence in the Near Infra-Red (NIR) region from a [Yb(Tren-Me-3,2-HOPO)(H2O)2] complex, and hence the direct measurement of q for the archetypical member of this family of compounds.
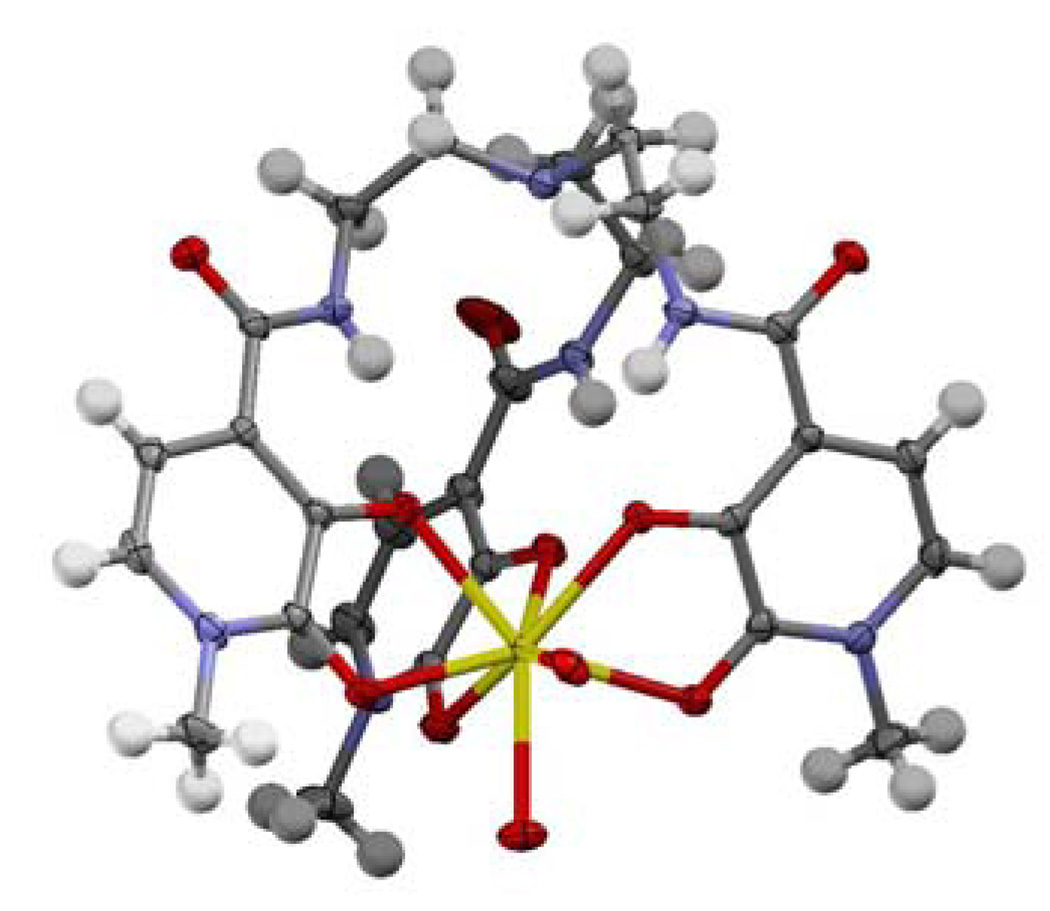
The [Yb(Tren-Me-3,2-HOPO)(H2O)2] complex was prepared in analytically pure form using standard methodologies in MeOH as solvent with excess pyridine as a base to ensure deprotonation of the phenolic oxygens (see Supp. Info. for more details). X-ray quality crystals of the complex were grown by slow evaporation (over several months) of a 10 µM aqueous solution in 0.1 M TRIS buffer at pH 7.4. The resulting structure12 is shown in Fig. 1, with full crystal data in CIF format given in the Supp. Info. The metal center is eight coordinate (CN = 8), and the structure is isomorphous with that previously reported for the Tren-Me-3,2-HOPO ligand with Gd(III).2 Coordinate bond lengths to the metal center are typical (avg. Yb-O (phenolate) ~ 2.293 Å, avg. Yb-O (keto) ~ 2.349 Å) and are slightly shorter by ca. 2.5 % than those observed for Gd(III) (avg. Gd-O (phenolate) ~ 2.358 Å, avg.. Gd-O (keto) ~ 2.408 Å) as to be expected due to the well known lanthanide contraction.13 The presence of an intermolecular H-bonding interaction between the amide N-H and phenolate oxygen common to this structural motif is noted, with an average N-H---O separation of ca. 1.96 Å and the orientation of the tertiary amine lone pair from the Tren cap is directed toward the Yb(III) metal center. Other than the differing identities of co-crystallized solvent, the structures are essentially identical in all other aspects.
Figure 1.
A view of the asymmetric unit for the [Yb(Tren-Me-3,2-HOPO)(H2O)2]·2H2O complex. Non-coordinated solvent water molecules omitted for clarity and thermal ellipsoids are drawn at the 50 % probability level.
An important structural parameter which can also be obtained from the crystallographic data is the shape measure, SM,14,15 defined as;
This is a measure of the agreement in the variance of dihedral angles along all edges between the polyhedron derived from the crystal structure and the idealized cases. For the eight coordinate geometry, the three most common idealized coordination polyhedra are the bicapped trigonal prismatic (C2v), square antiprismatic (D4d) and trigonal dodecahedral (D2d) geometries, and the lowest value of SM for the three pairs represents the best fit to the closest idealized geometry. The resulting shape analysis results for the Yb(III) and Gd(III) complexes are summarized in Table 1. The coordination geometry of the Gd(III) was previously2 visually interpreted as a distorted bicapped trigonal prism (C2v). However, inspection of the shape analysis results reveal this complex is in fact closer to the trigonal dodecahedral (D2d) geometry, and hence is isostructural with the results obtained herein for the new Yb(III) analogue.
Table 1.
Summary of shape analysis results for Yb(III) and Gd(III) complexes of Tren-Me-3,2-HOPO.
| Shape Measure (SM) | |||
|---|---|---|---|
| Complex | D4d | C2v | D2d |
| [Yb(Tren-Me-3,2-HOPO)(H2O)2] | 14.35 | 11.42 | 5.45 |
| [Gd(Tren-Me-3,2-HOPO)(H2O)2] | 16.04 | 11.50 | 6.52 |
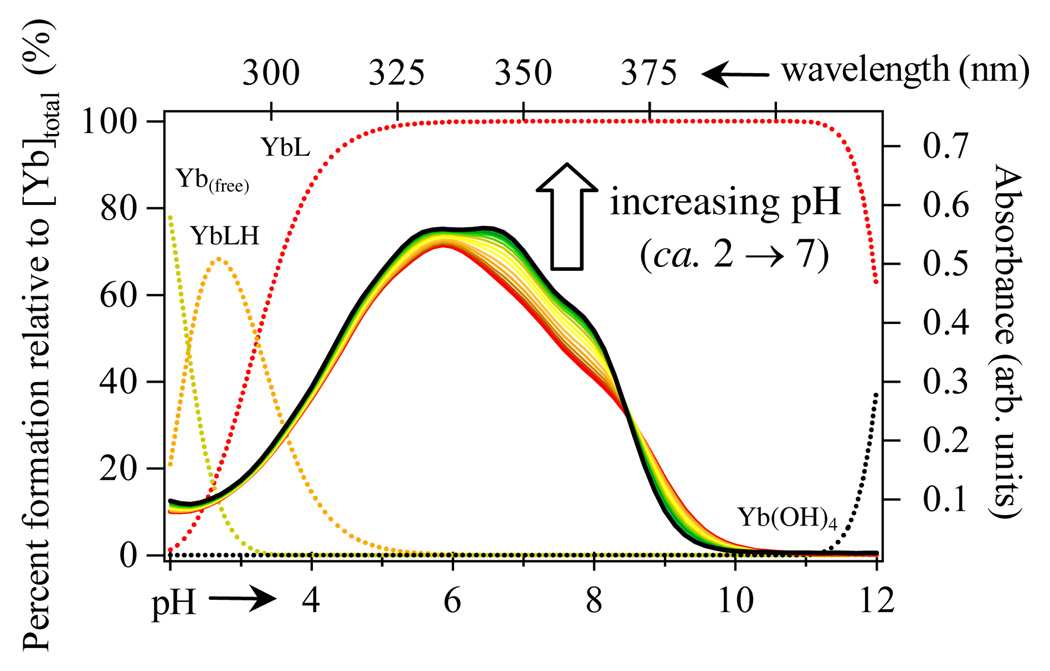
Our attempts to determine the pM17 of the complex by competition titration versus DTPA were unsuccessful, due to evidence of ternary complex formation (see Fig S1, Supp Info.). Instead, the aqueous stability was assessed by spectrophotometric titration as detailed elsewhere,16 utilizing the reported pKa’s of the Tren-Me-3,2-HOPO ligand.2 Resulting absorption spectra versus pH are shown in Fig. 2, and the stability constants determined for β110 and β111 were 20.81(2) and 24.04(2) respectively, with the site of protonation assigned to the amine backbone in accordance with previous studies.2 These data yielded a pM17 with Yb(III) of 20.8 which is comparable to the previously reported2 pGd = 20.3 with the same ligand, the slight increase being consistent with other ligand systems (eg. DTPA, EDTA, etc), and attributable to the decreasing size and increasing Lewis acidity of the metal.
Figure 2.
Speciation diagram of ca. 25 µM [Yb(Tren-Me-3,2-HOPO)(H2O)2] complex in aqueous solution (left, bottom axes) and overlaid changes in UV-Visible spectra (right, top axes) upon change in pH. red = free ligand spectrum (ca. pH ~ 2), black = fully formed complex (above ca.. pH 5.5 – 6).
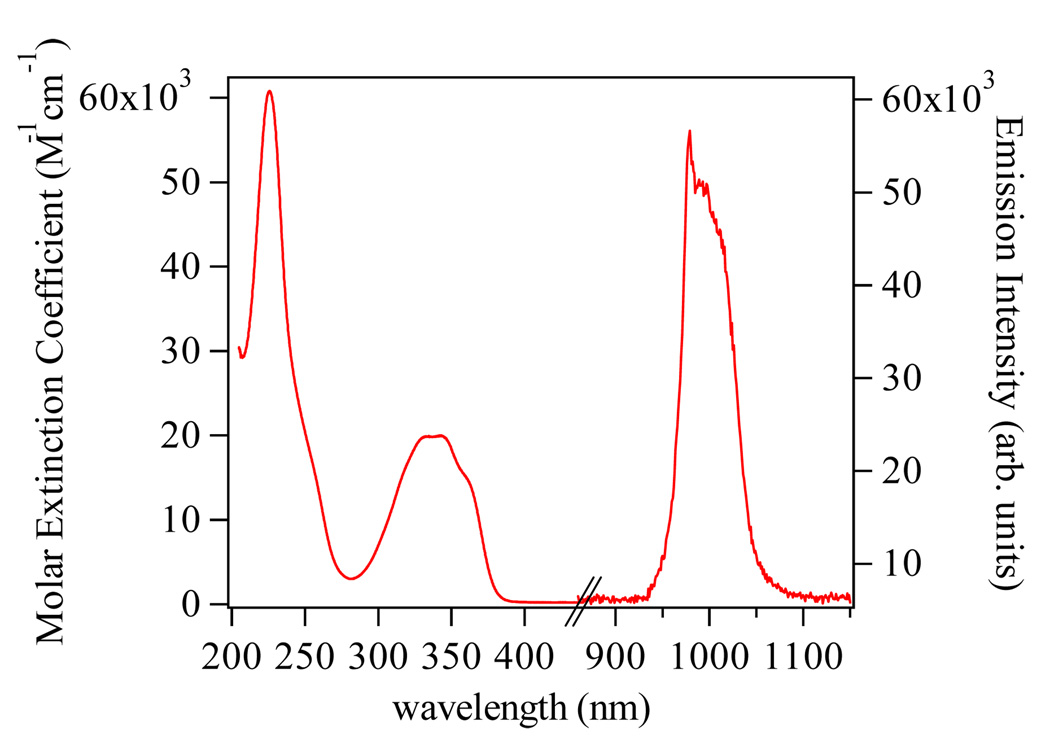
Having established the aqueous stability, we were interested in the photophysical properties of the Me-3,2-HOPO chromophore. The molar absorption and emission spectra for a ca. 10 µM solution of [Yb(Tren-Me-3,2-HOPO)(H2O)2] in 0.1 M TRIS buffer at pH 7.4 are shown in Fig. 3. The lowest energy ligand centered absorption maxima is located at ca. 345 nm (ε ~ 19,000 M−1 cm−1), in accordance with our previous reports, with a more intense ππ* band to higher energy at ca. 225 nm (ε ~ 60,000 M−1 cm−1). More importantly, our prediction that the Me-3,2-HOPO chromophore may sensitize Yb(III) was confirmed experimentally, and the characteristic metal centered 2F7/2 ← 2F5/2 emission band is clearly evident with a peak at ca. 980 nm, broadened due to crystal field splitting of this transition.
Figure 3.
Electronic absorption spectrum in 0.1 M TRIS buffer at pH 7.4 (left) and steady state Near Infra-Red (NIR) emission spectrum in D2O (right) (λex = 345 nm, 10 nm bandpass) for the [Yb(Tren-Me-3,2-HOPO)(H2O)2] complex.
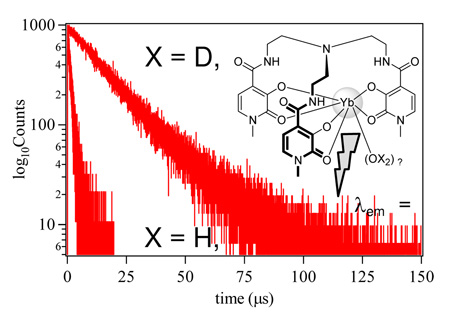
Lastly, to determine the hydration state, q, of the complex, we measured the observed NIR lifetime of the complex at 980 nm upon selective N2 laser excitation at 337.1 nm. The resulting τobs values obtained were 0.37 µsec and 8.06 µsec in H2O and D2O respectively. Hence, substitution of kH and kD into the empirical equation given by Beeby et al; q = A (kH - kD - B), with A = 1 µsec−1 and B = 0.2 µsec−1 yielded a value of q = 2.4. We take this non-integer result as a slight overestimate, and the residual can be accounted for by quenching due to adjacent vC=O and vC–H oscillators, which have a known11 strong quenching effect on NIR emission. In conclusion, we have confirmed a coordination number of eight (CN = 8) in aqueous solution for the archetypical Tren-Me-3,2-HOPO ligand by NIR luminescence techniques and are now working toward similar measurements with other ligand systems (eg. Tren-bis-HOPO-TAM).
Supplementary Material
ACKNOWLEDGEMENT
This work was partially supported by the NIH (Grant HL69832) and the Director, Office of Science, Office of Basic Energy Sciences, and the Division of Chemical Sciences, Geosciences, and Biosciences of the U.S. Department of Energy at LBNL under Contract No. DE-AC02-05CH11231.
Footnotes
Supporting Information Available: Detailed synthesis of [Yb(Tren-Me-3,2-HOPO)(H2O)2], X-ray crystallographic file (in CIF format), additional experimental details for conditional stability constant and photophysical characterization. These files are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Part 25 in the series: High Relaxivity Gadolinium MRI Agents. For the previous paper in this series, see: Datta A, Hooker JM, Botta M, Francis MB, Aime S, Raymond KN. J. Amer. Chem. Soc. 2008;130:2546. doi: 10.1021/ja0765363.
- 2.Xu J, Franklin SJ, Whisenhunt DW, Jr, Raymond KN. J. Amer. Chem. Soc. 1995;117:7245. [Google Scholar]
- 3.Pierre VC, Botta M, Aime S, Raymond KN. J. Amer. Chem. Soc. 2006;128:5344. doi: 10.1021/ja057805x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werner EJ, Avedano S, Botta M, Hay BP, Moore EG, Aime S, Raymond KN. J. Amer. Chem. Soc. 2007;129:1870. doi: 10.1021/ja068026z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datta A, Hooker JM, Botta M, Francis MB, Aime S, Raymond KN. J. Amer. Chem. Soc. 2008;130:2546. doi: 10.1021/ja0765363. [DOI] [PubMed] [Google Scholar]
- 6.Pierre VC, Botta M, Aime S, Raymond KN. J. Amer. Chem. Soc. 2006;128:9272. doi: 10.1021/ja061323j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierre VC, Botta M, Raymond KN. J. Amer. Chem. Soc. 2005;127:504. doi: 10.1021/ja045263y. [DOI] [PubMed] [Google Scholar]
- 8.Moore EG, Xu J, Jocher CJ, Werner EJ, Raymond KN. J. Amer. Chem. Soc. 2006;128:10648. doi: 10.1021/ja062597+. [DOI] [PubMed] [Google Scholar]
- 9.Jocher CJ, Moore EG, Xu J, Avedano S, Botta M, Aime S, Raymond KN. Inorg. Chem. 2007;46:9182. doi: 10.1021/ic700985j. [DOI] [PubMed] [Google Scholar]
- 10.Pierre VC, Botta M, Aime S, Raymond KN. Inorg. Chem. 2006;45:8355. doi: 10.1021/ic061262q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beeby A, Clarkson IM, Dickins RS, Faulkner S, Parker D, Royle L, de Sousa AS, Williams JAG, Woods M. J. Chem. Soc., Perkin Trans. 1999;2:493. [Google Scholar]
- 12.Crystal data: C27H30N7O9Yb (769.62 g/mol), Triclinic, a = 10.1056 (7) Å, b = 10.6100 (7) Å, c = 16.8122 (11) Å, α = 84.157 (1), β = 79.139 (1), γ =66.176 (1), V = 1618.81(8) Å3, space group P 1 (No. 2), Z = 2, T = 189 (2) K, λ = 0.71073 Å, ρdiff = 1.58 g/cm3, µ = 2.95 mm−1, R1(F0) = 0.028, wR2 (F02) = 0.063, GOF = 1.021.
- 13.Seitz M, Oliver AG, Raymond KN. J. Amer. Chem. Soc. 2007;129:11153. doi: 10.1021/ja072750f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kepert DL. Prog. Inorg. Chem. 1978;24:179. [Google Scholar]
- 15.Porai-Koshits MA, Aslanov LA. Zhurnal Strukturnoi Khimii. 1972;13:266. [Google Scholar]
- 16.Xu J, O'Sullivan B, Raymond KN. Inorg. Chem. 2002;41:6731. doi: 10.1021/ic025610+. [DOI] [PubMed] [Google Scholar]
- 17.pM = −log10[M]free. Defined as the negative log of the free metal concentration at pH 7.4 with 10 µM [L]total and 1 µM [M]total.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.